ABSTRACT
Objective: Ultraviolet B (UVB) irradiation is the initial etiological factor for various skin disorders, including erythema, sunburn, photoaging, and photocarcinogenesis. Pterostilbene (Pter) displayed remarkable antioxidant, anti-inflammatory, and anticarcinogenic activities. This study aimed to investigate the effective mechanism of Pter against UVB-induced photodamage in immortalized human keratinocytes.
Methods: Human keratinocytes were pretreated with Pter (5 and 10 μM) for 24 h prior to UVB irradiation (300 mJ/cm2). Harvested cells were analyzed by MTT, DCFH-DA, comet, western blotting, luciferase promoter, small interference RNA transfection, and quantitative real-time polymerase chain reaction assay.
Results: Pter significantly attenuated UVB-induced cell death and reactive oxygen species (ROS) generation, and effectively increased nuclear translocation of NF-E2-related factor-2 (Nrf2), expression of Nrf2-dependent antioxidant enzymes, and DNA repair activity. Moreover, the protective effects of Pter were abolished by small interference RNA-mediated Nrf2 silencing. Furthermore, Pter was also found to induce the phosphorylation of Nrf2 and the known phosphatidylinositol-3-kinase (PI3K) phosphorylated kinase, Akt. The specific inhibitor of PI3K, LY294002, successfully abrogated Pter-induced Nrf2 phosphorylation, activation of Nrf2-antioxidant response element pathway, ROS scavenging ability, and DNA repair activity.
Conclusion: The present study indicated that Pter effectively protected against UVB-induced photodamage by increasing endogenous defense mechanisms, scavenging UVB-induced ROS, and aiding in damaged DNA repair through a PI3K-dependent activation of Nrf2/ARE pathway.
Introduction
Ultraviolet B (UVB), wavelength 290–320 nm, is the initial etiological factor that induces a variety of cutaneous pathologies, including erythema, sunburn, premature skin aging, and skin tumors [Citation1,Citation2]. Cumulative UVB irradiation induces excessive production of reactive oxygen species (ROS), which causes oxidative modification of cellular macromolecules, such as lipids, proteins, and nucleic acids, leading to cell death and skin disorders, such as inflammation, premature aging, and even skin cancers [Citation3,Citation4].
Skin is provided with a complicated antioxidant system to eliminate ROS. NF-E2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway is recognized as the central defense mechanism against oxidative stress, which regulates the expression of a battery of detoxifying/antioxidant genes [Citation5]. Nrf2 disrupts from its repressor Kelch-like ECH-associated protein 1 (Keap1), rapidly undergoes nuclear translocation, and transactivates the ARE-related genes [Citation6], when induced by antioxidants, such as pterostilbene (Pter) [Citation7]. This process can promote ROS scavenging, maintain redox homeostasis, inhibit inflammation, and repair damaged DNA, thereby enhancing cell survival in pro-oxidative environments [Citation8]. Thus, using exogenous antioxidants for the activation of Nrf2/ARE pathway was thought to be a rational approach for improving skin’s protective ability against external insults [Citation9].
Pter, a natural resveratrol analog [Citation10], is an active compound isolated from blueberries and Pterocarpus marsupium heartwood [Citation11]. Pter has been shown to possess preventive and therapeutic properties against numerous human diseases, including neurological, cardiovascular, metabolic, and hematologic disorders, due to its ability to reduce ROS production [Citation12,Citation13]. Many mechanisms and cellular targets are involved in the antioxidant and anti-tumor effects of Pter, such as signaling of Notch1 [Citation14], Wnt [Citation15], adenosine monophosphate-activated protein kinase (AMPK) [Citation16], and ROS [Citation17]. Pter has been found to prevent UVB-induced skin acute damage and carcinogenesis in hairless mice [Citation18]. However, the mechanism and cellular signaling pathways underlying the action of Pter have still not been fully understood. Therefore, the present study explored the protective role and possible mechanism of Pter in UVB-irradiated immortalized human keratinocytes (HaCaT) cells, especially focusing on the activation of the Nrf2/ARE pathway by Pter.
Materials and methods
Cell culture and UVB irradiation
The HaCaT cell culture and UVB (300 mJ/cm2) irradiation were performed as described previously [Citation19]. Briefly, after washing twice with phosphate-buffered saline (PBS), the cells were irradiated with a thin cover of PBS (0.4 mL in 6-well plates) to avoid drying, using a UVB-TL/12 lamp (Philips, Amsterdam, Netherlands). A UV dosimeter (Sigma High-Tech Co., Shanghai, China) was used to monitor the irradiation dose. After UVB irradiation, PBS was replaced with a fresh medium.
MTT assay
Pter (1 mM; Sigma, St. Louis, MO, USA) was prepared in dimethyl sulfoxide. HaCaT cells were treated with Pter (5 and 10 μM) for 24 h, followed by UVB irradiation. The MTT assay was performed as described in a previous study [Citation19].
ARE luciferase reporter assay
ARE luciferase reporter plasmid (8× ARE) was constructed as described by Lieder et al. [Citation20]. HaCaT cells were transiently transfected with ARE luciferase reporter plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 24 h, transfected cells were transferred to a fresh medium containing Pter or dimethyl sulfoxide as a negative control. Luciferase activities were obtained using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA). The inhibitors used were LY294002 [phosphatidylinositol-3-kinase (PI3K) inhibitor], SB203580 [p38 mitogen-activated protein kinase (MAPK) inhibitor], u0126 [extracellular signal–regulated kinase (ERK) 1/2 inhibitor], and SP600125 [c-Jun N-terminal kinase (JNK) inhibitor]. The cells were incubated for 1 h prior to Pter treatment.
Measurement of intracellular ROS
The cells were treated with Pter for 24 h, followed by UVB irradiation, and then re-incubated for 4 h. The cells in each well were incubated with 100 μM DCFH-DA for 30 min and placed under a fluorescence microscope (Olympus, Tokyo, Japan). Fluorescence was measured using the Guava Flow Cell fluorometer (Millipore, Billerica, MA, USA) with excitation and emission wavelengths of 485 and 530 nm.
Quantitative real-time RT-PCR
The total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and cDNA was generated with M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Relative gene expression analysis was performed using the Step-One Plus™ Real-Time PCR System (Invitrogen, Carlsbad, CA, USA). The sets of primers used are shown in .
Table 1. Quantitative RT-PCR primer sequences.
Small interference RNA silencing
Transfection was performed with Nrf2-targeted small interference RNA (siRNA) (CAAACUGACAGAAGUUGACAAUUAU) or scramble siRNA using Lipofectamine 2000.
Western blotting
Cytoplasmic and nuclear protein samples were prepared using NEPER Nuclear and Cytoplasmic Extraction Reagents Kit (Thermo Scientific, Waltham, MA, USA). After incubating with primary antibodies against Nrf2, phospho-Nrf2 and lamin A (Santa Cruz, Dallas, TX, USA), phospho-Akt, total Akt, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Abcam, Cambridge, UK), the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Promega, Madison, WI, USA), and visualized using an ECL Western Blotting Substrate (Thermo Scientific, Waltham, MA, USA).
Comet assay
Comet assay was performed as described in a previous study [Citation19]. Briefly, the cells were treated with Pter prior to UVB irradiation, incubated for 24 h, and subsequently mixed with 0.5% low-melting point agarose. Then they were placed on slides pre-coated with normal agarose, lysed, and placed in an electrophoresis chamber with an alkaline buffer, followed by electrophoresis. The slides were stained with ethidium bromide for detection using the fluorescent microscope. The comet image was quantitatively analyzed using the CASP software.
Statistics
All the data were expressed as the mean ± standard deviation (SD) and analyzed using a one-way analysis (GraphPad Software, La Jolla, CA, USA). A P value < 0.05 was considered as statistically significant.
Results
Pter protected against UVB-induced cell death through activation of Nrf2
The MTT assay showed that 5–10 μM Pter had no obvious effect on the cell viability ((A)). Since Nrf2 plays a key role in the defense of cells against oxidative stress, the effectiveness of Pter (5 and 10 μM) on the modulation of cytosolic and nuclear Nrf2 protein was first investigated. As shown in (B,C), Nrf2 translocation into the nucleus increased as the concentration of added Pter increased.
Figure 1. Nrf2 silencing abolished the protective effect of Pter. (A) Viability of HaCaT cells incubated for 24 h in different Pter concentrations. Data are presented as percentage of the untreated control ± SD (n = 6); ns = P > 0.05, ***P < 0.001. (B) Pter-induced Nrf2 nuclear accumulation. HaCaT cell treatment with Pter for 24 h. The indicated proteins were detected by western blotting. GAPDH and lamin A were used to confirm the purity of the cytosolic and nuclear extracts, respectively. (C) Relative protein level of Nrf2. Quantitative densitometric data were expressed as fold change, the vehicle was set to 1. Values are mean ± SD (n = 3); *P < 0.05, ***P < 0.001. (D) Nrf2 was silenced by transfecting with siRNA. HaCaT cells were transfected with a specific siRNA against Nrf2 (siNrf2) or a non-silencing control (NC-siRNA). The knocking down efficiency was demonstrated by western blotting using GAPDH as a loading control. (E) Quantification densitometric data from (D). Values are mean ± SD (n = 3); ns = P > 0.05, ***P < 0.001, compared with non-transfection control. (F) Cell viability in HaCaT cells treated as indicated. Data are presented as percentage of the untreated control ± SD (n = 6); ns = P > 0.05, ***P < 0.001.
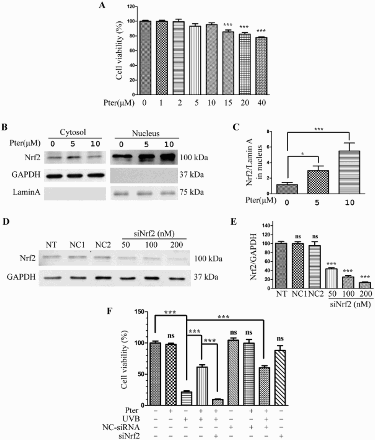
The cell viability was measured after siRNA knockdown of Nrf2 to determine whether protective effects against UVB-induced cell death were mediated by Nrf2. The efficiency of Nrf2 knocking down was determined by western blotting using GAPDH as a control ((D,E)). Pter significantly prevented the UVB-induced reduction in cell viability, whereas the protective effect of Pter was abolished by Nrf2 silencing. However, transfection with the same amount of siNrf2 or nonspecific control siRNA (NC-siRNA) alone without UVB irradiation had no obvious effect on the cell viability ((F)).
Pter-activated Nrf2/ARE through PI3K
Nrf2 is known to be activated by multiple signaling kinases, such as p38 MAPK, PI3K, and ERK [Citation21–23]. Hence, related kinase inhibitors were utilized to explore the mechanism of Nrf2 activation by Pter. As shown in (A), the increased activity of the ARE reporter induced by Pter (5 and 10 μM) was completely abrogated by LY294002, an inhibitor of PI3K. However, no obvious effects of inhibitors of MAPK, ERK, and JNK on Pter-induced ARE activation were detected (data not shown).
Figure 2. Pter activated Nrf2/ARE via PI3K. After transfection with luciferase reporter plasmid for 24 h, HaCaT cells were pretreated for 1 h with LY294002 prior to Pter treatment. (A) Luciferase activity data are presented as percentage of the untreated control ± SD (n = 6); ns = P > 0.05, ***P < 0.001. (B) The indicated proteins were detected by western blotting, with laminA as the loading control. (C) The levels of phosphorylated Nrf2 were quantified by densitometric scanning. Values are mean ± SD (n = 3); **P < 0.01, ***P < 0.001. (D) Cell viability in HaCaT cells treated as indicated. Data are presented as percentage of the untreated control ± SD (n = 6); ns = P > 0.05, *P < 0.05, ***P < 0.001.
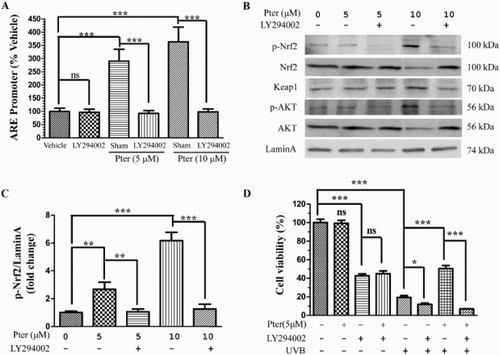
The phosphorylation of Nrf2 and the known PI3K phosphorylated kinase, Akt was analyzed by western blotting to further confirm that the PI3K pathway was activated directly by Pter. The increase in the phosphorylation of Nrf2 and Akt was observed in the Pter-treated cells, whereas pretreatment with LY294002 attenuated the Pter-induced phosphorylation of Nrf2 and Akt ((B,C)). Moreover, the effect of Pter on the Keap1 protein level was also analyzed. It is notable that Pter had no obvious effect on the Keap1 protein level compared with the untreated control.
The possible link between the protective effects of Pter against UVB-induced cell death and PI3K pathway was investigated by assessing the effect of LY294002 on the cell viability. As shown in (D), inhibition of PI3K diminished the protective effect of Pter in HaCaT cells.
Pter scavenged UVB-induced ROS via PI3K
The UVB-induced excessive ROS production is identified as the key factor of photo-damage. As shown in (A,B), treatment with Pter prior to UVB irradiation significantly reduced the UVB-induced ROS generation. However, LY294002, the PI3K inhibitor, abolished Pter-mediated inhibition of ROS production.
Figure 3. Pter scavenged UVB-induced ROS via PI3K. HaCaT cells were treated with vehicle, Pter, and LY294002 prior to UVB irradiation. (A) Representative intracellular ROS images and recordings. (B) Quantitative fluorescence data from (A) were presented as mean ± SD (n = 3); ***P < 0.001. (C) Quantitative RT-PCR data of indicated Nrf2 target genes. The relative mRNA levels of Nrf2 target genes were represented as fold change; the vehicle was set to 1 with gapdh and actin for normalization. Values are mean ± SD (n = 3), *P < 0.05.
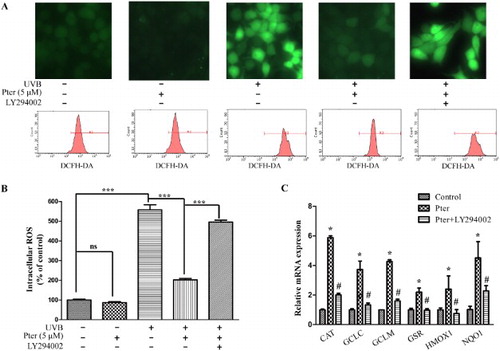
A previous study indicated that Pter decreased oxidative damage by activating endogenous antioxidant enzymes [Citation18]. Quantitative RT-PCR was used to detect mRNA expression of some known Nrf2-dependent ARE target genes, including catalase, glutamate-cysteine ligase catalytic subunit, glutamate-cysteine ligase, modifier subunit, glutathione reductase, heme oxygenase 1, and NAD(P)H:quinone oxidoreductase 1 to confirm that antioxidant enzymes were induced by the activation of Nrf2 by Pter through PI3K. These target genes were upregulated by Pter treatment apparently. However, LY294002 reversed the upregulation caused by Pter ((C)).
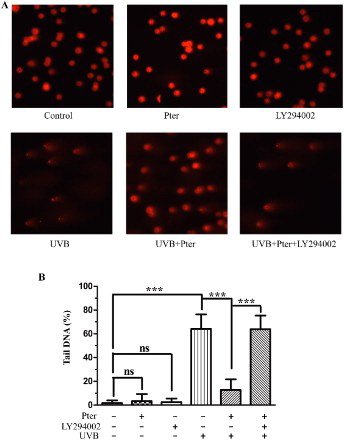
Pter prevented UVB-induced DNA damage by PI3K
UV-induced DNA damage is the decisive factor for photoaging and skin cancer. The strand breaks of DNA damage were measured by the comet assay ((A,B)). Treatment with Pter or LY294002 alone has no observable effect on DNA. Pter pretreatment prior to UVB treatment significantly reduced the UVB-induced DNA comet tail compared with the UV-irradiated control. However, Pter-induced DNA repair activity was effectively inhibited by LY294002.
Discussion
The incidence of skin disorders caused by UV, such as inflammation, immunosuppression, photoaging, and carcinogenesis, increases with aging and in UV irradiation due to depletion of the ozone layer [Citation1,Citation2,Citation24]. Indeed, various plant polyphenols have been shown to possess anti-inflammatory, immunomodulatory, and antioxidant properties to protect from UV-induced photo-damage [Citation25]. The photoprotective property of topical application of Pter on mouse skin has been reported [Citation18]. However, the mechanisms responsible for the protective effects of Pter have not been elucidated fully. The present study investigated the role of Nrf2/ARE pathway in Pter-mediated protective effects against UVB-induced photo-damage and the underlying signaling pathway.
The Nrf2/ARE pathway plays a critical role in defense mechanism against oxidative stress [Citation5,Citation8,Citation9]. Nrf2 deficiency enhances UVB-induced skin inflammation, oxidative DNA damage, and extracellular matrix damage [Citation26]. Conversely, genetic or pharmacological Nrf2 activation protects against UV-mediated skin carcinogenesis [Citation27]. More recently, Sirerol et al. [Citation18] observed the close relationship between the photoprotective effect of Pter and its ability to modulate the Nrf2-dependent antioxidant response. Furthermore, it has been reported that irradiation of mouse skin with UVB (100 or 300 mJ/cm2) failed to activate Nrf2 although elevated levels of ROS [Citation28]. The present results showed that Pter significantly increased Nrf2 translocation into the nucleus, expression of Nrf2-dependent antioxidant enzymes, ROS scavenging ability, and DNA repair activity. Moreover, Pter failed to protect the Nrf2-knockdown cells from UVB-induced oxidative stress. These results confirmed that the photoprotective effect of Pter against UVB-induced photo-damage was mainly mediated by Nrf2 activation.
Activation of Nrf2 results from the disruption of Keap1–Nrf2 complex and subsequent nuclear translocation of Nrf2, which begins with either the modification of Keap1 cysteine thiols [Citation5] or the phosphorylation of Nrf2 by multiple signaling kinases, such as MAPK, PI3K/Akt, protein kinase C, and AMPK [Citation21–23]. The present data showed that Pter significantly increased the ARE activity and protein level of phosphorylated Nrf2 and Akt. However, the Pter-induced ARE activity and phosphorylation of Nrf2 were successfully abolished by LY294002, a selective inhibitor of PI3K. These results suggested that Pter-activated PI3k pathway and subsequently inducted Nrf2. Furthermore, treatment with LY294002 also successfully abrogated Pter-induced upregulation of Nrf2/ARE-dependent genes, ROS scavenging ability, and DNA repair activity. These results demonstrated that Pter effectively activated Nrf2/ARE via PI3K signaling, and subsequently increased endogenous defense mechanisms, scavenged UVB-induced ROS, and promoted DNA repair activity, thereby protecting against UVB-induced photo-damage.
It is well known that Pter has much more powerful antioxidant, anti-inflammatory, and anticancer effects than resveratrol, despite sharing a similar structure [Citation10,Citation18,Citation29,Citation30]. The better activity of Pter over resveratrol may, in part, be explained by the replacement of two hydroxyl groups on resveratrol by methoxy groups on Pter [Citation10]. The methoxy substitution in Pter enhanced its lipophilicity and increased membrane permeability [Citation31]. Besides differences in bioavailablity, the difference in underlying mechanisms of action and downstream signaling pathways between Pter and resveratrol were also suggested. Chang et al. [Citation29] observed that Pter, not resveratrol, upregulated the manganese superoxide dismutase expression, inhibited nuclear factor κβ, and decreased the phosphorylation of JNK and tau by increased peroxisome proliferator-activated receptor alpha expression in SAMP8 mice. Rimando et al. [Citation32] achieved the similar observations in hypercholesterolemic hamsters that Pter, not resveratrol, activated the peroxisome proliferator-activated receptor alpha. Another study indicated that Pter exhibited a different downstream signaling pathway compared with piceatannol, another analog of resveratrol [Citation33]. The photoprotective ability of resveratrol against UVA-induced oxidative damage resulted from the nuclear accumulation of Nrf2 through the downregulation of Keap1 protein in HaCaT cells [Citation34]. However, the present results showed that Pter activated the PI3K signaling pathway subsequently inducing phosphorylation and translocation of Nrf2, without altered the expression of Keap1 protein. It is widely known that the PI3K/Akt is a vital pathway for cell survival against multiple apoptotic insults because it regulates many apoptotic-related factors [Citation35]. Thus, it is conceivable that the superior photoprotective capacity of Pter over resveratrol may be closely linked to the cell survival signaling pathway triggered by Pter. However, further studies are still needed to determine the detailed interaction between the photoprotective effect of Pter and the survival signaling pathway.
Taken together, Pter effectively activated the PI3K signaling pathway, subsequently promoted the Nrf2 nuclear translocation, and induced the expression of antioxidant enzymes, thereby protecting against the UVB-induced oxidative stress.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52(6):1119–1136. doi: 10.1111/j.1751-1097.1990.tb08452.x
- Ichihashi M, Ueda M, Budiyanto A, et al. UV-induced skin damage. Toxicology. 2003;189(1-2):21–39. doi: 10.1016/S0300-483X(03)00150-1
- Heck DE, Vetrano AM, Mariano TM, et al. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem. 2003;278(25):22432–22436. doi: 10.1074/jbc.C300048200
- Schafer M, Werner S. Nrf2 – A regulator of keratinocyte redox signaling. Free Radic Biol Med. 2015;88(Pt B):243–252. doi: 10.1016/j.freeradbiomed.2015.04.018
- Itoh K. Protective mechanism against oxidative stress by Keap1/Nrf2 pathway. Seikagaku. 2006;78(2):79–92.
- Bhakkiyalakshmi E, Dineshkumar K, Karthik S, et al. Pterostilbene-mediated Nrf2 activation: mechanistic insights on Keap1: Nrf2 interface. Bioorg Med Chem. 2016;24(16):3378–3386. doi: 10.1016/j.bmc.2016.05.011
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046
- Johnson J, Maher P, Hanneken A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Invest Ophthalmol Vis Sci. 2009;50(5):2398–2406. doi: 10.1167/iovs.08-2088
- Estrela JM, Ortega A, Mena S, et al. Pterostilbene: biomedical applications. Crit Rev Clin Lab Sci. 2013;50(3):65–78. doi: 10.3109/10408363.2013.805182
- Lin HS, Yue BD, Ho PC. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed Chromatogr. 2009;23(12):1308–1315. doi: 10.1002/bmc.1254
- McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res. 2012;173(2):e53–e61. doi: 10.1016/j.jss.2011.09.054
- McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev. 2013;2013:575482. doi: 10.1155/2013/575482
- Yang Y, Yan X, Duan W, et al. Pterostilbene exerts antitumor activity via the Notch1 signaling pathway in human lung adenocarcinoma cells. PLoS One. 2013;8(5):e62652. doi: 10.1371/journal.pone.0062652
- Zhang W, Sviripa V, Kril LM, et al. Fluorinated N,N-dialkylaminostilbenes for Wnt pathway inhibition and colon cancer repression. J Med Chem. 2011;54(5):1288–1297. doi: 10.1021/jm101248v
- Lin VC, Tsai YC, Lin JN, et al. Activation of AMPK by pterostilbene suppresses lipogenesis and cell-cycle progression in p53 positive and negative human prostate cancer cells. J Agric Food Chem. 2012;60(25):6399–6407. doi: 10.1021/jf301499e
- Chakraborty A, Bodipati N, Demonacos MK, et al. Long term induction by pterostilbene results in autophagy and cellular differentiation in MCF-7 cells via ROS dependent pathway. Mol Cell Endocrinol. 2012;355(1):25–40. doi: 10.1016/j.mce.2012.01.009
- Sirerol JA, Feddi F, Mena S, et al. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic Biol Med. 2015;85:1–11. doi: 10.1016/j.freeradbiomed.2015.03.027
- Li H, Gao A, Jiang N, et al. Protective effect of curcumin against acute ultraviolet B irradiation-induced photo-damage. Photochem Photobiol. 2016;92(6):808–815.
- Lieder F, Reisen F, Geppert T, et al. Identification of UV-protective activators of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) by combining a chemical library screen with computer-based virtual screening. J Biol Chem. 2012;287(39):33001–33013. doi: 10.1074/jbc.M112.383430
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36(10):1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075
- Na HK, Kim EH, Jung JH, et al. (-)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys. 2008;476(2):171–177. doi: 10.1016/j.abb.2008.04.003
- Tang Y, Jacobi A, Vater C, et al. Salvianolic acid B protects human endothelial progenitor cells against oxidative stress-mediated dysfunction by modulating Akt/mTOR/4EBP1, p38 MAPK/ATF2, and ERK1/2 signaling pathways. Biochem Pharmacol. 2014;90(1):34–49. doi: 10.1016/j.bcp.2014.04.008
- O’Shaughnessy JA, Kelloff GJ, Gordon GB, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8(2):314–346.
- Korkina LG, Pastore S, Dellambra E, et al. New molecular and cellular targets for chemoprevention and treatment of skin tumors by plant polyphenols: a critical review. Curr Med Chem. 2013;20(7):852–868.
- Saw CL, Yang AY, Huang MT, et al. Nrf2 null enhances UVB-induced skin inflammation and extracellular matrix damages. Cell Biosci. 2014;4:39–45. doi: 10.1186/2045-3701-4-39
- Knatko EV, Ibbotson SH, Zhang Y, et al. Nrf2 activation protects against solar-simulated ultraviolet radiation in mice and humans. Cancer Prev Res (Phila). 2015;8(6):475–486. doi: 10.1158/1940-6207.CAPR-14-0362
- Durchdewald M, Beyer TA, Johnson DA, et al. Electrophilic chemicals but not UV irradiation or reactive oxygen species activate Nrf2 in keratinocytes in vitro and in vivo. J Invest Dermatol. 2007;127(3):646–653. doi: 10.1038/sj.jid.5700585
- Chang J, Rimando A, Pallas M, et al. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol Aging. 2012;33(9):2062–2071. doi: 10.1016/j.neurobiolaging.2011.08.015
- Chiou YS, Tsai ML, Nagabhushanam K, et al. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem. 2011;59(6):2725–2733. doi: 10.1021/jf2000103
- Yeo SC, Ho PC, Lin HS. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: the impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol Nutr Food Res. 2013;57(6):1015–1025. doi: 10.1002/mnfr.201200651
- Rimando AM, Nagmani R, Feller DR, et al. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem. 2005;53(9):3403–3407. doi: 10.1021/jf0580364
- Fu Z, Yang J, Wei Y, et al. Effects of piceatannol and pterostilbene against beta-amyloid-induced apoptosis on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct. 2016;7(2):1014–1023.
- Liu Y, Chan F, Sun H, et al. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur J Pharmacol. 2011;650(1):130–137. doi: 10.1016/j.ejphar.2010.10.009
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14(5):381–395. doi: 10.1016/S0898-6568(01)00271-6