ABSTRACT
Objectives: In congestive heart failure (CHF), men are younger, more likely to have reduced ejection fraction (HF-rEF), and to be diabetic compared to women. Despite this, sex differences in oxidative stress have yet to be explored in CHF.
Methods: Data from 67 males and 63 females hospitalized for CHF were collected. Static oxidation–reduction potential (sORP), a relative indicator of oxidative stress, and capacity ORP (icORP), a relative indicator of antioxidant capacity, were measured from plasma samples. We examined whether sex modified the relationship between ORP and hospital discharge disposition (poor outcome: death, hospice), along with other demographics, medications, and diagnostic parameters.
Results: Males with poor outcomes had higher sORP and icORP values than females (P < 0.05). For those with a good outcome, there were no differences between the sexes (P > 0.05). Males were younger and more likely to have HF-rEF and diabetes. Controlling for these variables did not account for the sex differences in ORP measures. Regardless of sex, higher creatinine was related to higher sORP and icORP, while lower magnesium and potassium were related to higher sORP and icORP, respectively.
Discussion: Increases in sORP during CHF are partially affected by sex and acute outcomes, but are also related to variables without sexual biases.
Introduction
The generation of reactive oxygen species (ROS) and hydrogen peroxide (H2O2) during heart failure have important physiological roles including mediating myocyte autophagy and contributing to vascular homeostasis by regulating angiotensin II [Citation1,Citation2]. The pathological generation of these pro-oxidants can lead to a state of oxidative stress, defined most recently by Sies in his review of oxidative stress, as ‘An imbalance between oxidants and anti- oxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage’ [Citation3]. Oxidative stress in cardiovascular disease has been implicated in vascular inflammation, impaired endothelial function and vascular tone, cardiomyocyte apoptosis, and ultimately heart failure [Citation2,Citation4,Citation5].
The presence of oxidative stress in heart failure has been identified by the increased generation of ROS via increased NADPH oxidase (NOX), xanthine oxidase, myeloperoxidase (MPO), and other pro-oxidant enzyme activities [Citation4,Citation6–12], leading to specific foot-prints of oxidative damage. Decreases in antioxidant enzymes, such as superoxide dismutase (SOD) and the elevated presence of DNA and lipid oxidation have also been reported [Citation4,Citation10,Citation13–16]. While these studies have provided valuable insight into the role of oxidative stress in congestive heart failure (CHF), they did not account for possible differences between the sexes. In all these studies, data were combined across sex and less than one-third of the patients included were female, therefore any changes that occur in males only were diluted by the minority presence of females and changes in females alone remain to be established.
Cardiovascular disease has definite sex differences [Citation17–19]. Compared to females, heart failure in males has been associated with lower ejection fraction and slower heart rate; males may also have elevated levels of brain natriuretic peptide (BNP), higher creatinine and galectin-3 levels, higher diastolic blood pressure (dBP), and are more likely to be diabetic [Citation11,Citation20–22]. Thus, in light of the paucity of information regarding sex differences, oxidative stress, and CHF, we conducted a study with the purpose of measuring a global biomarker of oxidative stress in both sexes hospitalized for CHF to determine if oxidative stress is differentially expressed in males and females under these conditions and if there is a difference based on acute outcome.
Because oxidative stress can result from multiple sources of imbalance in the redox system, we sought to use a more global measure to provide insight into the oxidative state of CHF patients – one which was not limited to measuring specific ROS, lipid peroxidation, antioxidant activity or status, or thiol–thiol disulfide reactions. To achieve this, we measured oxidation–reduction potential (ORP), which is a measure of a biological sample’s tendency for losing electrons (oxidizing) or gaining electrons (reducing) regardless of the donating or receiving agent [Citation23]. The measurement of ORP is governed by the Nernst equation; the equation relates the measured potential to the log ratio of all oxidants to all antioxidants, not to just thiols and thiol disulfides, as in thiol-redox assays employed for the classical measure of oxidative stress [Citation24,Citation25]. Therefore, measures of ORP provide a relative indicator of the balance between all pro- and anti-oxidants which, consistent with the recent definitions, is indicative of oxidative stress [Citation3,Citation26,Citation27]. ORP has traditionally been used in water quality testing [Citation28–30] but has also been applied to medical biologics for assessing relative change in oxidative stress [Citation23,Citation31–38]. In the current study, we used ORP as an indicator of relative plasma oxidative stress to illuminate potential differences between men and women hospitalized for CHF.
Materials and methods
Patients
Patient participants were those admitted to Swedish Medical Center (Englewood, CO, U.S.A.) with a primary diagnosis of CHF between January 2015 and June 2015. CHF patients younger than 18 years of age or who had cognitive deficits were excluded. Consent for participation was given by the patient or their legally authorized representative. The study was approved by the HealthOne Institutional Review Board and in accordance with institutional guidelines. Patients without a stored blood plasma sample for admission day were excluded. Total sample size was 130 patients.
Data collection
The following patient data were collected: sex, age, presence of a pacemaker, diabetes, and the use of medications that could impact heart failure (beta blockers, diuretics, NSAIDS, statins, ACE inhibitors, calcium channel blockers, and aldosterone antagonists). Additional data included blood pressure, heart rate, ejection fraction, respiratory rate, hematocrit, serum creatinine, BNP, galectin-3, sodium, potassium, and magnesium levels. During hospitalization, the maintenance, addition, or removal of CHF medications were recorded. Ejection fraction data were dichotomized into preserved ejection fraction (>40%; HF-pEF) and reduced ejection fraction (< = 40%; HF-rEF). Lastly, acute outcomes, as defined by a good outcome in which patients were discharged to home or care facility versus a poor outcome in which patients died or were discharged to hospice, were recorded. Patients with incomplete data (i.e. information regarding pace maker or ejection fraction) were not excluded from analyses but did result in lower cohort subject numbers when analyzing these variables.
Measuring oxidative stress
Relative oxidative stress was determined electrochemically, by measuring oxidation–reduction potential (ORP). ORP was measured using the RedoxSYS System (Aytu BioScience, Englewood, CO U.S.A.) using previously described methods for biological samples [Citation31,Citation38]. ORP was measured using excess blood plasma taken during routine daily blood draws as part of standard care. No additional blood draw was required to measure ORP. Blood samples, collected using vacutainer tubes containing sodium heparin (a.k.a. green tops), were processed to plasma within 1 h of collection. Plasma samples were then stored at –80°C for no longer than 10 months until ORP was measured and were not previously thawed prior to analyses. Samples were thawed to room temperature for 30 minutes at which point 30 μL of plasma were added to the sensor strip pre-inserted into the analyzer. The analyzer begins measuring automatically when the sample is detected. The RedoxSYS System provides two measures of ORP- static ORP (sORP) measured in millivolts (mV) and capacity ORP (cORP) measured in microcoulombs (μC). SORP measures the current ratio in activity of oxidizing to reducing elements in the sample, a relative indicator of oxidative stress. The higher the sORP value the more oxidizing activity is present and thus, potentially elevated plasma oxidative stress.
The cORP value represents a sample’s capacity to accommodate an oxidative insult. To measure cORP, the sample is oxidized electrochemically via a specified and increasing current between the working and counter electrodes of the RedoxSYS System until an inflection in the ORP is reached. The total amount of energy delivered to the sample is calculated and is proportional to the sum activity of the combined antioxidants present in the sample measured. It is expressed as μC (microcoulombs). Because cORP values are not normally distributed, the data underwent an inverse transformation so the lower the icORP (μC–1), the greater the plasma antioxidant capacity.
Study statistics
Measures of ORP on admit day and day 2 were compared across sex and acute outcome using a 3-way mixed model ANOVA with day as the repeated measure. To determine sex differences in the remaining variables, t-tests were used for continuous variables and chi-squares were used for categorical variables. ORP values were then analyzed against variables that changed significantly by sex to determine if sex differences in ORP could be accounted for by other sex defined variables. This was done using Pearson r correlations for continuous variables and ANOVA for categorical variables. In these analyses, data were collapsed across acute outcome.
Results
Overall there were 130 patients admitted for CHF over the 6-month study period with 51.5% male. Differences by gender in our covariates and outcomes are presented in . There was a significant age difference between the male and female CHF patients, with males being significantly younger. Males also had a lower ejection fraction, higher dBP, higher hematocrit levels, and were more likely to be diabetic than the female group. Females had a slightly higher proportion of poor acute outcomes (11/63) than males (5/67), however this did not reach significance. In the remaining variables, there were no significant differences based on sex.
Table 1. Demographics and descriptive statistics between sexes.
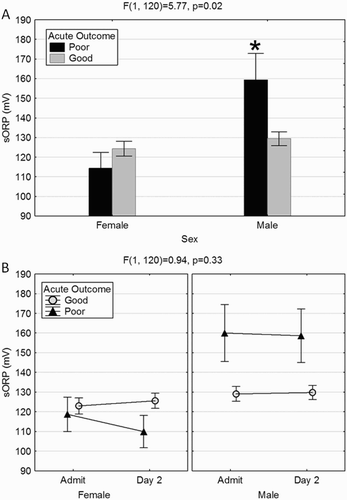
In general, males had significantly higher sORP values than females (F(1,120) = 9.13, P = 0.003), however the magnitude of the effect was governed by acute outcome ((A)). Males with poor acute outcome had higher sORP values than males with good outcomes and females overall (P < 0.05). SORP values did not significantly change between admission and day 2, regardless of sex and/or outcome ((B)). Males with a poor acute outcome had higher sORP on both days.
Figure 1. A poor acute outcome in males is associated with elevated sORP. (A) Males with poor acute outcome had significantly higher sORP values than those with a good outcome and compared to females over all. (B) The elevation in males with a poor outcome was evident in both the admission and day 2 sORP values. While not significant, females with a poor acute outcome appear to have a decrease in sORP values on day 2. *Significantly higher than all other groups (P < 0.05).
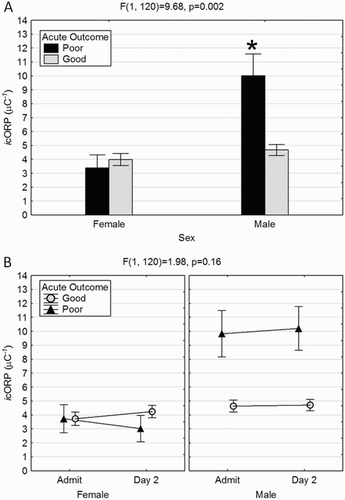
Sex differences in plasma antioxidant capacity were also determined by acute outcome. Relative antioxidant capacity was significantly decreased (elevated icORP) in males with poor acute outcome ((A)). There were no significant changes in icORP across days, again, regardless of sex or acute outcome ((B)).
Figure 2. Loss of antioxidant capacity in males with poor acute outcome. (A). Similar to sORP, males with a poor acute outcome had significantly higher icORP values indicating a relative loss of antioxidant capacity not seen in males with a good outcome or overall in females. (B) There were no differences in icORP values between admission and day 2 regardless of sex or outcome. Males with poor acute outcomes had elevated icORP values on both days.
To determine if sex differences in ORP values were merely a reflection of the age difference, an age-matched subset of data was extracted. In this group, differences in sORP and icORP values continued to be determined by both sex and acute outcome (F(1,75) = 8.30, P = 0.005 and F(1,75) = 12.13, P = 0.0008, respectively). Age-matched males with poor acute outcome continued to have significantly higher sORP and icORP values than age-matched males with a good outcome and both groups of age-matched females ().
Table 2. ORP values for age-matched males and females support the notion that males with poor acute outcomes have higher sORP and lower icORP values compared to females.
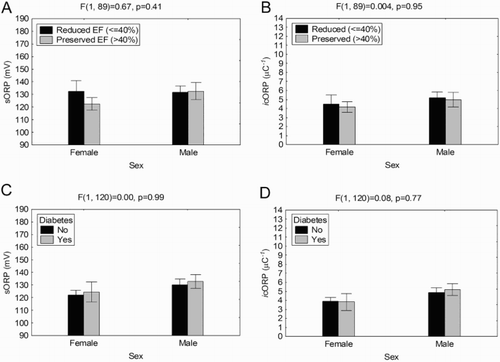
Reduced ejection fraction (HF-rEF) was disproportionately associated with males whereas females were more likely to have preserved ejection fraction (HF-pEF) (). The level of ejection fraction did not significantly alter sORP or icORP values in either males or females ((A,B)). Similarly, diabetes was more frequent in males than females (). There were no significant effects of diabetes on ORP values in either males or females ((C,D)), thus the sex differences in ORP values were not a function of diabetes or the level of ejection fraction.
Figure 3. Traditionally sex-biased variables do not account for the differences in ORP between males and females. (A, B) Average sORP and icORP were not significantly different based on reduced or preserved EF, regardless of sex. (C, D) Having diabetes did not significantly alter overall sORP or icORP values regardless of sex.
Males had higher dBP than females (). Correlations between ORP values and dBP were not significant (r = 0.12 and r = 0.10 for sORP and icORP, respectively). Hematocrit levels were also higher in males than females (), however there were no significant correlations between hematocrit levels and ORP values (r = 0.08 and r = 0.15 for sORP and icORP, respectively). Therefore, while men had higher dBP and hematocrit levels, these were not associated with the higher sORP and icORP values observed in males.
The number of males with and without pacemakers was proportional to the number of females with and without pacemakers. The proportion of those with regular heartbeat among men and women were also proportional (). There were no significant differences in sORP or icORP in those with or without pacemakers in either sex (F(1,114) = 0.04, P = 0.85 and F(1,114) = 0.38, P = 0.54, respectively, data not shown). No significant differences in sORP or icORP emerged as a result of heartbeat either (F(1,116) = 0.05, P = 0.83 and F(1,116) = 0.25, P = 0.62, respectively; data not shown).
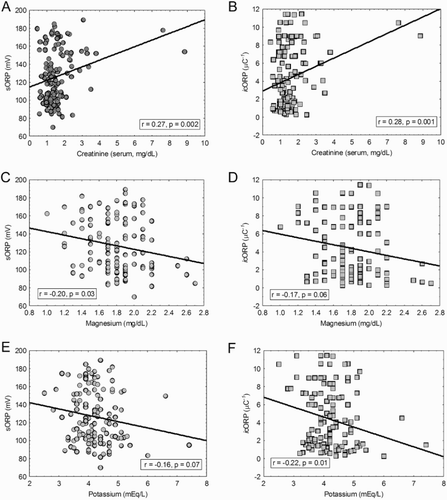
Neither sORP nor icORP were correlated with systolic BP, heart rate, respiration, BNP, galectin-3, or sodium levels (data not shown). Creatinine was correlated with both admission day sORP and icORP, such that as creatinine levels increased both ORP measures also increased ((A,B)). Magnesium levels were negatively correlated with sORP values but not with icORP values ((C,D)). As sORP values increased, the magnesium decreased. Potassium levels were negatively correlated with icORP but did not reach significance in sORP ((E,F)). Higher icORP values were related to lower potassium levels. There were no sexual biases found in these variables ().
Figure 4. ORP values changed with changes in creatinine, magnesium, and potassium. (A, B) Higher creatinine values were related to higher admit day sORP and icORP values. (C, D) Higher magnesium levels were significantly associated with lower admit day sORP values, and lesser so with icORP. (E, F) Increasing potassium levels were more strongly related with decreasing admit day icORP than sORP. Correlations were run and graphed across sex.
There were no sexual biases found in the distribution of cardiac medicines. The numbers of males and females in which medications were (i) maintained during hospitalization, (ii) added during hospitalizations, and (iii) taken off during hospitalization, or (iv) were never on, were not significantly different (). In addition, there were no significant changes in ORP values with the use of such medications (). There was insufficient patient data to analyze sexual biases and changes in ORP as a function of calcium channel blockers (n = 50) or aldosterone antagonists (n = 46).
Table 3. The presence, absence, or removal of cardiac medications were evenly distributed across males and females and did not alter ORP values.
Discussion
ORP values were significantly elevated in males who died or were discharged to hospice. Males with a good acute outcome and females, regardless of outcome, had similar plasma sORP and icORP values. Controlling for other sexually biased variables – age, ejection fraction, diabetes, dBP, and hematocrit levels, did not abolish the sex differences in ORP values. This is in agreement with an earlier study that also did not find any relationship between their measure of serum oxidative stress and diabetes, ejection fraction and heart rate in CHF patients [Citation6]. There were significant relationships between overall ORP values and serum creatinine, magnesium, and potassium levels, but these were not sex specific changes.
Under healthy conditions, there have been mixed reports regarding sexual biases in measures of oxidative stress. One study found that males have lower oxidant levels than females; however, antioxidant levels were similar [Citation39]. Another found no significant difference in oxidative stress between the sexes but did find an effect of diabetes, with otherwise healthy diabetic females having higher levels of oxidative stress [Citation40]. Our study found no effect of diabetes on the relative measures determined here, however the study population were all hospitalized CHF patients, suggesting that changes in the plasma redox system during CHF may over shadow healthy diabetic differences.
Women facing a poor acute outcome have the opposite response in ORP measures than men in the same situation. This difference may not necessarily be a result of sex hormones as most of the women in this study were of age to be post-menopausal and Brunelli et al. [Citation39] found no significant difference in oxidative stress markers between young and aged women, at least under healthy conditions. In those living with coronary artery disease (CAD), however, older females have increased markers of oxidative stress compared to males with CAD [Citation41], thus under stress conditions, it is possible that sex differences become more pronounced.
Previous studies have identified differences between sexes with cardiovascular disease [Citation16–18]. The differences in baseline characteristics between the male and female patients of this study are also similar to what has been previously reported in the literature for higher diastolic blood pressure (dBP), hematocrit levels, ejection fraction, and diabetes [Citation10,Citation19–21]. There have also been reports that biomarkers, such as BNP, have a sexual bias in predicting cardiac outcome. BNP was predictive for men with acute decompensated heart failure but was not predictive for women [Citation42]. Thus, it is not unreasonable to assume that because there are sex differences in the symptoms of CHF, there would be sex-biased biomarkers for CHF, as found in the present study in which higher sORP was associated with poor outcomes for males with heart failure but not for females. Whether increased oxidative stress is causal to poor outcomes in males or merely relational is unknown at this time. The controlled generation of free radicals has a protective role during heart failure [Citation1,Citation2], but it is possible that the over production as well as the under production of these elements could contribute to a poor acute outcome creating the contrast observed between males and females.
Measures of ORP provide a holistic relative indicator of oxidative stress but does specify from where the redox imbalance occurs- over generation of a specific oxidant or an under production of a specific antioxidant. Because our samples were stored at –80°C for up to 10 months, it is possible that this storage time may preferentially affect specific antioxidants or pro-oxidants and this may be differentially affected by the pathology examined here. The total antioxidant capacity (TAC) of breast milk decreased when stored at –80°C for 3 months but there was no change in total oxidation status (TOS) of breast milk, and in colostrum there was no effect of storage at all [Citation43]. However in the current study, CHF patients were admitted randomly into the study (i.e. both males and females were admitted across the entire study period) and acute outcome was also random (i.e. poor acute outcomes occurred throughout the study period and were not limited to an earlier or later period). Under these sampling conditions, storage effects are anticipated to drop out statistically because the storage variability is distributed randomly within and across groups. The significant elevation in sORP and icORP in men with poor acute outcome was great enough to supersede the possible variability produced by storage. Thus, our data continue to suggest that there is a sexual bias in blood plasma sORP and icORP levels that manifests itself in those patients with poor acute outcomes.
The current study used blood plasma to measure a global marker of oxidative stress. Blood may reflect the systemic oxidative state but may not accurately reflect oxidative changes within the tissue itself, such as in cardiac tissue. A meta-analysis by Margaritelis et al. [Citation44], however, found good agreement between blood and tissue for most measures of oxidative stress, specifically in cardiac tissue. For example, there were strong correlations between blood and heart tissue for oxidized glutathione (GSSG), xanthine oxidase (XO), lipid and protein oxidation, reduced glutathione (GSH), and catalase [Citation45]. Thus, it is likely that blood plasma sORP and icORP levels are also representative of the oxidative changes in the heart occurring during CHF.
Of note is that one of the studies included in the meta-analysis found that liver tissue did not correlate with blood specific markers of oxidative stress. For example, liver and blood did not agree on enzymatic antioxidants (GSH and catalyze) even though they correlated on oxidative markers (GSSG and XO) and markers of oxidative damage [Citation45]. Small molecule antioxidants (i.e. ascorbic acid) or measures of ROS levels were not included in the analysis [Citation45]. This comparison should make us mindful that there is variability in the expression of redox system components and measuring a single or even small group of these factors may not be representative of the actual redox status, regardless of whether it is systemic or within the tissues. This underscores the need for a global measure of the redox system, like ORP, to identify relative changes in oxidative stress.
Conclusion
In males that face a poor acute outcome, there was an unparalleled rise in sORP measures and a loss in antioxidant capacity (increased icORP values) at hospital admission and which continued through at least day 2, relative to females with a poor acute outcome. In those with good acute outcomes, there were no differences between males and females. Sex differences in ORP measures were independent of other sex-biased variables, such as age, reduced EF, and diabetes. Other non-biased variables were also related to ORP measures. Changes in creatinine, magnesium, and potassium influenced ORP levels regardless of sex.
Acknowledgments
The authors gratefully acknowledge the collection of patient samples and maintenance of patient records by Rachel Aumann, R.N., and Anita Leyden, R.N.
Disclosure statement
Dr. Bjugstad was a paid employee and Dr. Bar-Or is a paid consultant for Aytu BioScience, Inc. The remaining co-authors declare that they have no competing interests.
ORCID
Kimberly B. Bjugstad http://orcid.org/0000-0002-0473-5413
Jeffrey Lalama http://orcid.org/0000-0001-7134-4052
Additional information
Funding
References
- Li B, Chi RF, Qin FZ, et al. Distinct changes of myocyte autophagy during myocardial hypertrophy and heart failure: association with oxidative stress. Exp Physiol. 2016;101:1050–1063. doi: 10.1113/EP085586
- Shimokawa H, Satoh K. Light and dark of reactive oxygen species for vascular function: 2014 ASVB (Asian society of vascular biology). J Cardiovasc Pharmacol. 2015;65:412–418. doi: 10.1097/FJC.0000000000000159
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002
- Ellidag HY, Eren E, Yilmaz N, et al. Oxidative stress and ischemia-modified albumin in chronic ischemic heart failure. Redox Rep. 2014;19:118–123. doi: 10.1179/1351000213Y.0000000083
- Narasimhan M, Rajasekaran NS. Reductive potential – a savior turns stressor in protein aggregation cardiomyopathy. Biochim Biophys Acta. 2015;1852:53–60. doi: 10.1016/j.bbadis.2014.11.010
- Amir O, Paz H, Rogowski O, et al. Serum oxidative stress level correlates with clinical parameters in chronic systolic heart failure patients. Clin Cardiol. 2009;32:199–203. doi: 10.1002/clc.20317
- Heymes C, Bendall JK, Ratajczak P, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/S0735-1097(03)00471-6
- Ijsselmuiden AJ, Musters RJ, de Ruiter G, et al. Circulating white blood cells and platelets amplify oxidative stress in heart failure. Nat Clin Pract Cardiovasc Med. 2008;5:811–820. doi: 10.1038/ncpcardio1364
- Karabacak M, Dogan A, Tayyar S, et al. Oxidative stress status increase in patients with nonischemic heart failure. Med Princ Pract. 2014;23:532–537. doi: 10.1159/000365512
- Landmesser U, Spiekermann S, Dikalov S, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.CIR.0000041431.57222.AF
- Meyer S, van der Meer P, van Deursen VM, et al. Neurohormonal and clinical sex differences in heart failure. Eur Heart J. 2013;34:2538–2547. doi: 10.1093/eurheartj/eht152
- Ng LL, Pathik B, Loke IW, et al. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am Heart J. 2006;152:94–101. doi: 10.1016/j.ahj.2005.09.020
- Castro PF, Diaz-Araya G, Nettle D, et al. Effects of early decrease in oxidative stress after medical therapy in patients with class IV congestive heart failure. Am J Cardiol. 2002;89:236–239. doi: 10.1016/S0002-9149(01)02211-1
- Diaz-Velez CR, Garcia-Castineiras S, Mendoza-Ramos E, et al. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am Heart J. 1996;131:146–152. doi: 10.1016/S0002-8703(96)90063-0
- Kobayashi S, Susa T, Tanaka T, et al. Urinary 8-hydroxy-2'-deoxyguanosine reflects symptomatic status and severity of systolic dysfunction in patients with chronic heart failure. Eur J Heart Fail. 2011;13:29–36. doi: 10.1093/eurjhf/hfq178
- Kono Y, Nakamura K, Kimura H, et al. Elevated levels of oxidative DNA damage in serum and myocardium of patients with heart failure. Circ J. 2006;70:1001–1005. doi: 10.1253/circj.70.1001
- Garcia M, Mulvagh SL, Bairey Merz CN, et al. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118:1273–1293. doi: 10.1161/CIRCRESAHA.116.307547
- Motiwala SR, Sarma A, Januzzi JL, et al. Biomarkers in ACS and heart failure: should men and women be interpreted differently? Clin Chem. 2014;60:35–43. doi: 10.1373/clinchem.2013.202531
- Fazal L, Azibani F, Vodovar N, et al. Effects of biological sex on the pathophysiology of the heart. Br J Pharmacol. 2014;171:555–566. doi: 10.1111/bph.12279
- Ho JE, Enserro D, Brouwers FP, et al. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail. 2016;9:e003116 doi: 10.1161/CIRCHEARTFAILURE.115.003116
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/S0735-1097(02)02059-4
- Sakata Y, Miyata S, Nochioka K, et al. Gender differences in clinical characteristics, treatment and long-term outcome in patients with stage C/D heart failure in Japan. Report from the CHART-2 study. Circ J. 2014;78:428–435. doi: 10.1253/circj.CJ-13-1009
- Shapiro HM. Redox balance in the body: an approach to quantitation. J Surg Res.1972;13:138–152. doi: 10.1016/0022-4804(72)90057-1
- Roede JR, Uppal K, Liang Y, et al. Characterization of plasma thiol redox potential in a common marmoset model of aging. Redox Biol. 2013;1:387–393. doi: 10.1016/j.redox.2013.06.003
- Go YM, Jones DP. Thiol/disulfide redox states in signaling and sensing. Crit Rev Biochem Mol Biol. 2013;48:173–181. doi: 10.3109/10409238.2013.764840
- Halliwell B. Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomed J. 2014;37:99–105.
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865
- Schmelke FC. The oxidation potential concept of chlorination. J Am Water Works Assoc. 1933;25:695–703.
- World Health O. Guidelines for safe recreational water environments. Volume 2: swimming pools and similar environments. In: World Health O, editor. Geneva, Switzerland: WHO Press; 2006. p. 146.
- World Health O. Guidelines for drinking-water quality. In: World Health O, editor. 4th ed. Geneva, Switzerland: WHO Press; 2011. p. 564.
- Bjugstad KB, Rael LT, Levy S, et al. Oxidation-reduction potential as a biomarker for severity and acute outcome in traumatic brain injury. Oxid Med Cell Longev. 2016;5:1–9. doi: 10.1155/2016/6974257
- Rael LT, Bar-Or R, Kelly MT, et al. Assessment of oxidative stress in patients with an isolated traumatic brain injury using disposable electrochemical test strips. Electroanalysis. 2015;27:2567–2573. Epub 2015 Jul 14. doi: 10.1002/elan.201500178
- Rael LT, Bar-Or R, Mains CW, et al. Plasma oxidation-reduction potential and protein oxidation in traumatic brain injury. J. Neurotrauma. 2009;26:1203–1211. doi: 10.1089/neu.2008.0816
- Spanidis Y, Goutzourelas N, Stagos D, et al. Variations in oxidative stress markers in elite basketball players at the beginning and end of a season. Exp Ther Med. 2016;11:147–153. doi: 10.3892/etm.2015.2843
- Spanidis Y, Mpesios A, Stagos D, et al. Assessment of the redox status in patients with metabolic syndrome and type 2 diabetes reveals great variations. Exp Ther Med. 2016;11:895–903. doi: 10.3892/etm.2016.2968
- Stagos D, Goutzourelas N, Bar-Or D, et al. Application of a new oxidation-reduction potential assessment method in strenuous exercise-induced oxidative stress. Redox Rep. 2015;20:154–162. doi: 10.1179/1351000214Y.0000000118
- Zhi L, Hu X, Han C. Biphasic changes (overreduction and overoxidation) of plasma redox status and clinical implications in early stage of severe burns. J Crit Care. 2014;29:1063–1068. doi: 10.1016/j.jcrc.2014.06.013
- Bjugstad KB, Fanale C, Wagner J, et al. A 24 h delay in the redox response distinguishes the most severe stroke patients from less severe stroke patients. J Neurol Neurophysiol. 2016;7:10. doi: 10.4172/2155-9562.1000395
- Brunelli E, Domanico F, La Russa D, et al. Sex differences in oxidative stress biomarkers. Curr Drug Targets. 2014;15:811–815. doi: 10.2174/1389450115666140624112317
- Marra G, Cotroneo P, Pitocco D, et al. Early increase of oxidative stress and reduced antioxidant defenses in patients with uncomplicated type 1 diabetes: a case for gender difference. Diabetes Care. 2002;25:370–375. doi: 10.2337/diacare.25.2.370
- Vassalle C, Maffei S, Boni C, et al. Gender-related differences in oxidative stress levels among elderly patients with coronary artery disease. Fertil Steril. 2008;89:608–613. doi: 10.1016/j.fertnstert.2007.03.052
- Nakada Y, Kawakami R, Nakano T, et al. Sex differences in clinical characteristics and long-term outcome in acute decompensated heart failure patients with preserved and reduced ejection fraction. Am J Physiol Heart Circ Physiol. 2016;310:H813–H820.
- Sari FN, Akdag A, Dizdar EA, et al. Antioxidant capacity of fresh and stored breast milk: is –80 degrees C optimal temperature for freeze storage? J Matern Fetal Neonatal Med. 2012;25:777–782. doi: 10.3109/14767058.2011.592230
- Margaritelis NV, Veskoukis AS, Paschalis V, et al. Blood reflects tissue oxidative stress: a systematic review. Biomarkers. 2015;20:97–108. doi: 10.3109/1354750X.2014.1002807
- Veskoukis AS, Nikolaidis MG, Kyparos A, et al. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic Biol Med. 2009;47:1371–1374. doi: 10.1016/j.freeradbiomed.2009.07.014
