ABSTRACT
Objective: This study aimed to evaluate the activity of delta-aminolevulinate dehydratase (δ-ALA-D) and oxidative stress biomarkers in pregnant women with gestational diabetes mellitus (GDM), in order to demonstrate the involvement of oxidative stress in this condition, which presents pathophysiology still undetermined.
Methods: δ-ALA-D activity, lipid peroxidation estimated as the levels of thiobarbituric acid reactive substances (TBARS), protein (P-SH) and non-protein thiol (NP-SH) content, catalase (CAT) activity and concentration of vitamin C (VIT C) in samples of pregnant women with GDM (n = 48) and in healthy pregnant women (n = 30), who constituted the control group.
Results: The δ-ALA-D activity was significantly lower in pregnant women with GDM compared to controls, as well as levels of thiols, VIT C and CAT activity. Lipid peroxidation was higher in GDM group.
Discussion: The results suggest that the main factor for the increase in oxidative stress and reduced δ-ALA-D activity in diabetic pregnant women is gestational hyperglycemic environment, which changed the redox balance and interfered on mechanism of the δ-ALA-D activity in relation to normoglycemic pregnant women.
1. Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance, to varying degrees of intensity, which is usually diagnosed in the second or third trimester of pregnancy [Citation1]. This period of pregnancy is physiologically characterized by peripheral resistance to insulin action, mainly due to increased maternal adiposity and the production of diabetogenic hormones by the placenta [Citation2]. In normal pregnancy, the pancreatic β-cells increase insulin secretion to compensate this resistance, thereby regulating the glycemic levels [Citation3]. However, in GDM there is a failure to cope with the high insulin demand, primarily due to dysfunction of pancreatic beta cells and insulin resistance, indicating that GDM operates similarly to type 2 diabetes (T2DM) and that hormonal changes of pregnancy may indicate a woman’s increased susceptibility to T2DM [Citation4,Citation5].
Maternal hyperglycemia during pregnancy may result in complications for the baby, such as macrosomia, malformations, neonatal hypoglycemia and increased fetal mortality [Citation6]. For pregnant women there are greater risks of having high blood pressure, infections and, cesarean delivery, and of developing DM2 after pregnancy [Citation7].
The development of GDM is not fully understood, but there is evidence that maternal hyperglycemia is associated with increased oxidative stress [Citation8], which may also be involved in the pathophysiology and maternal and fetal complications of GDM [Citation9]. Oxidative stress occurs when the production of reactive species, particularly reactive oxygen species (ROS), exceed the capacity of the antioxidant defense system. This process can cause damage and change the functions of biomolecules, such as lipids, proteins and DNA [Citation10].
In order to protect the organism from the deleterious effects of ROS, the antioxidant system is divided into enzymatic and non-enzymatic antioxidants. Enzymatic antioxidants are mainly comprised of superoxide dismutase (SOD), which converts the superoxide radical (O2−.) into hydrogen peroxide (H2O2), and glutathione peroxidase (GPx) and catalase (CAT), both of which degrade H2O2 [Citation10,Citation11]. Non-enzymatic antioxidants can be endogenous, such as reduced glutathione (GSH), the main representative of the thiols (-SH), which are responsible for the detoxification of oxygen radicals [Citation12], or exogenous, such as vitamins, minerals and phenolic compounds [Citation13]. Vitamin C (VIT C) is an important water-soluble antioxidant, which donates electrons to break the chain reaction of lipid peroxidation and regenerates vitamin E in order to protect cell membranes from oxidative damage [Citation11].
Another important enzyme involved in oxidative stress is δ-aminolevulinic acid dehydratase (δ-ALA-D) [Citation14]. It is an essential enzyme for aerobic organisms, because it participates in heme biosynthesis, catalyzing the condensation of two δ-aminolevulinic acid molecules (δ-ALA) to form porphobilinogen (PBG) [Citation15]. δ-ALA-D contains thiol groups, which are required for its activity, since in pro-oxidant conditions such as hyperglycemia, these groups are oxidized and the enzyme is inhibited [Citation16]. When inhibited, δ-ALA-D affects heme synthesis and consequently hemoproteins, such as hemoglobin, CAT and peroxidases [Citation17]. Another consequence is the accumulation of δ-ALA substrate, a precursor of ROS [Citation14]. Due to this behavior, δ-ALA-D has been suggested as a biomarker of oxidative stress in diseases such as T2DM [Citation18,Citation19].
Previous studies have demonstrated an increase in lipid peroxidation by measuring malondialdehyde (MDA) in the maternal circulation of women with GDM, as well as a decrease in the levels of antioxidants CAT, GSH and VIT C [Citation20–22]. However, the number of studies assessing oxidative/antioxidant status in women with GDM is still relatively limited. Similarly, there are no reports in the literature about δ-ALA-D activity in these pregnant women. Therefore, the aim of this study was to evaluate the activity of δ-ALA-D, due to its high sensitivity to oxidative situations, and its relation to biomarkers of oxidative stress in women with GDM.
2. Materials and methods
2.1. Study population
This study included 48 women with GDM and receiving high-risk prenatal care at the University Hospital of Santa Maria. The control group consisted of 30 healthy women with low-risk pregnancies receiving care at the basic health unit (BHU) Wilson Paulo Noal. All pregnant women were in their third trimester. Diabetic pregnant women were controlling diabetes only with diet.
All the pregnant women underwent oral glucose tolerance test (75 g OGTT) screening between 24 and 28 weeks of pregnancy. In all cases, GDM was diagnosed according to the American Diabetes Association criteria [Citation23]. The diagnosis of GDM is made when any of the following plasma glucose values are exceeded: Fasting: ≥ 92 mg/dL (5.1 mmol/L), 1 h: ≥ 180 mg/dL (10.0 mmol/L) and 2 h: ≥ 153 mg/dL (8.5 mmol/L). Exclusion criteria for all participants included chronic systemic diseases, pre-existing diabetes (type 1 or type 2), treatment with insulin or hypoglycemiants, preeclampsia, multiple pregnancies, smoking, alcoholism and use of drugs or supplements.
Information regarding each participant was obtained through a complete questionnaire that requested data about personal characteristics, medical and dietary history. Anthropometric measurements were determined. Body mass index (BMI) was calculated as weight by height squared (kg/m²). In addition, systolic and diastolic blood pressures were measured by a calibrated mercury sphygmomanometer.
The study was approved by the Human Ethics Committee of the Federal University of Santa Maria, RS, Brazil (No. 33665314.4.0000.5346) and carried out in accordance with the Declaration of Helsinki (2000). All pregnant women received an explanation about the study before signing an informed consent form.
2.2. Laboratory assessments
Blood samples were collected from all pregnant women in the morning after an 8-hour overnight fasting period. The collection was made by venipuncture in into Vacutainer® tubes (BD Diagnostics, Plymouth, UK) containing anticoagulants. The EDTA blood tube was used for hemogram and measurement of the glycated hemoglobin. Fasting glucose was measured in a tube containing sodium fluoride. Tube containing heparin was used to obtain whole blood, plasma and erythrocytes for the determination of oxidative stress parameters. Analysis of the hemogram was determined in Sysmex® XE-5000 (Sysmex, Kobe, Japan). Plasma glucose was measured by hexokinase/glucose-6-phosphate dehydrogenase method (Glucose – Dimension®RxLMax® – Siemens/USA). The glycated hemoglobin A1c (HbA1c) was analyzed only in diabetic pregnant women using the cation-exchange HPLC method with a D-10 analyzer (Bio-Rad Laboratories, Hercules, CA, USA).
δ-ALA-D activity was assessed in whole blood with heparin by the method of Berlin and Schaller [Citation24]. The final product of the reaction, measured at 555 nm and the results were expressed in U/L (PBG nmol/h/mg Hb). The δ-ALA-D reactivation index was estimated using the equation: A-B/A*100, where A = absorbance of δ-ALA-D with dithiothreitol (DTT) and B = absorbance of δ-ALA-D without DTT.
Lipid peroxidation was estimated in plasma and in erythrocytes by measuring thiobarbituric acid reactive substances (TBARS) according to the method of Lapenna et al. [Citation25]. The reaction products were measured spectrophotometrically at 532 nm and the results were expressed in nmol TBARS/mL of plasma and in nmol TBARS/mL of erythrocytes.
The concentration of protein thiol (P-SH) and non-protein thiol level (NP-SH) was determined by spectrophotometry at 412 nm, as described by Boyne and Ellman [Citation26] modified by Jacques-Silva et al. [Citation27]. The P-SH results were expressed as nmol of P-SH/mL plasma and NP-SH levels were expressed as nmol of NP-SH/mL erythrocytes.
CAT activity was quantified spectrophotometrically in erythrocytes by the method of Aebi [Citation28], which consists of monitoring the decomposition of H2O2 at 240 nm. The enzymatic activity was expressed as K/mg·Hb.
VIT C levels in plasma were determined according to Galley et al. [Citation29] with some modifications by Jacques-Silva et al. The VIT C levels were measured at 520 nm and expressed as μg vit C/mL plasma.
2.3. Statistical analysis
Statistical analysis was done with GraphPad Prism, version 6.0 (GraphPad Software, San Diego, CA, USA). The Shapiro–Wilk test was used for assessing the distribution of the variables. Variables with normal distribution were compared by Student’s t-test and the results represented as mean ± standard deviation (SD). The Mann–Whitney test was used for variables with no-normal distribution and the results expressed as median (interquartile range). Correlations were assessed by Spearman’s correlation coefficient. Values of p < 0.05 were considered significant.
3. Results
3.1. Demographic, clinical and laboratory parameters of the studied groups
Demographic, clinical and laboratory parameters of women with GDM and controls are shown in . Significantly higher fasting glucose levels were found in women with GDM when compared to controls (p < 0.0001). The other parameters analyzed showed no significant differences among pregnant women (p > 0.05).
Table 1. Demographic, clinical and laboratory parameters of the studied groups.
3.2. δ-ALA-D activity and reactivation index
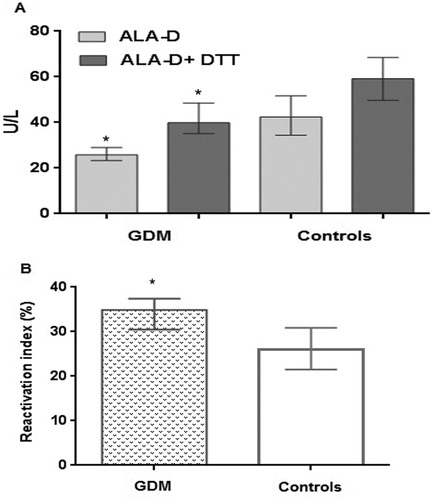
δ-ALA-D activity and its reactivation index are presented in (A–B). There was a decrease of δ-ALA-D activity in women with GDM, in the presence or absence of DTT reducing agent, when compared to the control group (A) (p < 0.05). Furthermore, the reactivation index of δ-ALA-D was significantly higher in women with GDM than in the control group (B).
Figure 1. (A) Activity of δ-ALA-D enzyme in women with GDM and controls. Data were expressed as median (interquartile range) in U/L. (B) Reactivation index of δ-ALA-D enzyme in women with GDM and controls. Data were expressed as median (interquartile range) in %. Statistically significant differences from the controls were determined by Mann–Whitney test (* p < 0.0001).
3.3. Measures of oxidative stress biomarkers
Biomarkers of oxidative stress are shown in . As can be observed, the TBARS levels of both plasma and erythrocytes were significantly higher in women with GDM than in the control group (p < 0.0001). In relation to the antioxidants, the levels of P-SH and NP-SH were lower in GDM than in the control group (p < 0.001). Similarly, VIT C levels (p < 0.01) and CAT activity (p < 0.05) were lower in GDM.
Table 2. Biomarkers of oxidative stress in women with GDM and controls.
3.4. Correlations between analyzed parameters
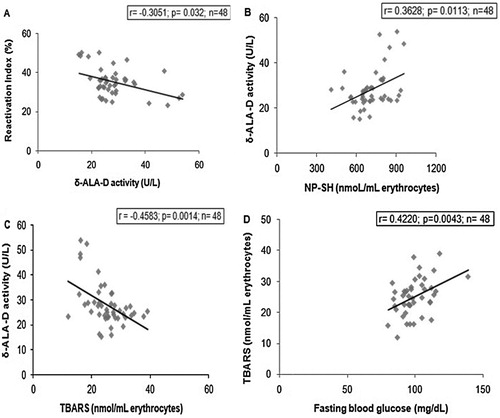
Significant correlations between the parameters analyzed in women with GDM are shown in (A–D). We observed a negative correlation between δ-ALA-D activity and the reactivation index ((A)), a positive correlation between δ-ALA-D activity and NP-SH levels ((B)) and a negative correlation between δ-ALA-D activity and TBARS levels in erythrocytes ((C)). Furthermore, TBARS levels in erythrocytes were also positively correlated with fasting glucose levels ((D)).
Figure 2. Spearman correlations in women with GDM (n = 48). (A) Correlation between δ-ALA-D activity and the reactivation index (r = −0.3051, p = 0.0032), (B) Correlation between δ-ALA-D activity and NP-SH levels (r = 0.3628, p = 0.0113), (C) Correlation between δ-ALA-D activity and TBARS levels in erythrocytes (r = −0.4583, p = 0.0014), (D) Correlation between TBARS levels in erythrocytes and fasting glucose levels in women with GDM (r = 0.4220, p = 0.0043).
4. Discussion
Pregnancy is a physiological period with increased susceptibility to insulin resistance and increased oxidative stress, because the placenta acts in the production of diabetogenic hormones and contributes to the generation of ROS, creating an environment rich in mitochondria with high oxygen pressure [Citation2,Citation30]. In normal pregnancy, the ROS production rate is compensated by an increased synthesis of antioxidants [Citation31]. However, when the pregnancy is complicated by diabetes, excessive production of ROS overpowers antioxidant defenses, leading to increased oxidative stress [Citation9,Citation22].
In this line, the results obtained in our study demonstrate for the first time in the literature that δ-ALA-D activity in women with GDM was significantly reduced when compared to controls. Moreover, this enzyme was associated with increased oxidative stress in GDM.
As expected, women with GDM showed a significant increase in fasting glucose levels when compared to controls (). This result may be due to an inadequate secretion of insulin or reduced sensitivity to insulin, similar to typical T2DM abnormalities [Citation4]. There is also evidence that women with GDM form advanced glycation end-products (AGEs) [Citation32,Citation33]. These AGEs are generally formed in chronic processes of hyperglycemia, as in T2DM and may generate an increased ROS and oxidative stress [Citation33]. Based on these metabolic characteristics of T2DM present in GDM, δ-ALA-D may behave similarly in these two types of diabetes, since it is sensitive to oxidative conditions such as hyperglycemia [Citation16].
The lower activity of δ-ALA-D in diabetic pregnant women when compared to controls ((A)) may be associated with the disproportionate glycemic levels of these women [Citation30]. This finding corroborates the fact that δ-ALA-D is sensitive to hyperglycemic conditions, as in T2DM [Citation18,Citation19].
In order to verify whether δ-ALA-D was inhibited by oxidation of thiol groups, we evaluated the reactivation index, through a reaction with DTT [Citation34]. In women with GDM, the reactivation index was significantly higher when compared to the controls ((B)), in addition to being negatively correlated with δ-ALA-D activity (A). This indicates that the thiol groups were more oxidized in GDM, resulting in reduced δ-ALA-D activity, since these groups (-SH) are vital components for its functioning [Citation34]. Furthermore, inhibition of δ-ALA-D leads to the accumulation of its δ-ALA substrate, which further increases the amount of ROS and is associated with the oxidation of lipid membranes and antioxidant depletion [Citation35], also contributing to increased oxidative stress in women with GDM.
Regarding the analysis of protein thiol (P-SH) and non-protein (NP-SH) groups, both were decreased in diabetic pregnant women when compared to controls (). This result is in agreement with Rajdl et al. [Citation20] who attributed the reduced GSH to oxidative stress of GDM. A negative correlation between δ-ALA-D activity and NP-SH was also observed in these pregnant women ((B)), suggesting that non-protein thiol, main constituents of GSH [Citation12], are easily oxidized in conditions of oxidative stress. Furthermore, GSH may be related to the reduced δ-ALA-D activity, as it is dependent on thiol groups, which may reduce or even inhibit its activity when they are oxidized.
In order to analyze lipid damage, MDA was assessed using a TBARS assay and a significant increase of lipid peroxidation was observed in women with GDM when compared to controls (). Previous studies showed similar results in women with GDM [Citation20,Citation22]. Moreover, δ-ALA-D activity was negatively correlated with TBARS levels in erythrocytes ((C)). This suggests that ROS produced in GDM, besides damaging the lipid membrane [Citation8], may be responsible for oxidation of δ-ALA-D thiol groups, resulting in inhibition of this enzyme and in an increase of the total load of ROS in the organism [Citation35].
Some studies suggest that the increase in ROS in GDM is due to hyperglycemia, which in different pathways leads to oxidative stress [Citation21,Citation36]. In fact, our results corroborate with this argument, because a positive correlation between TBARS in erythrocytes and fasting glucose levels in women with GDM was observed ((D)). In addition, antioxidants such as CAT and VIT C were reduced in women with GDM when compared to controls (). The reduction of CAT may reflect an adaptive and protective response of the antioxidant system to oxidative stress [Citation9,Citation21]. Furthermore, CAT may be reduced due to it being a hemoprotein [Citation17] and since δ-ALA-D enzyme was reduced, this may have affected the synthesis of heme and its hemoproteins, such as CAT. The reduction of VIT C observed in women with GDM is in agreement with other studies [Citation22,Citation37], suggesting that this antioxidant is consumed during oxidative reactions, since it is the first line of defense in aqueous solution.
5. Conclusion
In summary this study demonstrated that there was a greater oxidative stress in women with GDM, different from the physiological stress of a normal pregnancy. The data indicate the increase in oxidative stress accompanied by decrease in δ-ALA-D activity, which was shown to be sensitive to the hyperglycemic environment that emerged during pregnancy. Thus, the use of δ-ALA-D together with other markers of oxidative stress may be important to assess metabolic processes that are debilitated, as in GDM.
Acknowledgments
This study was supported by Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Programa de Iniciação Científica Para o Hospital Universitário de Santa Maria (PROIC-HUSM), Núcleo de Educação Permanente em Saúde (NEPeS) and Federal University of Santa Maria (UFSM), Brazil. Also, we thank all the pregnant women who participated in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Fabiane Rodrigues http://orcid.org/0000-0002-2836-4792
Walter S. Neme http://orcid.org/0000-0003-0250-7932
References
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2016;39:13–22. doi:10.2337/dc15-S005 doi: 10.2337/dci15-0030
- Padilha PC, Sena AB, Nogueira JL, et al. Nutritional therapy in gestational diabetes. Rev Nutr. 2010;23:95–105. doi:10.1590/S1415-52732010000100011.
- Salzer L, Tenenbaum-Gavish K, Hod M. Metabolic disorder of pregnancy (understanding pathophysiology of diabetes and preeclampsia). Best Pract Res Clin Obstet Gynaecol. 2015;29:328–338. doi:10.1016/j.bpobgyn.2014.09.008.
- Baz B, Riveline JP, Gautier JF. Endocrinology of pregnancy: gestational diabetes mellitus – definition, aetiological and clinical aspects. Eur J Endocrinol. 2016;174:43–51. doi:10.1530/EJE-15-0378.
- Buchanan TA, Xiang A, Kjos SL, et al. What is gestational diabetes? Diabetes Care. 2007;30:105–111. doi:10.2337/dc07-s201.
- Wójcik M, Chmielewska-Kassassir M, Grzywnowicz K, et al. The relationship between adipose tissue-derived hormones and gestational diabetes mellitus (GDM). Endokrynol Pol. 2014;65:132–142. doi:10.5603/EP.
- Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi:10.1016/S0140-6736(09)60731-5.
- Karacay Ö, Sepici-Dincel A, Karcaaltincaba D, et al. A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24–36 weeks of gestation. Diabetes Res Clin Pract. 2010;89:231–238. doi:10.1016/j.diabres.2010.04.015.
- López-Tinoco C, Roca M, García-Valero A, et al. Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetol. 2013;50:201–208. doi:10.1007/s00592-011-0264-2.
- Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi:10.1097/WOX.0b013e3182439613.
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi:10.1039/C4RA13315C.
- Yang Y, Guan X. Rapid and thiol-specific high-throughput assay for simultaneous relative quantification of total thiols, protein thiols, and nonprotein thiols in cells. Anal Chem. 2015;87:649–655. doi:10.1021/ac503411p.
- Barbosa KBF, Costa NMB, Alfenas RCG, et al. Oxidative stress: concept, implications and modulating factors. Rev Nutr. 2010;23:629–643. doi:10.1590/S1415-52732010000400013.
- Rocha JBT, Saraiva RA, Garcia SC, et al. Aminolevulinate dehydratase (δ-ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Toxicol Res (Camb). 2012;1:85–102. doi:10.1039/C2TX20014G.
- Kayaaltı Z, Sert S, Kaya-Akyüzlü D, et al. Association between delta-aminolevulinic acid dehydratase polymorphism and placental lead levels. Environ Toxicol Pharmacol. 2016;41:147–151. doi:10.1016/j.etap.2015.11.017.
- Schmatz R, Perreira LB, Stefanello N, et al. Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie. 2012;94:374–383. doi:10.1016/j.biochi.2011.08.005.
- Heinemann IU, Jahn M, Jahn D. The biochemistry of heme biosynthesis. Arch Biochem Biophys. 2008;474:238–251. doi:10.1016/j.abb.2008.02.015.
- Bonfanti G, Ceolin RB, Valcorte T, et al. δ-Aminolevulinate dehydratase activity in type 2 diabetic patients and its association with lipid profile and oxidative stress. Clin Biochem. 2011;44:1105–1109. doi:10.1016/j.clinbiochem.2011.06.980.
- Souza JB, Rocha JBT, Nogueira CW, et al. Delta-aminolevulinate dehydratase (δ-ALA-D) activity in diabetes and hypothyroidism. Clin Biochem. 2007;40:321–325. doi:10.1016/j.clinbiochem.2006.11.016.
- Rajdl D, Racek J, Steinerová A, et al. Markers of oxidative stress in diabetic mothers and their infants during delivery. Physiol Res. 2005;54:429–436. http://www.ncbi.nlm.nih.gov/pubmed/15588143
- Biri A, Onan A, Devrim E, et al. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27:327–332. doi:10.1016/j.placenta.2005.01.002.
- Shang M, Zhao J, Yang L, et al. Oxidative stress and antioxidant status in women with gestational diabetes mellitus diagnosed by IADPSG criteria. Diabetes Res Clin Pract. 2015;109:404–410. doi:10.1016/j.diabres.2015.05.010.
- Association AD. Standards of medical care in diabetes. Diabetes Care. 2014;37:14–80. doi:10.2337/dc14-S014.
- Berlin A, Schaller KH. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z Klin Chem Klin Biochem. 1974;12:389–390. http://www.ncbi.nlm.nih.gov/pubmed/4428852
- Lapenna D, Ciofani G, Pierdomenico SD, et al. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic Biol Med. 2001;31:331–335. doi:10.1016/S0891-5849(01)00584-6.
- Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46:639–653. doi:10.1016/0003-2697(72)90335-1.
- Jacques-Silva MC, Nogueira CW, Broch LC, et al. Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol. 2001;88:119–125. doi:10.1034/j.1600-0773.2001.d01-92.x.
- Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi:10.1016/S0076-6879(84)05016-3.
- Galley HF, Davies MJ, Webster NR. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic Biol Med. 1996;20:139–143. doi:10.1016/0891-5849(95)02022-5.
- Agarwal A, Aponte-Mellado A, Premkumar BJ, et al. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:1, doi:10.1186/1477-7827-10-49.
- Leal CAM, Schetinger MRC, Leal DBR, et al. Oxidative stress and antioxidant defenses in pregnant women. Redox Rep. 2011;16:230–236. doi:10.1179/2301351000211Y.0000000013 doi: 10.1179/1351000211Y.0000000013
- Harsem NK, Braekke K, Torjussen T K, et al. Advanced glycation end products in pregnancies complicated with diabetes mellitus or preeclampsia. Hypertens Pregnancy. 2008;27:374–386. doi:10.1080/10641950802000968.
- Guosheng L, Hongmei S, Chuan N, et al. The relationship of serum AGE levels in diabetic mothers with adverse fetal outcome. J Perinatol. 2009;29:483–488. doi:10.1038/jp.2009.12.
- Valentini J, Schmitt GC, Grotto D, et al. Human erythrocyte δ-aminolevulinate dehydratase activity and oxidative stress in hemodialysis patients. Clin Biochem. 2007;40:591–594. doi:10.1016/j.clinbiochem.2007.02.007.
- Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927–945. doi:10.1016/S0891-5849(00)00413-5.
- Arribas L, Almansa I, Miranda M, et al. Serum malondialdehyde concentration and glutathione peroxidase activity in a longitudinal study of gestational diabetes. PLoS One. 2016;11:e0155353, doi:10.1371/journal.pone.0155353.
- Suhail M, Patil S, Khan S, et al. Antioxidant vitamins and lipoperoxidation in non-pregnant, pregnant, and gestational diabetic women: erythrocytes osmotic fragility profiles. J Clin Med Res. 2010;2:266–273. doi:10.4021/jocmr454w.
