ABSTRACT
Objectives: To unveil the role of SIRT1 in limiting oxidative stress in psoriasis and to further discuss the therapeutic prospects of salidroside in psoriasis.
Methods: Literature from 2002 to 2019 was searched with “psoriasis”, “oxidative stress”, “SIRT1”, “salidroside” as the key words. Then, Oxidative stress in psoriasis and the role of SIRT1 were summarized and the potential role of salidroside in the disease was speculated.
Results: Oxidative stress might contribute to the pathogenesis of psoriasis. High levels of ROS produced during oxidative stress lead to the release of inflammatory mediators, that, in turn, induce angiogenesis and excessive proliferation of keratinocytes. SIRT1 is a member of the sirtuin family, of which the activation lead to the inhibition of such oxidative stress signaling pathways MAPK, NF-κB, and STAT3, down-regulation of inflammatory factors, suppression of inflammation and keratinocyte hyperproliferation, and inhibition of angiogenesis. Salidroside, the main ingredient of Rhodiola, is known to exert antioxidant roles, which has been attributed to SIRT1 activation.
Conclusion: Salidroside might inhibit oxidative stress singling pathways via SIRT1 activation, and could be as an ideal candidate for management of psoriasis.
Introduction
Psoriasis is a chronic inflammatory disease that affects 2%–5% of the world’s population. It is characterized by erythema, plaques, and scales, and is sometimes accompanied by itching [Citation1]. The histological hallmarks of psoriasis include angiogenesis, abnormal keratinocyte proliferation, and infiltration of inflammatory cells. Some investigators have highlighted the genetic factors involved in the pathogenesis of psoriasis. For example, TNF-α gene polymorphisms, including the SNP +489 allele, have been significantly associated with psoriatic arthritis susceptibility and severity [Citation2]. Moreover, other previous studies have established their association with autoimmune diseases and environmental factors. Recently, it has been reported that the generation of oxidative stress could promote psoriasis through the MAPK, NF-κB, and STAT3 pathways and that it can be alleviated by inhibition of these three pathways [Citation3,Citation4].
Salidroside – the main ingredient of Rhodiola – has anti-aging and antioxidant properties. Recent reports have emphasized its protective effects on the nervous and cardiovascular systems, and in the treatment of tumors and inflammatory diseases [Citation5]. Salidroside reportedly plays an antioxidant role via activation of SIRT1, which could serve as a new target for treating psoriasis. This review aims to elucidate the role of SIRT1 in mediating oxidative stress in psoriasis and to discuss the potential therapeutic role of salidroside in psoriasis.
Oxidative stress in psoriasis and the role of SIRT1
Oxidative stress is generated due to an imbalance between levels of reactive oxygen species (ROS) and antioxidants [Citation6]. ROS mainly include superoxide anion (O2−), hydroxyl radical (HO−), hydrogen peroxide (H2O2), and lipid radicals, which are the byproducts of cellular metabolism [Citation7]. Some enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidases (GSH-px), function as antioxidants, protecting cells from oxidative damage [Citation8].
Oxidative stress is an essential factor that induces and aggravates psoriasis, which is known to be induced by a variety of factors – alcohol consumption, smoking, infection, drugs, obesity, cell metabolism, immune response, and pathological state [Citation9]. Previous reports showed that the total antioxidant capacity (TAC) in patients with psoriasis is lower than that in healthy subjects [Citation10–12]. Although severe oxidative stress leads directly to cell death, recent research has demonstrated that mild oxidative stress plays a more critical role in the pathogenesis of psoriasis than severe oxidative stress [Citation4].
Generation of ROS is a crucial step in the induction of oxidative stress in psoriasis. ROS generally act as second messengers during this process and lead to an increase in the levels of malondialdehyde (MDA), NO, HO−, and inducible nitric oxide synthase (iNOS), and decrease in the levels of SOD, CAT, and GSH-px [Citation3,Citation9,Citation13]. Elevated levels of oxidative products result in the activation of Th1 and Th17 cells and keratinocytes through the MAPK, NF-κB, and JAK-STAT pathways [Citation12,Citation14]. This results in a cascade of inflammatory cytokines and growth factors, such as IFN-γ, IL-2, TNF-α, and TNF-β, produced by Th11; IL-17, IL-22, IL-23, and TNF-α, by Th17; and antimicrobial peptide (AMP), TNF-α, IL-6, IL-8, polymorphonuclears (PMN), and VGEF, by activated keratinocytes [Citation12,Citation15]. These inflammatory mediators further activate T cells and mast cells to give rise to a self-amplifying loop that results in keratinocyte overproliferation, neutrophil recruitment, hypervascular hyperplasia, and sustained skin inflammation [Citation15,Citation16]. Angiogenesis – a marker of psoriasis pathogenesis – could also be promoted by angiogenic mediators like VEGF, TNF-α, IL-8, and IL-17 [Citation16,Citation17]. As reported, oxidative stress and a high level of TNF-α induce endothelial dysfunction, which can consequently contribute to autoimmune and cardiovascular diseases [Citation18,Citation19].
Sirtuins are a family of proteins that are involved in multiple cellular functions related to metabolism, cell cycle, aging, inflammation, apoptosis, cell proliferation, and DNA repair [Citation20–22]. Among them, SIRT1 is the most well studied. SIRT1 is a highly conserved NAD+-dependent histone deacetylase that mediates the antioxidative stress in psoriasis [Citation23,Citation24]. It was reported that SIRT1 could promote the differentiation of human keratinocytes [Citation25] and inhibit keratinocyte proliferation [Citation26]. Moreover, SIRT1 protects fibroblasts of individuals with psoriasis from oxidative stress-induced apoptosis and restores both mitochondrial function and redox balance via downregulation of MAPK signaling [Citation12]. Additionally, Wang Y et al. reported that chemerin exacerbated psoriasiform dermatitis in a mouse model and that it can induce inflammatory response and promote NF-κB activation by inhibiting the activity of ROS-induced SIRT1 [Citation27]. Recently, a study found that the expression levels of SIRT1-5 were downregulated, while those of SIRT6 and SIRT7 were upregulated in psoriasis skin lesions [Citation28]. According to the literature, many compounds, such as dioscin, grape seed oil, luteolin, resveratrol, methyl ferulic acid, and STR1720, can activate or/and upregulate SIRT1 [Citation29].
SIRT1/MAPK/AP-1 signaling cascade
MAPKs are a family of serine-threonine protein kinases that are involved in signal transduction, cell differentiation, proliferation, apoptosis, and immune response. Members of this family include – extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and the p38 MAPKs [Citation4,Citation30]. AP-1 is an important eukaryotic transcription factor that is activated by the three MAPK pathways and regulates various inflammatory factors such as TNF-α, IL-6, and MCP-1ex. The involvement of the MAPK/AP-1 pathway in oxidative stress has been extensively investigated [Citation31–34]. It has been demonstrated that there is increased activation of ERK1/2, p38 MAPK, and JNK in lesional psoriatic skin [Citation35–37], and JNK is involved in the differentiation and proliferation of keratinocytes [Citation38]. Reports suggest that activation of SIRT1 protects the heart from oxidative stress and cushions the brain from alcohol-induced neurodegeneration via inhibition of the p38 MAPK pathway [Citation39,Citation40]. Studies indicate that carnosic acid protects normal mouse hepatocytes against oxidative stress-induced cytotoxicity by regulating ERK1/2 via SIRT1 [Citation41]. When SRT2014, an agonist of SIRT1, was used to treat moderate to severe psoriasis, it was observed that the expression of known IL-17 and TNF-α responsive genes and that of genes involved in keratinocyte differentiation were significantly downregulated [Citation42].
SIRT1/NF-κB relationship
Nuclear factor-κB (NF-κB) is an essential inflammatory mediator in the pathogenesis of psoriasis; increased expression of NF-κB has been demonstrated in psoriatic lesions [Citation4]. ROS can activate NF-κB through the phosphorylation of the inhibitor of kappa B kinase (IKK) complex [Citation43,Citation44]. Studies indicate that H2O2, imported via AQP3, participates in the activation of the NF-κB signaling pathway in keratinocytes and is involved in the pathogenesis of psoriasis [Citation45]. Altered NF-κB signaling disrupts the balance of apoptotic signals, leading to the upregulation of cyclins and survivins, thereby inhibiting apoptosis [Citation44]. Furthermore, NF-κB induces the production of IL-17 and TNF-α, thereby enhancing the downstream inflammatory response [Citation44,Citation46]. SIRT1 has been shown to suppress NF-κB signaling through deacetylation of the p65 subunit of NF-κB and resulting in the reduction of inflammatory responses [Citation47]; overexpression of SIRT1 reduced the expression of IL-1β, IL-8, and TNF- α [Citation27]. It has been recognized that SIRT1 can inhibit NF-κB signaling through AMPK, PGC-1α, and PPARα; concomitantly, NF-κB also downregulated SIRT1 expression and signaling via ROS, IFN-γ, and PARP-1 under oxidative stress conditions [Citation48].
SIRT1/STAT3 interaction
Besides MAPK/AP-1 and NF-κB pathways, STAT3 also plays an essential role in psoriasis. STAT3 has been implicated in the regulation of fundamental biological processes such as cell proliferation, differentiation, oncogenesis, survival, and apoptosis [Citation49]. In resting cells, STAT3 is localized in the cytoplasm. When stimulated by ROS, STAT3 gets activated through phosphorylation at Tyrosine 705 (Tyr705), following which it enters the nucleus and regulates gene expression. STAT3 is mainly phosphorylated by JAK and the epidermal growth factor receptor kinase; however, Src and ERK may also be involved in STAT3 phosphorylation [Citation50]. In addition, MAPK, ERK, and protein kinase have been demonstrated to enhance STAT3 gene transcription [Citation50,Citation51]. Thus, MAPK and STAT3 synergistically promote the development of psoriasis. A variety of studies have demonstrated that STAT3 is over-active in psoriatic lesions, where it promotes the excessive proliferation of keratinocytes and the production of IL-6, IL-8, IL-23, and IL-17. These cytokines, in turn, trigger the Th17 and STAT3 signaling pathway, thereby resulting in continuous inflammation [Citation52]. Ki67, keratin16, and keratin17 are markers of excessive proliferation of keratinocytes. Bin Zhang et al. (Citation2016), reported that compared to that in healthy skin, the activity of STAT3 is high in psoriatic lesions, and there is an excessive proliferation of keratinocytes as is evidenced by high levels of Ki67, keratin16, and keratin17 staining. They also reported that stimulation with IL-6 and IL-22 results in increased phosphorylation of STAT3. In parallel, the downstream genes of STAT3 such as survivin, cyclin D1, and the Bcl family were upregulated [Citation53]. There is compelling evidence that SIRT1 counteracts the IL-22-induced STAT3 activity by deacetylating STAT3 and represses the downstream gene expression, thereby inhibiting keratinocyte proliferation and migration [Citation54]. Besides, SIRT1 has been shown to reduce the severity of lesions in the Aldara-induced psoriasis model via negatively regulating STAT3 activation. Moreover, a significant reduction in SIRT1 levels and conversely, an increase in PY-STAT3 were detected in psoriatic mouse models as compared with that in healthy mice [Citation55].
In summary, activation of SIRT1 can counteract the damage caused by oxidative stress by inhibiting the MAPK, NF-κB, and STAT3 pathways (), leading to the alleviation of the pathological injury in psoriasis. Therefore, SIRT1 can serve as a new therapeutic target for this disease. However, to date, most studies have focused on antioxidants such as dimethyl fumarate, curcumin, and propylthiouracil for treating psoriasis [Citation56–58]. Besides, studies using specific antibodies for the treatment of psoriasis have confirmed that biological agents are indeed effective against this condition [Citation59,Citation60]. However, these medicines have limited clinical applications due to adverse reactions, high cost, and inaccurate long-term efficacy. Thus, it is vital to develop a cheap and effective treatment with limited side effects for psoriasis.
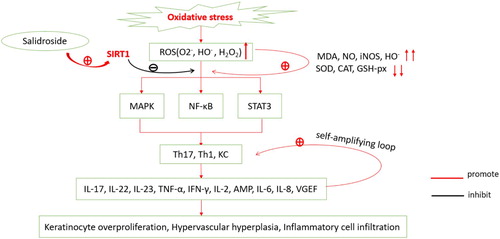
Figure 1. Psoriatic oxidative stress mechanism. Reactive oxygen species (ROS) including superoxide anion (O2−), hydroxyl radical (HO−), hydrogen peroxide (H2O2) are increased in oxidative stress in psoriasis, resulting in the increase of malondialdehyde (MDA), NO, HO− and inducible nitric oxide synthase (iNOS); on the contrary, levels of SOD, CAT and GSH-px are decreased. The increased oxidative products lead to the activation of Th1, Th17, and keratinocyte cells through MAPK, NF-κB, JAK-STAT pathways, resulting in overproduction of IL-17, IL-22, IL-23, TNF-α, IFN-γ, IL-2, AMP, IL-6, IL-8, and VGEF. These inflammatory factors further activate Th1, Th17, and keratinocytes, forming a self-amplifying loop, eventually leading to keratinocyte overproliferation, hypervascular hyperplasia, and inflammatory cell infiltration. Salidroside, the activator of SIRT1, resists oxidative stress by inhibiting MAPK, NF-κB, and STAT3 pathways.
Salidroside
Recently, a detailed review of the pharmacological effects of salidroside in various diseases has demonstrated its anti-aging, anti-inflammatory, antioxidation, anti-cancer, and liver protection properties [Citation61]. It is also non-toxic to astrocytes [Citation62], improves hind limb motor function and reduces tissue damage in spinal cord injury by reducing the phosphorylation of NF-κB, ERK, and p38 and by reducing the production of inflammatory cytokines such as IL-1β, IL-6, and TNF-α [Citation62]. Previous studies showed that salidroside can prevent DMBA/TPA-induced skin cancer in mouse models by inhibiting inflammation and promoting apoptosis [Citation63]. Xiang-jun Fan et al. indicated that salidroside was able to inhibit the growth of HT-29 human colorectal cancer cells and induce cell apoptosis, possibly via the PI3 K/Akt/mTOR signaling pathway [Citation64]. Studies have also shown that salidroside protected retinal pigment epithelial cells from oxidative stress induced by H2O2 [Citation65]. Besides, salidroside reduces the production of LPS-induced proinflammatory cytokines and mediators and attenuates LPS-induced acute lung injury by inhibiting the JAK2-STAT3 signaling pathway [Citation66].
In addition, salidroside could act as an activator of SIRT1 to exert its antioxidant effects. Chun-Yang Wang et al. proved that salidroside increased SOD activity and GSH levels and reduced the phosphorylation of MAPK by activating SIRT1; they also found that it protects SH-SY5Y cells from apoptosis and oxidative stress induced by MPP+ [Citation67]. Similarly, salidroside protects against kainic acid-induced status epilepticus through suppression of oxidative stress, and this effect might be mediated by the AMPK/SIRT1/FoxO1 pathway [Citation68]. It has also been reported to protect human umbilical vein endothelial cells (HUVECs) from oxidative stress induced by ox-LDL through increasing SIRT1, FoxO1, and SOD expression and to simultaneously down-regulate MDA and LDH [Citation69]. Meanwhile, another research concluded that salidroside protects HUVECs against ox-LDL injury through inhibiting oxidative stress and improving mitochondrial dysfunction, which were dependent on AMPK/SIRT1 pathway activation [Citation70]. Furthermore, Xu N et al. concluded that salidroside decreased the expression of inflammatory cytokines and improved LPS-induced learning memory impairments, which were involved in the SIRT1-dependent Nrf-2/HO-1/NF-κB pathway [Citation71].
However, the antioxidative mechanism of salidroside in psoriasis has not yet been reported. In our review, we highlight the role of SIRT1 in reducing oxidative stress in psoriasis. The activation of SIRT1 could effectively inhibit MAPK, NF-κB, and STAT3 phosphorylation, thereby decreasing the expression of downstream genes and secretion of inflammatory cytokines, eventually blocking the development of psoriasis. Salidroside, the activator of SIRT1, may cure psoriasis resistant to oxidative stress damage by inhibiting the MAPK, NF-κB, and STAT3 pathways; however, this property of salidroside needs further research.
Conclusion
This review, overall, indicates that psoriasis is a common dermatitis associated with oxidative stress mediated by MAPK, NF-κB, and STAT3 signaling cascade. Salidroside, the activator of SIRT1, may cure psoriasis by blocking these three targets. Thus, salidroside, alone or in combination with other compounds, could be an ideal candidate for the management. However, to date, no research on salidroside as a therapeutic option to treat psoriasis has been reported. Hence, further investigation is required to validate the effects of salidroside on psoriasis pathophysiology and further verify the effect of SIRT1 on MAPK, NF-κB, and STAT3 pathways in the skin.
Acknowledgments
We thank Editage for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Fengli Xu http://orcid.org/0000-0002-9882-8900
References
- Yadav K, Singh D. Protein biomarker for psoriasis: a systematic review on their role in the pathomechanism, diagnosis, potential targets and treatment of psoriasis. Int J Biol Macromol. 2018;118:1796–1810. doi:10.1016/j.ijbiomac.2018.07.021.
- Murdaca G, Gulli R, Spanò F, et al. TNF-α gene polymorphisms: association with disease susceptibility and response to anti-TNF-α treatment in psoriatic arthritis. J Invest Dermatol. 2014;134:2503–2509. doi:10.1038/jid.2014.123.
- Lai R, Xian D, Xiong X, et al. Proanthocyanidins: novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018;23:130–135. doi:10.1080/13510002.2018.1462027.
- Lin X, Huang T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free Radic Res. 2016;50:585–595. doi:10.3109/10715762.2016.1162301.
- Chiang HM, Chen HC, Wu CS, et al. Rhodiola plants: chemistry and biological activity. J Food Drug Anal. 2015;23:359–369. doi:10.1016/j.jfda.2015.04.007.
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biodoi:10.1016/j.redox.2015.01.002.
- Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol. 2015;33:8–13. doi:10.1016/j.ceb.2014.09.010.
- Harrison DG. Basic science: pathophysiology: oxidative stress. J Am Soc Hypertens. 2014;8:601–603. doi:10.1016/j.jash.2014.07.002.
- Péter I, Jagicza A, Ajtay Z, et al. Psoriasis and oxidative stress. Orv Hetil. 2016;157:1781–1785. doi:10.1556/650.2016.30589.
- Gabr SA, Al-Ghadir AH. Role of cellular oxidative stress and cytochrome c in the pathogenesis of psoriasis. Arch Dermatol Res. 2012;304:451–457. doi:10.1007/s00403-012-1230-8.
- Barygina VV, Becatti M, Soldi G, et al. Altered redox status in the blood of psoriatic patients: involvement of NADPH oxidase and role of anti-TNF-α therapy. Redox Rep. 2013;18:100–106. doi:10.1179/1351000213Y.0000000045.
- Becatti M, Barygina V, Mannucci A, et al. Sirt1 protects against oxidative stress-induced apoptosis in fibroblasts from psoriatic patients: a new insight into the pathogenetic mechanisms of psoriasis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19061572.
- Houshang N, Reza K, Masoud S, et al. Antioxidant status in patients with psoriasis. Cell Biochem Funct. 2014;32:268–273. doi: 10.1002/cbf.3011
- Lei Y, Wang K, Deng L, et al. Redox regulation of inflammation: old elements, a new story. Med Res Rev. 2015;35:306–340. doi:10.1002/med.21330.
- Georgescu SR, Tampa M, Caruntu C, et al. Advances in understanding the immunological pathways in psoriasis. Int J Mol Sci. 2019;20:739.doi:10.3390/ijms20030739.
- Richarz NA, Boada A, Carrascosa JM. Angiogenesis in dermatology - insights of molecular mechanisms and latest developments. Actas Dermosifiliogr. 2017;108:515–523. doi:10.1016/j.ad.2016.12.001.
- Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177
- Murdaca G, Spanò F, Cagnati P. Free radicals and endothelial dysfunction: potential positive effects of TNF-α inhibitors. Redox Rep. 2013;18:95–99. doi:10.1179/1351000213Y.0000000046.
- Murdaca G, Colombo BM, Cagnati P, et al. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis. 2012;224:309–317. doi:10.1016/j.atherosclerosis.2012.05.013.
- Garcia-Peterson LM, Wilking-Busch MJ, Ndiaye MA, et al. Sirtuins in skin and skin cancers. Skin Pharmacol Physiol. 2017;30:216–224. doi:10.1159/000477417.
- Serravallo M, Jagdeo J, Glick SA, et al. Sirtuins in dermatology: applications for future research and therapeutics. Arch Dermatol Res. 2013;305:269–282. doi:10.1007/s00403-013-1320-2.
- Vachharajani VT, Liu T, Wang X, et al. Sirtuins link inflammation and metabolism. J Immunol Res. 2016;2016:1–10. doi:10.1155/2016/8167273.
- Zhou M, Luo J. Role of sirtuin 1 in the pathogenesis of ocular disease (review). Int J Mol Med. 2018;42:13–20. doi:10.3892/ijmm.2018.3623.
- Singh CK, Chhabra G, Ndiaye MA, et al. The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28:643–661. doi:10.1089/ars.2017.7290.
- Blander G, Bhimavarapu A, Mammone T, et al. SIRT1 promotes differentiation of normal human keratinocytes. J Invest Dermatol. 2009;129:41–49. doi:10.1038/jid.2008.179.
- Wu Z, Uchi H, Morino-Koga S, et al. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J Dermatol Sci. 2014;75:16–23. doi:10.1016/j.jdermsci.2014.03.004.
- Wang Y, Huo J, Zhang D, et al. Chemerin/ChemR23 axis triggers an inflammatory response in keratinocytes through ROS-sirt1-NF-κB signaling. J Cell Biochem. 2019;120:6459–6470. doi:10.1002/jcb.27936.
- Fan X, Yan K, Meng Q, et al. Abnormal expression of SIRTs in psoriasis: decreased expression of SIRT 1-5 and increased expression of SIRT 6 and 7. Int J Mol Med. 2019;44:157–171. doi:10.3892/ijmm.2019.4173.
- Yan T, Huang J, Nisar MF, et al. The beneficial roles of SIRT1 in drug-induced liver injury. Oxid Med Cell Longev. 2019;2019:8506195. doi:10.1155/2019/8506195.
- Kyriakis JM, Kyriakis JM. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi:10.1152/physrev.00028.2011.
- Corsini E, Galbiati V, Nikitovic D, et al. Role of oxidative stress in chemical allergens induced skin cells activation. Food Chem Toxicol. 2013;61:74–81. doi:10.1016/j.fct.2013.02.038.
- Koren Carmi I, Haj R, Yehuda H, et al. The role of oxidation in FSL-1 induced signaling pathways of an atopic dermatitis model in HaCaT keratinocytes. Adv Exp Med Biol. 2015;849:1–10. doi:10.1007/5584_2014_98.
- Bak DH, Lee E, Lee BC, et al. Therapeutic potential of topically administered γ-AlOOH on 2,4-dinitrochlorobenzene-induced atopic dermatitis-like lesions in Balb/c mice. Exp Dermatol. 2019;28:169–176. doi:10.1111/exd.13865.
- Koçtürk S, Yüksel Egrilmez M, Aktan Ş, et al. Melatonin attenuates the detrimental effects of UVA-irradiation in human dermal fibroblasts by suppressing oxidative damage and MAPK/AP-1 signal pathway in vitro. Photodermatol Photoimmunol Photomed. 2019. doi:10.1111/phpp.12456.
- Yu XJ, Li CY, Dai HY, et al. Expression and localization of the activated mitogen-activated protein kinase in lesional psoriatic skin. Exp Mol Pathol. 2007;83:413–418. doi:10.1016/j.yexmp.2007.05.002.
- Johansen C, Kragballe K, Westergaard M, et al. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br J Dermatol. 2005;152:37–42. doi:10.1111/j.1365-2133.2004.06304.x.
- Takahashi H, Ibe M, Nakamura S, et al. Extracellular regulated kinase and c-Jun N-terminal kinase are activated in psoriatic involved epidermis. J. Dermatol. Sci. 2002;30:94–99. doi: 10.1016/S0923-1811(02)00064-6
- Schumacher M, Schuster C, Rogon ZM, et al. Efficient keratinocyte differentiation strictly depends on JNK-induced soluble factors in fibroblasts. J Invest Dermatol. 2014;134:1332–1341. doi:10.1038/jid.2013.535.
- Yang B, Xu B, Zhao H, et al. Dioscin protects against coronary heart disease by reducing oxidative stress and inflammation via Sirt1/Nrf2 and p38 MAPK pathways. Mol Med Rep. 2018;18:973–980. doi:10.3892/mmr.2018.9024.
- Gu X, Cai Z, Cai M, et al. AMPK/SIRT1/p38 MAPK signaling pathway regulates alcohol-induced neurodegeneration by resveratrol. Mol Med Rep. 2018;17:5402–5408. doi:10.3892/mmr.2018.8482.
- Wang T, Takikawa Y. Carnosic acid protects normal mouse hepatocytes against H2 O2 -induced cytotoxicity via sirtuin 1-mediated signaling. Hepatol Res. 2016;46:239–246. doi:10.1111/hepr.12563.
- Krueger JG, Suárez-Fariñas M, Cueto I, et al. A randomized, placebo-controlled study of SRT2104, a SIRT1 activator, in patients with moderate to severe psoriasis. PLoS One. 2015;10:e0142081. doi:10.1371/journal.pone.0142081.
- Nguyen TT, Ung TT, Li S, et al. Metformin inhibits lithocholic acid-induced interleukin 8 upregulation in colorectal cancer cells by suppressing ROS production and NF-kB activity. Sci Rep. 2019;9:2003. doi:10.1038/s41598-019-38778-2.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89–94. doi:10.1016/j.jdermsci.2012.11.002.
- Hara-Chikuma M, Satooka H, Watanabe S, et al. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat Commun. 2015;6:7454. doi:10.1038/ncomms8454.
- Abdou AG, Hanout HM. Evaluation of survivin and NF-kappaB in psoriasis, an immunohistochemical study. J Cutan Pathol. 2008;35:445–451. doi:10.1111/j.1600-0560.2007.00841.x.
- Yang H, Zhang W, Pan H, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS One. 2012;7:e46364. doi:10.1371/journal.pone.0046364.
- Kauppinen A, Suuronen T, Ojala J, et al. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi:10.1016/j.cellsig.2013.06.007.
- Sano S, Chan KS, DiGiovanni J. Impact of Stat3 activation upon skin biology: a dichotomy of its role between homeostasis and diseases. J Dermatol Sci. 2008;50:1–14. doi:10.1016/j.jdermsci.2007.05.016.
- Zhou Q, Mrowietz U, Rostami-Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med. 2009;47:891–905. doi:10.1016/j.freeradbiomed.2009.06.033.
- Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi:10.1111/j.1749-6632.2009.04911.x.
- Calautti E, Avalle L. Psoriasis: A STAT3-Centric View. Int J Mol Sci. 2018: 19. doi:10.3390/ijms19010171.
- Zhang B, Xie S, Su Z, et al. Heme oxygenase-1 induction attenuates imiquimod-induced psoriasiform inflammation by negative regulation of Stat3 signaling. Sci Rep. 2016;6:21132. doi:10.1038/srep21132.
- Sestito R, Madonna S, Scarponi C, et al. STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB J. 2011;25:916–927. doi:10.1096/fj.10-172288.
- Xie S, Su Z, Zhang B, et al. SIRT1 activation ameliorates aldara-induced psoriasiform phenotype and histology in mice. J Invest Dermatol. 2015;135:1915–1918. doi:10.1038/jid.2015.82.
- Bovenschen HJ, Langewouters AM, van de Kerkhof PCM. Dimethylfumarate for psoriasis: Pronounced effects on lesional T-cell subsets, epidermal proliferation and differentiation, but not on natural killer T cells in immunohistochemical study. Am J Clin Dermatol. 2010;11:343–350. doi:10.2165/11533240-000000000-00000.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:1–7. doi:10.1155/2015/283634.
- Gnanaraj P, Dayalan H, Elango T, et al. Downregulation of involucrin in psoriatic lesions following therapy with propylthiouracil, an anti-thyroid thioureylene: immunohistochemistry and gene expression analysis. Int J Dermatol. 2015;54:302–306. doi:10.1111/ijd.12565.
- Visvanathan S, Baum P, Vinisko R, et al. Psoriatic skin molecular and histopathologic profiles after treatment with risankizumab versus ustekinumab. J Allergy Clin Immunol. 2018. doi:10.1016/j.jaci.2018.11.042.
- Ortiz Salvador JM, Saneleuterio Temporal M, Magdaleno Tapial J, et al. A prospective multi-center study assessing effectiveness and safety of secukinumab in a real-life setting in 158 patients. J Am Acad Dermatol. 2019. doi:10.1016/j.jaad.2019.02.062.
- Zhuang W, Yue L, Dang X, et al. Rosenroot (Rhodiola): potential applications in aging-related diseases. Aging Dis. 2019;10:134–146. doi:10.14336/AD.2018.0511.
- Su Y, Zong S, Wei C, et al. Salidroside promotes rat spinal cord injury recovery by inhibiting inflammatory cytokine expression and NF-κB and MAPK signaling pathways. J Cell Physiol. 2019;234:14259–14269. doi:10.1002/jcp.28124.
- Kong YH. Salidroside prevents skin carcinogenesis induced by DMBA/TPA in a mouse model through suppression of inflammation and promotion of apoptosis. Oncol Rep. 2018;39:2513–2526. doi:10.3892/or.2018.6381.
- Fan XJ, Wang Y, Wang L, et al. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep. 2016;36:3559–3567. doi:10.3892/or.2016.5138.
- Yin Y, Liu D. Salidroside prevents hydroperoxide-induced oxidative stress and apoptosis in retinal pigment epithelium cells. Exp Ther Med. 2018;16:2363–2368. doi:10.3892/etm.2018.6494.
- Qi Z, Qi S, Ling L, et al. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol. 2016;35:265–271. doi:10.1016/j.intimp.2016.04.004.
- Wang CY, Sun ZN, Wang MX, et al. SIRT1 mediates salidroside-elicited protective effects against MPP+-induced apoptosis and oxidative stress in SH-SY5Y cells: involvement in suppressing MAPK pathways. Cell Biol Int. 2018;42:84–94. doi:10.1002/cbin.10864.
- Si PP, Zhen JL, Cai YL, et al. Salidroside protects against kainic acid-induced status epilepticus via suppressing oxidative stress. Neurosci Lett. 2016;618:19–24. doi:10.1016/j.neulet.2016.02.056.
- Zhu Z, Li J, Zhang X. Salidroside protects against ox-LDL-induced endothelial injury by enhancing autophagy mediated by SIRT1-FoxO1 pathway. BMC Complement Altern Med. 2019;19:111. doi:10.1186/s12906-019-2526-4.
- Zhao D, Sun X, Lv S, et al. Salidroside attenuates oxidized low-density lipoprotein-induced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int J Mol Med. 2019;43:2279–2290. doi:10.3892/ijmm.2019.4153.
- Xu N, Huang F, Jian C, et al. Neuroprotective effect of salidroside against central nervous system inflammation-induced cognitive deficits: A pivotal role of sirtuin 1-dependent Nrf-2/HO-1/NF-κB pathway. Phytother Res. 2019;33:1438–1447. doi:10.1002/ptr.6335.
