ABSTRACT
Objective
Ginger (Zingiber officinale Roscoe) is considered to be one of the most commonly consumed dietary condiments of the world. The present study was designed to explicate the protective role of zingerone; an active ingredient of ginger in complete Freund’s adjuvant (FCA)-immunized arthritic rats.
Methods
24 Wistar rats were divided into 4 groups with 6 rats each. Group I as control followed by group II, III and IV were treated with single intradermal injection of FCA (0.1 ml = 100 µg) to induce rheumatoid arthritis. Group III and IV were also administered with zingerone orally at 25 mg/kg b.w for 3 weeks at two different time points.
Results
Adjuvant-treated rats exhibited a significant increase in lipid peroxidation and a reduction in the enzymatic antioxidants such as SOD, catalase and GPx, in the liver and joint tissues. Moreover, FCA inoculation resulted in the increase in levels of NF-κB, TGF-β, TNF-α, IL-1β, IL-6 and Hs-CRP and a decrease in IL-10 levels. Zingerone significantly reduced the levels of NF-κB, TGF-β, TNF-α, IL-1β, IL-6 and Hs-CRP and markedly increased IL-10 levels. Levels of antioxidant enzymes were also restored by zingerone treatment.
Discussion
Oral administration of zingerone ameliorated inflammatory outburst and decreased oxidative stress, suggesting its role in the prevention of rheumatoid arthritis. Further mechanistic insights are necessary to study the exact mechanism involved.
Introduction
Arthritis is a disease with multiple factors and has been reported to affect nearly 1% adult population of the world [Citation1, Citation2]. It is a progressive inflammatory disease resulting in the destruction of bone and cartilage through the involvement of synovial membranes of joints [Citation3, Citation4]. Although the main target of disease process in arthritis is joints, the disease is recognized as a non-organ-specific autoimmune disease, because of the occurrence of extra-articular signs, such as subcutaneous nodules, vasculitis and pulmonary fibrosis [Citation5]. The initial phase of the disease results in strong inflammation of joints by the recognition of self-protein epitopes by auto-reactive T and B cell clones [Citation6]. The activated Th1 and Th17 cells coordinate this process by the secretion of cytokines. The cytokines, in turn, activate innate immune cells leading to pain, bone degradation and reduction in cartilage repair [Citation7, Citation8].
Many cytokines contribute to rheumatoid arthritis and act as the key factors responsible for the loss of ability of tissues forming joints by activating destructive processes [Citation9]. Tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β) and interleukin one beta (IL-1β) are the key inflammatory molecules responsible for the pathophysiological alterations taking place during the course of rheumatoid arthritis and increased levels of these inflammatory markers are observed in synovial fluid, synovial membrane, cartilage and sub-chondral bone layer [Citation10]. Moreover, in joint cells, IL-1β induces its own secretion and stimulates synthesis of other molecules such as TNF-α, interleukin six (IL-6), interleukin eight (IL-8), and CCL chemokine [Citation11, Citation12].
During the disease process, IL-1β stimulates reactive oxygen species (ROS) production along with decreased expression of oxidative enzymes, thereby accelerating further damage of articular cartilage in the joint affected by the disease [Citation13]. TNF-α and IL-1β act in a synergic manner in many phenomena that take place in the course of rheumatoid arthritis [Citation14]. This effect is the result of the activation of similar intracellular signaling pathways, which trigger inflammation and destruction of joint tissues [Citation15]. So these reactive oxygen species (ROS) and mediators of inflammation act in complete coordination.
A number of drugs that are being currently used to relieve pain and inflammation of damaged joint include non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, disease-modifying anti-rheumatoid drugs (DMARDs), biologicals such as TNF-α and IL antagonists [Citation16–18]. However, these drugs have lost their role because of the safety concerns and associated side effects such as gastrointestinal or cardiac toxicity [Citation19, Citation20], immune suppression, osteoporosis, and metabolic disorders [Citation21, Citation22], high incidence of infection rate and huge cost [Citation23, Citation24].
Due to the limitations of the above therapeutic approaches, there is a need for the identification of biomolecules from plants or natural sources devoid of toxic effects. The medicinal plants and herbs are playing an essential role in the health and vitality of human beings and animals. Hence, several phytomedicines (medicinal plants or herbs) are now used for the prevention and cure of arthritis. In the recent past, many of these herbs and compounds obtained from plant sources have received attention as an alternate source of medicine [Citation25].
Zingiber officinale is a monocotyledonous medicinal plant, native to India or Southeast Asia, from where it reached to rest of the world [Citation26]. Both fresh and dried ginger has found its use as a food additive and dietary spice as well as a phytomedicines [Citation27]. Several studies have reported the effectiveness of compounds isolated from ginger against inflammatory diseases [Citation28]. Zingerone, one of the active components, isolated from Zingiber officinale, is a phenolic alkanone that contains a vanilloid (4-hydroxy-3-methoxyphenyl) group in its structure with many pharmacological properties including antioxidant [Citation29], anti-inflammatory [Citation30], anticancer [Citation31], antimicrobial [Citation32] and antidiabetic activity [Citation33].
Since no literature is available on the potential role of zingerone on rheumatoid arthritis, the current study was contemplated as a first time investigation to decipher the mechanisms involving the promising anti-arthritic effect of zingerone by studying inflammatory markers, oxidative stress, and arthritic markers in an experimental model of Freund’s Complete Adjuvant (FCA)-induced rheumatoid arthritis.
Materials and methods
Experimental animals
Six- to eight-week-old, albino rats (140–160 g) of Wistar strain used in the study were purchased from Indian Institute of Integrated Medicine (IIIM) Jammu. The protocols were approved by the ‘Institutional Animal Ethical Committee (IAEC)’ (Vide no: AU/FVSc/VCC/1-3/19/815-16 dated: 23-11-2019) accredited by CPCSEA, New Delhi, India. Animals were kept in polypropylene cages in groups of three rats per cage at 25°C and had free access to standard diet and water.
Preparation of zingerone
Oral dose was prepared by dissolving 0.5 g of zingerone (Sigma Aldrich) in 100 ml of normal saline [Citation34].
Design of experiment
After acclimatization period the animals were divided into 4 groups with 6 rats in each group to assess the effect of zingerone on FCA-induced rheumatoid arthritis. Group I served as normal control and received diet + water ad libitum and normal saline orally for three weeks, group II which served as disease control, received single intra-dermal injection of FCA (0.1 ml = 100 µg) at the base of tail to induce rheumatoid arthritis. Group III (treatment group I) received FCA (0.1 ml) at the base of tail + zingerone (25 mg/kg b.w.; from the day of arthritis induction) in normal saline daily for three weeks by an oral gavage, while group IV (treatment group II) received single intra-dermal injection of FCA (0.1 ml) at the base of tail + zingerone (25 mg/kg b.w.; from day of arthritis onset) in normal saline daily for three weeks by an oral gavage ().
Table 1. Tabular design of experiment.
Measurement of arthritis
The degree of severity of arthritis was assessed in the affected paw by macroscopic scoring and grading system, as described by Banerjee et al. [Citation35]. In each affected paw, the severity of arthritis was graded on a subjective scale of 1–3 as redness and swelling (grade 1), deformity (grade 2) and ankylosis (grade 3). The progress of arthritis was assessed by measuring the paw thickness with the help of calipers.
Sample collection
After completion of the experiment all animals were sacrificed with light ether anesthesia. Blood was drawn by cardiac puncture and centrifuged at 3000 rpm (4°C) for the collection of serum. Liver and knee joints were removed for the preparation of homogenate and cell-free extract.
Liver homogenate and preparation of post-mitochondrial supernatant (PMS)
After removing the liver aseptically from all animals, it was homogenized in a chilled phosphate buffer (0.1 M, pH 7.4) containing potassium chloride (KCl; 1.17% w/v). The homogenate was used for lipid peroxidation (LPO) analysis. The homogenate was further centrifuged at 800× g for 5 min at 4°C in order to separate the nuclear debris. The supernatant was further centrifuged at 10000× g for 20 min at 4°C to get the post-mitochondrial supernatant for the analysis of antioxidant profile.
Preparation of cell-free extract of the knee joints
Knee joints from arthritic and non-arthritic rats were removed and cut into small pieces with the help of a blade and homogenized in 5 vol of 50 mM Tris HCl buffer, pH 7.4 containing 0.1 M NaCl and 0.1% Triton X-100 and 1 vol. of fine glass powder with the help of a mortar and pestle. The crude extract was sonicated for 20 sec. The homogenate was then centrifuged at 3000× g for 5 min, and the resulting supernatant was used for the estimation of LPO, immune markers and antioxidant profile.
Estimation of malondialdehyde (MDA)
LPO in liver homogenate and cell-free extract of joint was estimated by Wright et al. [Citation36]. LPO was expressed as nmoles of MDA formed/g tissue.
Estimation of superoxide dismutase (SOD) activity
The activity of SOD in post-mitochondrial supernatant of the liver and cell-free extract of joint was assayed by the method of Marklund and Marklund [Citation37] and was measured as units/mg protein.
Estimation of glutathione peroxidase (GPx) activity
The activity of GPx in post-mitochondrial supernatant of the liver and cell-free extract of joint was measured by Mohandas et al. The enzyme activity was recorded as nmoles of NADPH oxidized/mg protein [Citation38].
Estimation of catalase activity
Catalase activity in PMS of the liver and cell-free extract of joint was done by the method of Claiborne and the enzyme activity was measured as nmoles of H2O2 consumed/min/mg protein. [Citation39].
Estimation of nuclear factor-kappaB (NF-κB)
NF-κB was assessed by rat NF-κB ELISA-based kit (NF-κB p65 ELISA, Invitrogen Corporation, CA, USA) in the serum as pg/ml and cell-free extract of joint in pg/g tissue. The assay was carried out as per the instructions of the manufacturer.
Estimation of TNF-α and TGF-β
TNF-α and TGF-β levels were estimated using rat TNF-α and TGF-β ELISA-based kits of (eBioscience San Diego CA, USA) from the serum and cell-free extract of joint, respectively. The TNF-α and TGF-β levels were estimated as per the instructions of the manufacturer and were measured as pg/mg in cell-free extract of joint and as pg/ml in serum.
Estimation of interleukin-1 beta (IL-1β)
IL-1β levels were assayed by rat IL-1β ELISA-based kit (Qayee-Bio Korea). The estimation of IL-1β levels was carried out as per the instructions of the manufacturer and was measured as pg/mg in cell-free extract of joint and as pg/ml in serum.
Estimation of IL-6, IL-10 and high-sensitivity C-reactive protein (Hs-CRP)
IL-6, IL-10 and Hs-CRP were estimated using rat IL-6, IL-10 and Hs-CRP ELISA-based kits (DiacloneSAS, France) from the serum and cell-free extract of joint as per the directions of the manufacturer. The estimation of IL-6, IL-10 and Hs-CRP was carried out as per the directions of the manufacturer and was measured as pg/mg in cell-free extract of joint and as pg/ml in serum.
Statistical analysis
The experimental data obtained were expressed as mean ± standard deviation (SD). Differences between groups were analyzed by using one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls Test and the minimum criterion for difference was set at P < 0.05.
Results
Effect of zingerone and FCA on clinical severity of arthritis
After injecting FCA for the induction of rheumatoid arthritis, early symptoms of inflammation were visible in rats on day 10 after immunization with FCA. The affected joints showed redness, hotness, or swelling. All these signs peaked by day 17. Arthritic rats (disease control) showed an increase in thickness in paws and ankles which were evidenced by severe inflammation, edema and ankylosis. The inflammation and edema of paw was mild in zingerone-administered group III (treatment I) rats compared to diseased group II (diseased group), while zingerone-administered group IV (treatment II) also exhibited a decrease in inflammation and there were no signs of ankylosis compared to disease control.
Effect of zingerone and FCA on hepatic and joint oxidative stress parameters
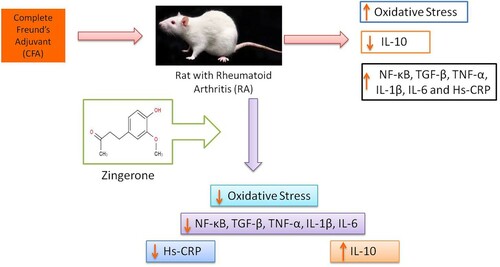
Immunization with FCA resulted in a significant (P < 0.01 and P < 0.001) increase in the MDA levels of hepatic and joint tissue, respectively compared to the control group ((A)). Zingerone treatment resulted in the reduction in the elevated hepatic MDA levels in group III and elevated MDA levels in joints of both the treatment groups compared with group II (disease control). FCA immunization significantly reduced (P < 0.01) the levels of SOD in hepatic and joint tissues of group II (disease control) compared to group I (normal control) ((B)). However, zingerone treatment significantly elevated the levels of SOD in hepatic and joint tissue of group III compared to disease control, while the group IV rats did not show any significant elevation of SOD in hepatic and joint tissue in comparison to group II rats. Furthermore, adjuvant treatment significantly reduced (P < 0.001) the activity of GPx and catalase in disease control in comparison to group I (Control). Zingerone treatment augmented the activities of GPx and catalase in both the treatment groups ((C,D)).
Figure 1. Effect of CFA and zingerone on LPO and activity of SOD, GPx and catalase. Values are presented as mean ± SD; n = 6 animals in each group. **indicates significance at P < 0.01 from the control group. ***indicates significance at P < 0.001 from the control group. #indicates significance at P < 0.05 from the CFA group. ##indicates significance at P < 0.01 from the CFA group. ###indicates significance at P < 0.001 from the CFA group. na = non-significant. LPO was expressed as nmoles of MDA formed/g tissue, SOD as units/mg protein, Gpx as nmoles of NADPH oxidized/mg protein and catalase as nmoles of H2O2 consumed/min/mg protein.
Effect of zingerone and FCA on inflammatory cytokine, TGF-β and Hs-CRP in joint
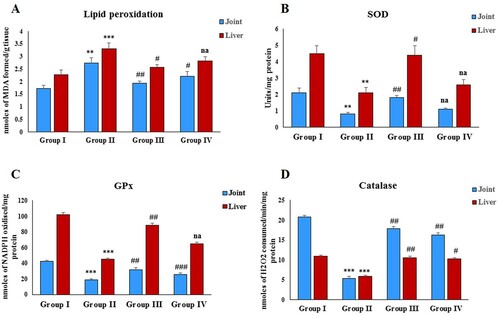
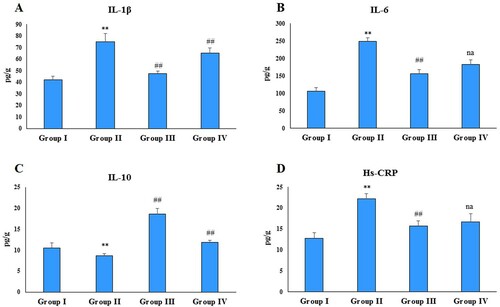
NF-κB, TNF-α, IL-1β, IL-6, TGF-β and Hs-CRP are the key players of inflammation and play a critical role in rheumatoid arthritis. Immunization with FCA significantly (P < 0.001, P < 0.001and P < 0.01) increased the NF-κB, TNF-α and TGF-β levels in the disease control group compared to the normal control group ((A–C)). Administration of zingerone significantly attenuated these levels in both the treatment groups compared to the disease control (group II). Treatment with FCA also resulted in a significant (P < 0.01) increase in the levels of IL-1β, IL-6 and Hs-CRP in the disease control (group II) compared to the control ((A,B,D)) and a significant (P < 0.01) decrease in the levels of IL-10 ((C)). Administration of zingerone reduced the levels of IL-1β, IL-6, Hs-CRP in the treatment group 1 compared to the disease control; however, group IV animals did not exhibit a significant decrease in these cytokines compared to the disease control. Furthermore, Zingerone treatment restored the levels of IL-10 in both the treatment groups compared to the disease control.
Figure 2. Effect of CFA and zingerone on NF-κB, TNF-α and TGF-β in cell-free extract of joint. Values are presented as mean ± SE; n = 6 animals in each group. **indicates significance at P < 0.01 from the control group. ***indicates significance at P < 0.001 from the control group. ##indicates significance at P < 0.01 from the CFA group. ### indicates significance at P < 0.001 from the CFA group. NF-κB, TNF-α and TGF-β were measured as pg/g protein.
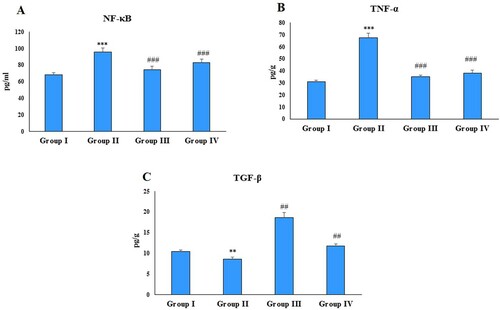
Figure 3. Effect of CFA and zingerone on IL-1β, IL-6, IL-10 and Hs-CRP in cell-free extract of joint. Values are presented as mean ± SE; n = 6 animals in each group. **indicates significance at P < 0.01 from the control group. ##indicates significance at P < 0.01 from the CFA group, na = non-significant. IL-1β, IL-6, IL-10 and Hs-CRP were measured as pg/g protein.
Effect of zingerone and FCA on inflammatory cytokines, TGF-β and Hs-CRP in serum
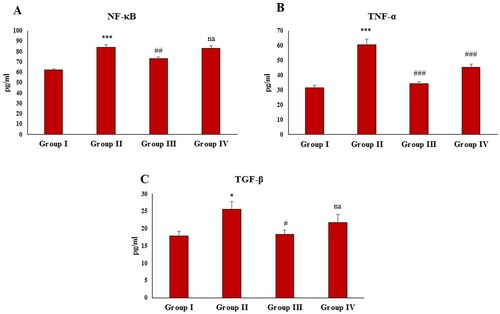
FCA treatment resulted in a significant (P < 0.001, P < 0.001 and P < 0.05) elevation in the NF-κB, TNF-α and TGF-β levels in group II ((A–C)). The administration of zingerone significantly restored the NF-κB, TNF-α and TGF-β levels in the treatment group 1 (group III) compared to the disease control. In the treatment group II (group IV), zingerone administration restored the levels of TNF-α level; however, zingerone treatment had no significant effect on NF-κB and TGF-β levels compared to the disease control.
Figure 4. Effect of CFA and zingerone on NF-κB, TNF-α, and TGF-β levels in serum. Values are presented as mean ± SE; n = 6 animals in each group. *indicates significance at P < 0.05 from the control group. ***indicates significance at P < 0.001 from the control group. #indicates significance at P < 0.05 from the CFA group. ##indicates significance at P < 0.01 from the CFA group. ### indicates significance at P < 0.001 from the CFA group. na = non-significant. NF-κB, TNF-α and TGF-β were measured as pg/ml.
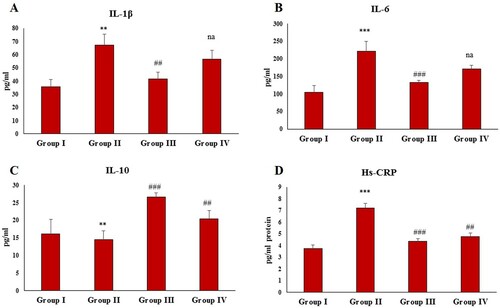
Administration of FCA also resulted in a significant (P < 0.01, P < 0.001 and P < 0.001) elevation in the serum levels of pro-inflammatory cytokines IL-1β, IL-6 and Hs-CRP in the disease control ((A,B,D)) and a significant (P < 0.001) decrease in IL-10 compared to the normal control ((C)). Zingerone treatment restored the levels of IL-1β, IL-6, Hs-CRP and IL-10 in the group III compared to the disease control, while as the group IV-treated rats did not show any significant alteration in IL-1β and IL-6 level compared to the group II. However, levels of Hs-CRP and IL-10 were restored significantly in the group IV compared to the group II with zingerone treatment.
Figure 5. Effect of CFA and zingerone on IL-β, IL-6, IL-10 and Hs-CRP levels in serum. Values are presented as mean ± SE; n = 6 animals in each group. **indicates significance at P < 0.01 from the control group. ***indicates significance at P < 0.001 from the control group. ##indicates significance at P < 0.01 the CFA group. ### indicates significance at P < 0.001 from the CFA group. na = non-significant. IL-1β, IL-6, IL-10 and Hs-CRP were measured as pg/g protein.
Discussion
Arthritis is a group of diseases considered to encompass more than a hundred inflammatory or degenerative conditions. Arthritis not only affects the joints, but also damages other organ systems that may have direct or indirect effect on the joints. Hence, it is essential to decipher the effect of drugs on arthritis by examining pathological and biochemical aspects which are mandatory for evaluating the effect of drugs [Citation40]. Osteoarthritis and rheumatoid arthritis are the most prevalent forms of arthritis resulting in pain, inflammation, mobilization of cells, increase in the tissue size that culminates in loss of defense and joint function [Citation41]. Rheumatoid arthritis is a chronic inflammatory disease that affects nearly 1% population in developed countries [Citation42]. Because of resemblance in symptoms, the adjuvant-induced arthritic rat model is widely used and accepted for assessing and understanding the effect of drugs and various agents in rheumatoid arthritis [Citation43, Citation44]. The traditional use of plants as medicines by ancient people provides the basis about which part of the plant may be useful for a specific ailment. Also, the increasing demand by consumers in natural therapies and products specifies that compositional characteristics of these products are prerequisite. Since the present treatment regimens available for the rheumatoid arthritis have adverse side effects and are quite expensive, products derived from plants without such disadvantages provide new opportunities. Compounds obtained from plants with a potential to alter or modify the progression of disease clearly have a protective or curative role against rheumatoid arthritis.
In the present study, zingerone was evaluated for its preventive and therapeutic role in FCA-induced rheumatoid arthritis in experimental animals, by mediating with inflammatory and oxidative processes. Improvement in the swelling of paw is the measure of anti-arthritic activity of a number of drugs. The monitoring of swelling in the paw is easy and a reliable method for evaluating the degree of inflammation [Citation45, Citation46]. As rheumatoid arthritis represents a chronic inflammatory condition, the increased paw edema, as a result of ligament and joint capsule swelling, involves accumulation of granulocytes and monocytes and increased activation of macrophages. These macrophages produce several cytokines, such as IL-6 and TNF-α linked with rheumatoid arthritis [Citation47]. In the present study, zingerone treatment demonstrated anti-arthritic potential. The elevation in the thickness of paw after intra-dermal immunization with FCA is the indicator of rheumatoid arthritis. The treatment with zingerone showed decrease in the thickness of paw and in the diameter of joint by interfering with the inflammatory mediators, indicating its anti-inflammatory ability in FCA-induced rheumatoid arthritis.
NF-κB is the key player for initiating and intensifying inflammation in rheumatoid arthritis [Citation48, Citation49]. NF-κB along with its inhibitory-kB (IκB) is located in the cytoplasm complex. On activation, inhibitory-κB kinase (IKK) through phosphorylation degrades IκB and activates NF-κB. The activated, NF-κB gets transferred to nucleus and triggers the synthesis of inflammatory mediators, TNF-α and iNOS. Therefore, regulation of NF-κB activity by blocking its transfer to the nucleus or through inhibition of its binding to DNA can be employed for the modulation of inflammation and cellular injury. A number of studies have revealed that zingerone attenuates NF-κB activation that results in the reduction of cell damage [Citation30]. Thus, this study was designed to examine the protective effect of zingerone on NF-κB activity in FCA-induced rheumatoid arthritis. In agreement with the stated arguments, in the present study, FCA inoculation resulted in elevated levels of NF-κB, which was corrected by zingerone. This role of zingerone in reducing the joint inflammation may be either due to its ability to prevent the transfer of NF-κB to the nucleus or inhibiting its binding to DNA. Moreover, NF-κB regulates innate and adaptive immunity by the activation of inflammatory cytokines such as, TNF-α, IL-1β, IL-6 and enzyme nitric oxide synthase and COX. Activation of NF-κB worsens the rheumatoid arthritis [Citation50]. Tumor necrosis factor alpha (TNF-α), in turn, stimulates production of other inflammatory markers, such as IL-1β and IL-6, which further facilitates infiltration of leukocytes and vasodilatation at the site of disease [Citation51]. In addition, these inflammatory cytokines mobilize neutrophils and monocytes toward the joint through activation of chemokines [Citation52]. To prevent the destruction of bone and cartilage, it is essential to inhibit TNF-α responsible for the activation of matrix metalloproteinase (MMPs) [Citation53]. The present study resulted in significant reduction in the levels of TNF-α, IL-1β and IL-6 in contrast to the diseased control group. So our findings are in concurrence with the results of Rehman et al. [Citation54] that zingerone treatment significantly prevents NF-κB activation and decreased levels of TNF-α, IL-6 and IL-1β in an experimental model of type 2 diabetes.
IL-10 is the immune-regulatory cytokine, which impedes inflammation and the damage of bone and cartilage during rheumatoid arthritis by suppressing the activation of NF-κB through inhibition of IKK [Citation55]. IL-10 not only improves the integrity of joint during rheumatoid arthritis, but also inhibits T helper cell-activated cytokines mostly TNF-α and IL-1 [Citation56]. The findings of the current study revealed a significant increase in the IL-10 levels in the zingerone treatment group compared to the disease control group.
TGF-β acts as a regulatory anti-inflammatory cytokine; however, in some conditions it may work as a pro-inflammatory molecule [Citation57, Citation58]. In rheumatoid arthritis, TGF-β has been reported to act as a pro-inflammatory cytokine with increased levels in plasma and synovial fluid [Citation59, Citation60]. The increase of TGF-β in the arthritic untreated group compared to the normal control group and the decreased level with the administration of zingerone indicates that it acts as a pro-inflammatory molecule in rheumatoid arthritis. So, our findings are in concurrence with the above findings. Moreover, serum CRP is the biomarker of systemic inflammation, which represents active inflammation. Increase in CRP is the reflex of severity and progression of arthritis [Citation61]. Increase in the levels of IL-6 and TNF-α further alleviates the levels of CRP as reported by Kumar et al. [Citation31]. In the present study, FCA resulted in an increase in CRP levels and treatment with zingerone not only reduced the FCA-induced CRP changes but also reduced inflammation, as indicated by a low level of CRP, similar observations have been reported by Kalaiselvan & Rasool [Citation62].
Oxidative stress adds to the progression of rheumatoid arthritis. Increased production of ROS causes damage to joints by the activation of matrixmetallo-proteinases and osteoclast activity [Citation63]. Reactive oxygen species (ROS) above thresh hold levels not only activate nuclear factor kappa-β (NF-κB) and pro-inflammatory cytokines but also damage the lipids, proteins, membranes, nucleic acids [Citation64] and precipitate destruction of the joint tissue [Citation65]. Peroxidation of lipids could contribute to cell damage by altering the properties of biological membranes and plays an essential role in the progress of disease. Additionally, certain products of lipid peroxidation have been reported to activate genetic over-expression of proteins such as cytokines [Citation66]. Zingerone is reported to prevent LPO and to possess SOD like activity [Citation67]. Similarly, in the present study, higher amounts of liver and joint MDA or lipid peroxides were observed in rats immunized with FCA which was significantly decreased with zingerone treatment, findings are in concurrence with Ahmad et al. [Citation58] and Oboh et al. [Citation68].
As reported previously, oxygen-free radicals result in oxidative stress and elevation in GPx and catalase activity in rheumatoid arthritis-induced rats, compared to the control. This imbalance in the activity of enzymatic antioxidants is corrected with zingerone treatment, thereby indicating antioxidant activity of zingerone in rheumatoid arthritis with decrease in the production of ROS and hence preventing the tissue damage by oxidative stress. These findings are in concurrence with the previous reports of Hemalatha et al. [Citation69]; Alvana et al. [Citation70], Rao and Rao [Citation71] and Rahmani et al. [Citation72] and Saleem et al. [Citation73]. The significant reduction in LPO and elevation in antioxidants (SOD, catalase and GPx), in arthritic rats that received zingerone from the day first of rheumatoid arthritis induction, emphasizes the role of zingerone in protecting the organ damage and bone loss in rheumatoid arthritis rat model by scavenging the free radicals.
Conclusion
As manifested from the results of the present investigation, zingerone supplementation reversed the FCA-induced changes by reducing lipid peroxidation, increasing the activities of enzymic antioxidants, and regulating the levels of pro-inflammatory cytokines and the inflammatory mediators. These biochemical and molecular findings reveal the potent antioxidant and anti-inflammatory properties of zingerone against FCA-induced rheumatoid arthritis, which suggests that the protective effect of zingerone probably might be through the attenuation of oxidative stress and inflammation (). Thus, on the basis of our findings, the combined antioxidant and anti-inflammatory properties of zingerone can prove to be a remedial measure for the prevention and treatment of arthritic joint diseases.
Acknowledgements
The authors acknowledge the Deanship of Scientific Research at King Saud University for funding this work through the Research Group Project no RGP-VPP-139. The authors are also thankful to the Division of Veterinary Biochemistry, Faculty of Veterinary Science and Animal Husbandry, SKUAST-Kashmir, Shuhama, J&K, India for all the support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Kazantseva MG, Highton J, Stamp LK, et al. Dendritic cells provide a potential link between smoking and inflammation in rheumatoid arthritis. Arthritis Res Ther. 2012;14(5):R208.
- Kurko J, Besenyei T, Laki J, et al. Genetics of rheumatoid arthritis — A comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):170–179.
- Soubrier M, Mathieu S, Payet S. Elderly-onset rheumatoid arthritis. Joint Bone Spine. 2010;77(4):290–296.
- Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–1322.
- Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2(5):1269–1275.
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108.
- Zhang L, Li JM, Liu XG, et al. Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. J Clin Immunol. 2011;31(4):606–614.
- Yang L, Karin M. Roles of tumor suppressors in regulating tumor-associated inflammation. Cell Death Differ. 2014;21(11):1677–1686.
- Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94.
- Sohn DH, Sokolove J, Sharpe O, et al. Tumor necrosis factor α release in peripheral blood mononuclear cells of cutaneous lupus and dermatomyositis patients. Arthritis Res Ther. 2012;14(1):R1.
- Huh YH, Lee G, Lee KB, et al. HIF-2α-induced chemokines stimulate motility of fibroblast-like synoviocytes and chondrocytes into the cartilage-pannus interface in experimental rheumatoid arthritis mouse models. Arthritis Res Ther. 2015;17:302.
- Alves CH, Farrell E, Vis M, et al. Animal models of bone loss in inflammatory arthritis: from cytokines in the bench to novel treatments for bone loss in the bedside—a comprehensive review. Clin Rev Allergy Immunol. 2016;51(1):27–47.
- Park C, Jeong JW, Lee DS, et al. Sargassum serratifolium extract attenuates interleukin-1β-induced oxidative stress and inflammatory response in chondrocytes by suppressing the activation of NF-κB, p38 MAPK, and PI3K/Akt. Int J Mol Sci. 2018;19(8):2308.
- Stannus O, Jones G, Cicuttini F, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441–1447.
- Marcu KB, Otero M, Olivotto E, et al. NF-κB Signaling: multiple angles to target OA. Curr Drug Targets. 2010;11(50):599–613.
- He Y, Wong AY, Chan EW, et al. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;l(14):298.
- Köhler BM, Günther J, Kaudewitz D, et al. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med. 2019;8(7):938.
- Yamanaka H, Tanaka Y, Takeuchi T, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther. 2016;18(1):34.
- Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol. 2020;180:114147.
- Scott PA, Kingsley GH, Smith CM, et al. Non-steroidal anti-inflammatory drugs and myocardial infarctions: comparative systematic review of evidence from observational studies and randomised controlled trials. Ann Rheum Dis. 2007;66(10):1296–1304.
- Baschant U, Lane NE, Tuckermann J. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8(11):645–655.
- Baschant U, Culemann S, Tuckermann J. Molecular determinants of glucocorticoid actions in inflammatory joint diseases. Mol Cell Endocrinol. 2013;380(1-2):108–118.
- Cohen MD, Keystone E. Rituximab for rheumatoid arthritis. Rheumatol Ther. 2015;2(2):99–111.
- Atzeni F, Batticciotto A, Masala IF, et al. Infections and biological therapy in patients with rheumatic diseases. Isr Med Assoc J. 2016;18:164–167.
- Dragos D, Gilca M, Gaman L, et al. Phytomedicine in joint disorders. Nutrients. 2017;9(1):70.
- Zhang M, Zhao R, Wang D, et al. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother Res. 2021;35(2):711–742.
- Huang FY, Deng T, Meng LX, et al. Dietary ginger as a traditional therapy for blood sugar control in patients with type 2 diabetes mellitus. Medicine (Baltimore). 2019;98(13):e15054.
- Mao QQ, Xu XY, Cao SY, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. 2019;8(6):185.
- Rajan I, Narayanan N, Rabindran R, et al. Zingerone protects against stannous chloride-induced and hydrogen peroxide-induced oxidative DNA damage in vitro. Biol Trace Elem Res. 2013;155(3):455–459.
- Kim MK, Chung SW, Kim DH, et al. Modulation of age-related NF-κB activation by dietary zingerone via MAPK pathway. Exp Gerontol. 2010;45(6):419–426.
- Kumar L, Chhibber S, Harjai K. Zingerone inhibit biofilm formation and improve antibiofilm efficacy of ciprofloxacin against Pseudomonas aeruginosa PAO1. Fitoterapia. 2013;90:73–78.
- Ahmad B, Rehman MU, Amin I, et al. A review on pharmacological properties of zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone). The Sci World J. 2015;2015:816364.
- Ahmad B, Rehman MU, Amin I, et al. Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) protects against alloxan-induced diabetes via alleviation of oxidative stress and inflammation: probable role of NF-kB activation. Saudi Pharm J. 2018;26(8):1137–1145.
- Kumar L, Chhibber S, Harjai K. Structural alterations in Pseudomonas aeruginosa by zingerone contribute to enhanced susceptibility to antibiotics, serum and phagocytes. Life Sci. 2014;117(1):24–32.
- Banerjee S, Haqqi TM, Luthra HS, et al. Possible role of V beta T cell receptor genes in susceptibility to collagen-induced arthritis in mice. J Exp Med. 1988;167(3):832–839.
- Wright JR, Colby HD, Miles PR. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys. 1981;206(2):296–304.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–474.
- Mohandas J, Marshall JJ, Duggin GG, et al. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Biochem Pharmacol. 1984;33(11):1801–1807.
- Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC handbook of methods for oxygen radical research. Boca Raton: CRC Press; 1985. p. 283–284.
- Rainsford KD. Adjuvant polyarthritis in rats: is this a satisfactory model for screening anti-arthritic drugs?. Agents Actions. 1982;12(4):452–458.
- Neugebauer V, Han JS, Adwanikar H, et al. Techniques for assessing knee joint pain in arthritis. Mol Pain. 2007;28(3):8.
- Amresh G, Singh PN, Rao CV. Antinociceptive and antiarthritic activity of cissampelos pareira roots. J Ethnopharmacol. 2007;111(3):531–536.
- Costa MD, De Sutter P, Gybels J, et al. Adjuvant-induced arthritis in rats: a possible animal model of chronic pain. Pain. 1981;10(2):173–185.
- Noguchi M, Kimoto A, Kobayashi S, et al. Effect of celecoxib, a cyclooxygenase-2 inhibitor, on the pathophysiology of adjuvant arthritis in rat. Eur J Pharmacol. 2005;513(3):229–235.
- Rajendran R, Krishnakumar E. Anti-arthritic activity of premna serratifolia linn., wood against adjuvant induced arthritis. Avicenna J Med Biotechnol. 2010;2(2):101–106.
- Shejawal N, Menon S, Shailajan S. A simple, sensitive and accurate method for rat paw volume measurement and its expediency in preclinical animal studies. Hum Exp Toxicol. 2014;33(2):123–129.
- Yu Y, Xiong Z, Lv Y, et al. In vivo evaluation of early disease progression by X-ray phase-contrast imaging in the adjuvant-induced arthritic rat. Skeletal Radiol. 2006;35(3):156–164.
- Woś I, Tabarkiewicz J. Effect of interleukin-6, -17, -21, -22, and -23 and STAT3 on signal transduction pathways and their inhibition in autoimmune arthritis. Immunol Res. 2021;69(1):26–42.
- Ilchovska DD, Barrow DM. An overview of the NF-κB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-κB ligand RANKL and related nutritional interventions. Autoimmun Rev. 2021;20(2):102741.
- Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
- Mateen S, Moin S, Zafar A, et al. Redox signaling in rheumatoid arthritis and the preventive role of polyphenols. Clin Chim Acta. 2016;463:4–10.
- Voon FL, Sulaiman MR, Akhtar MN, et al. Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) isolated from boesenbergia rotunda (L.) mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur J Pharmacol. 2017;794:127–134.
- Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculo Skelet Dis. 2010;2(5):247–256.
- Rehman MU, Rashid SM, Rasool S, et al. Zingerone (4-(4-hydroxy-3-methylphenyl)butan-2-one) ameliorates renal function via controlling oxidative burst and inflammation in experimental diabetic nephropathy. Arch Physiol Biochem. 2019;125(3):201–209.
- Uttra AM, Alamgeer, Shahzad M, et al. Ephedra gerardiana aqueous ethanolic extract and fractions attenuate Freund complete adjuvant induced arthritis in sprague dawley rats by downregulating PGE2, COX2, IL-1β, IL-6, TNF-α, NF-κB and upregulating IL-4 and IL-10. J Ethnopharmacol. 2018;224:482–496.
- Shabbir A, Shahzad M, Ali A, et al. Anti-arthritic activity of N′-[(2,4-dihydroxyphenyl)methylidene]-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-1(4H)-yl)acetohydrazide. Eur J Pharmacol. 2014;738:263–272.
- Cheon H YUSJ, Yoo DH, et al. Increased expression of pro-inflammatory cytokines and metalloproteinase-1 by TGF-β1 in synovial fibroblasts from rheumatoid arthritis and normal individuals. Clin Exp Immunol. 2002;127:547–552.
- Ahmad SB, Rehman MU, Fatima B, et al. Antifibrotic effects of D-limonene (5(1-methyl-4-[1-methylethenyl]) cyclohexane) in CCl4 induced liver toxicity in Wistar rats. Environ Toxicol. 2018;33(3):361–369.
- Gonzalo-Gil E, Galindo-Izquierdo M. Papel del factor de crecimiento transformador-beta (TGF-β) en la fisiopatología de la artritis reumatoide. Reumatol Clin. 2014;10(3):174–179.
- Pohlers D, Beyer A, Koczan D, et al. Constitutive upregulation of the transforming growth factor-β pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2007;9(3):R59.
- Rhodes B, Furnrohr BG, Vyse TJ. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7(5):282–289.
- Kalaiselvan S, Rasool MK. Triphala herbal extract suppresses inflammatory responses in LPS-stimulated RAW 264.7 macrophages and adjuvant-induced arthritic rats via inhibition of NF-κB pathway. J Immunotoxicol. 2016;13(4):509–525.
- Mititelu RR, Pădureanu R, Băcănoiu M, et al. Inflammatory and oxidative stress markers—mirror tools in rheumatoid arthritis. Biomedicines. 2020;8(5):125.
- Prescha A, Zabłocka-Słowińska K, Płaczkowska S, et al. Diet quality and its relationship with antioxidant status in patients with rheumatoid arthritis. Oxid Med Cell Longev. 2018;2018:1.
- Kurien BT, Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. 2008;7:567–573.
- Halliwell BA, Hoult JR, Blake DR. Oxidants, inflammation, and anti-inflammatory drugs. Faseb J. 1988;2(13):2867–2873.
- Krishnakantha TP, Lokesh BR. Scavenging of superoxide anions by spice principles. Indian J Biochem Biophys. 1993;30(2):133–144.
- Oboh G, Akinyemi AJ, Ademiluyi AO. Antioxidant and inhibitory effect of red ginger (Zingiberofficinale var. Rubra) and white ginger (Zingiberofficinale Roscoe) on Fe2+ induced lipid peroxidation in rat brain in vitro. Exp Toxicol Pathol. 2012;64(1-2):31–36.
- Hemalatha KL, Mainzen S, Prince P. Antihyperlipidaemic, antihypertrophic, and reducing effects of zingerone on experimentally induced myocardial infarcted rats. J Biochem Mol Toxic. 2015;29(4):182–188.
- Alavala S, Nalban N, Sangaraju R, et al. Anti-inflammatory effect of stevioside abates Freund’s complete adjuvant (FCA)-induced adjuvant arthritis in rats. Inflammopharmacology. 2020;28(6):1579–1597.
- Rao BN, Rao BS. Antogonistic effects of zingerone, a phenolic alkaline against radiation- induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro. Mutagenesis. 2010;25(6):577–587.
- Rahmani AH. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int J Physiol. 2014;6(2):125–136.
- Saleem A, Saleem M, Akhtar MF, et al. Moringa rivae leaf extracts attenuate complete Freund's adjuvant-induced arthritis in Wistar rats via modulation of inflammatory and oxidative stress biomarkers. Inflammopharmacology. 2020;28(1):139–151.