Abstract
Background
Crohn's disease (CD) is a chronic inflammatory disease without a specific cause. Inflammation in these patients can disturb the oxidants/antioxidants balance and results in oxidative stress that plays a destructive role. This study aimed to evaluate the gene expression of sod1, sod2, cat, nrf2 and gp91phox in CD patients before and after Azathioprine (Aza) consumption.
Method
Peripheral bloodmononuclear cells (PBMCs) were separated from CD patients (n= 15, mean age = 33.6 ± 1.8) before and after treatment with Aza and healthy controls (n= 15, mean age = 31.5 ± 1.2). The expression levels of sod1, sod2, cat, nrf2 and gp91phox were measured in byusing real-time qRT-PCR technique.
Result
The expression levels of gp91phox (P-value < 0.001), cat (P-value < 0.05), sod1 (P-value < 0.001), nrf2 (P-value < 0.001) were significantly increased compared to control group. Following treatment with Aza, the decreased expression levels of gp91phox (P-value < 0.05), cat (P-value < 0.05), sod1(P-value < 0.001) and nrf2 (P-value < 0.001) were observed in CD patients.
Conclusion
Overall, our results showed that prescription of Azathioprine can lead to the altered expression of redox system-related genes in patients with CD.
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) that affects different parts of the gastrointestinal tract, frequently terminal ileum [Citation1]. CD’s main clinical manifestations include fever, fatigue, abdominal pain, diarrhea, fistula, and anal lesions [Citation2]. With a rapidly increasing trend worldwide, CD incidence has been estimated at 0.06–29.3 per 100,000 [Citation3]. Decades of basic and clinical studies increased our knowledge about molecular mechanisms underlying CD, but its etiology is still unclear [Citation4].
Various genetic and environmental factors have been speculated to play a significant role in CD pathogenesis [Citation5]. Inflammatory conditions that are mainly immunologically mediated thought to underlie the development of CD [Citation6]. Altered host immune responses against intestinal microbiota followed by impaired epithelial-mucosal barrier result in releasing many inflammatory agents and subsequent tissue injury in CD [Citation7–9]. Of note, inflammation increases inflammatory cytokines production such as IL-1β, IL-6, TNF-α and IFN-γ and promotes oxidative stress [Citation10,Citation11]. As a result, significantly augmented the GP91PHOX activity during the immune responses could be noticed [Citation12].
GP91PHOX, also called NOX-2 is a transmembrane protein and acts as a catalytic subunit of NADPH oxidase. Activation of GP91PHOX leads to the generation of superoxide onions (O2•-) through electron transfer from NADPH to O2. This process is a crucial step in producing reactive oxygen species (ROS) [Citation13]. Redox-active molecules such as ROS have crucial functions in eradicating pathogens and regulating inflammatory processes [Citation14]. Exceed production of ROS causes cellular damage and aggravates autoimmune diseases’ pathobiology [Citation15,Citation16]. In normal situations, antioxidant proteins neutralize free radicals and inhibit ROS production by preventing cellular damages and tissue injury triggered by oxidative stress. For instance, superoxide dismutases (SODs) could convert O2•- to H2O2. Then, catalase reduces H2O2 to water [Citation17]. As a transcription factor, Nuclear factor erythroid 2-related factor 2 (NRF2) binds to antioxidant response element (ARE) sequences in the promoter of antioxidant enzymes genes, including NADPH quinone oxidoreductase1 (NQO-1), catalase (CAT), and SODs [Citation18]. Dysregulation of NRF2 has been documented in many autoimmune disorders [Citation19–21].
Since the elevated levels of ROS along with impairment of the antioxidant defense system result in cellular damages and tissue injury. In the present study, we aimed to evaluate the expression of redox system genes including sod1, sod2, cat, gp91phox and nrf2 in patients suffering from CD. Besides, we examined the effect of Azathioprine as an immunosuppressant drug on oxidative stress levels in these patients.
Material and method
Study subjects
A longitudinal follow-up study was conducted on fifteen CD patients (female = 12, male = 3, mean age = 33.6 ± 1.8), referred to the Imam Khomeini General Hospital, Tehran University of Medical Sciences, Tehran, Iran. Diagnosis of CD was confirmed by clinical symptoms, laboratory evaluations, colonoscopy examinations, and histological criteria. Also, the disease severity is estimated according to the CD Activity Index (CDAI). Fifteen healthy persons (female = 11, male = 4, mean age = 31.5 ± 1.2) without a history of autoimmune and immunodeficiency disorders were also enrolled in the current study (). Neither CD patients nor healthy individuals used antibiotics, probiotics, and immune suppressive drugs at least in the three months before the study. According to the gastroenterologist's advice, Azathioprine was prescribed to CD patients (50 mg per day) after three months from the first sampling. Then, the second sample was collected.
Table 1. Characteristics of CD patients and healthy controls.
All patients and controls were of Iranian origin. Voluntary participants were informed about the study's procedure, and informed consent was obtained from them. The study was approved by the ‘Ethics Committee of Tehran University of Medical Sciences IR.TUMS.SPH.REC.1398.060' and conducted in accordance with the Declaration of Helsinki.
RNA extraction
A whole blood sample was collected from all CD patients and controls. For isolation of peripheral blood mononuclear cells (PBMCs), whole blood was diluted 1:1 with PBS, the mixture gently layered over the Ficoll density-gradient media (Lymphodex Inno, Germany), and then centrifuged at 1000 g for 20 min. The layer containing PBMCs was transferred to a new tube, washed three times, and prepared for the RNA extraction procedure. According to the manufacturer's instructions, total RNA was extracted with an RNX-Plus extraction kit (RN7713C, sinaclon, Iran). RNA concentration and purity were checked by 260/280 nm absorbance ratio using NanoDrop (Thermo Fisher).
Real-time PCR
First, total RNA was treated with DNase I, RNase free (Thermo Fisher Scientific, U.S.A.) to avoid genomic DNA contamination. Then, using a first-strand cDNA synthesis kit (Thermo Fisher Scientific, U.S.A.), total RNA was reverse transcribed into complementary DNA (cDNA) in a reaction primed by a random hexamer according to the manufacturer's instructions. The mRNA levels of cat, gp91phox, nrf2, sod1 and sod2 were measured by RT-qPCR with appropriate primers (). Amplification was performed on Applied Biosystem and revealed with SYBR-Green master mix (Ampliqone, Denmark). The cycling condition was performed for desired genes, as mentioned in . Melting curve analysis indicated no primer-dimers and non-specific products in the assay [Citation22]. Data were analyzed, and threshold cycle (Ct) values were determined. The average expression levels of mRNA were normalized to 18 s rRNA using the 2−ΔΔCt method.
Table 2. Primers sequences and cycling condition used for gene expression analysis through real-time qRT-PCR.
Statistical analysis
Data were analyzed using SPSS statistical software (version 19.0, IBM, New York, U.S.A.). One-way ANOVA followed by turkey’s post hoc test was applied to compare data from patients and control groups. All tests were two-tailed. P-value < 0.05 was considered statistically significant.
Results
To determine whether the gp91phox expression level was altered in CD, we investigated mRNA levels of gp91phox using a quantitative RT–PCR assay. Gene expression analysis showed a significant increase in gp91phox mRNA levels in CD patients than in the control group (8.35 ± 1.88 vs. 1.25 ± 0.29; P-value < 0.001). Furthermore, mRNA levels of gp91phox were measured after the prescription of Azathioprine to patients, and the results demonstrated that expression of gp91phox significantly decreased to normal levels (6.44 ± 0.80 vs. 1.25 ± 0.29; P-value < 0.05) ((A)).
Figure 1. Elevated expression of oxidative stress markers in patients with CD (CD). Fold changing expression of GP91PHOX (A), NRF2 (B), CATALASE (C), SOD1 (D), SOD2 (E) were measured in the PBMCs of CD patients before and after prescription of Azathioprine (Aza). Data are shown as average ± standard deviation (SD). Comparison between CD, Azathioprine receiving CD patients (CD + Aza) groups and healthy controls (Ctrl) was performed by One-way ANOVA and Turkey’s post hoc test. *P-value < 0.05, **P-value < 0.01, ***P-value < 0.05, NS: non-significant.
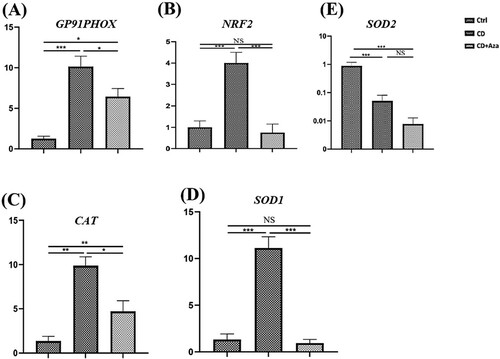
Redox regulation is enzymatically controlled. CAT, SOD1 and SOD2 have essential roles in scavenging free radicals [Citation17]. The imbalance between radical-generating and radical-scavenging systems considered as a potential mechanism in the pathophysiology of autoimmune disorders [Citation23]. Therefore, we measured mRNA levels of cat, sod1, sod2 and up-stream transcription factor nrf2 in CD patients. The results indicated that increased expression levels of cat (9.86 ± 2.5 vs. 1.36 ± 0.52; P-value < 0.05), SOD1 (12.76 ± 2.75 vs. 1.32 ± 0.33; P-value < 0.001), and nrf2 (18.27 ± 4.85 vs. 2.4 ± 0.74; P-value < 0.001) in patients compared with controls ((B–D)). Remarkably, treatment with Azathioprine resulted in a significant decrease in mRNA levels of cat (9.86 ± 2.5 vs. 4.7 ± 1.1; P-value < 0.05), sod1 (12.76 ± 2.75 vs. 0.92 ± 0.52; P-value < 0.001) and nrf2 (18.27 ± 4.85 vs. 2.04 ± 0.71; P-value < 0.001) in CD patients. Interestingly, expression levels of sod2 were significantly lower in patients compared with controls (0.05 ± 0.01 vs. 0.87 ± 0.22; P-value < 0.001) which showed decreased expression in patients compared to controls after treatment with Azathioprine. However, the difference was not statistically significant (0.05 ± 0.01 vs. 0.007 ± 0.002; P-value > 0.05) ((E)).
Discussion
Previous studies have shown that O2•- produced by GP91PHOX can lead to cell damage and tissue injury in many autoimmune diseases [Citation24]. There is cumulative evidence regarding the roles of oxidative stress in CD's pathogenesis [Citation25–27]. Superoxide radical is the progenitor of ROS, including OH-, RO-, ROO- and H2O2. Furthermore, superoxide radicals can react with highly reactive nitric oxide (NO-) molecules to generate the peroxynitrite anion (ONOO-), or so-called reactive oxygen and nitrogen species (RONS) [Citation28]. These highly reactive molecules can contribute to CD pathogenesis through DNA damage and lipid oxidation [Citation28]. In the present study, we observed higher mRNA levels of oxidative stress-related markers in CD patients than in the healthy group, which reduced following Azathioprine treatment.
Here, we showed a significantly higher expression level of gp91phox in CD patients than in healthy individuals. Bao et al. showed that GP91PHOX contributes to inflammation progression in a mouse model of acute colitis. Their results indicated that gp91phox-/- mice produce lower levels of pro-inflammatory cytokines compared with WT mice [Citation29]. Also, increased expression of gp91phox has been observed in colitis's rat model after stimulation with serotonin [Citation30].
In physiological condition, endogenous anti-oxidative defense components such as SODs and CAT eliminate excess ROS to prevent oxidative stress-related damage [Citation31]. There are three different superoxide dismutase genes in the eukaryotic genome: sod1 and sod3 encode cytoplasmic and extracellular Cu/Zn SOD, respectively and sod2 encodes mitochondrial Mn-SOD [Citation32,Citation33]. Our data revealed significantly higher mRNA levels of sod1 and cat in CD patients compared to healthy controls. But, lower level of sod2 was observed in patients. Similarly, Dincer et al. suggested augmented expression of sods in blood cells of IBD patients [Citation34]. In contrast, others reported decreased SODs and CAT activity in CD and ulcerative colitis [Citation35,Citation36]. It seems the lower sod2 levels in Crohn's patients are related to impaired mitochondrial structure and function. Nazli et al. and Söderholm et al. have reported that isolated enterocytes from patients with IBD exhibit swollen mitochondria with abnormal cristae [Citation37,Citation38]. Also, Rodenburg et al. demonstrated an abnormal mitochondrial structure in experimental mice models of colitis [Citation39]. Cellular stress and bioenergetic failure are indicative of these morphological shifts. Indeed, patients with CD have decreased ATP levels and short-chain fatty acids (SCFA) β-oxidation deficiencies. However, it is uncertain if the reported changes in the mitochondrial structure and function are due to disease or play a role in pathogenesis [Citation40].
NRF2 is recognized as a critical regulator that controls the transcriptional activation of genes involved in endogenous antioxidant biosynthesis [Citation41]. It binds to ARE sequences in the promoter of target genes [Citation18]. In normal oxidative conditions, NRF2 is constitutively ubiquitinated by Kelch-like ECH-associated protein 1 (Keap1) and Cullin-3 E3 ligase and degraded by the 26S proteasomal pathway [Citation42]. Under oxidative stress and exposure to ROS, NRF2 translocates to the nucleus and induces transcriptional activation of endogenous antioxidant defense system genes such as Sods and cat [Citation18,Citation43,Citation44]. Here, we indicated higher levels of nrf2 in CD patients compared to controls. These findings suggested that CD patients show higher levels of NRF2 dependent antioxidants (SOD1 and CAT) in response to increased ROS molecules. However, increased mRNA levels of sod1 and cat cannot explain the activity function of their proteins.
Azathioprine is a purine analog that commonly use to manage IBD, such as CD [Citation45]. The main mechanism of Azathioprine that contributes to immunosuppression is its association with suppression of DNA replication. Hypoxanthine-guanine phosphoribosyl transferase (HPRT) and thiopurine methyltransferase (TPMT) enzymes convert Aza to active metabolites, including mercaptopurine (6-MP) and thioguanine (6-TGN) which inhibit the synthesis of an essential purine for DNA and RNA production [Citation46]. Consequently, Aza may result in decreased expression of gp91phox, sod2, nrf2 and its downstream genes including cat and sod1 through suppressing purine metabolism in white blood cells.
On the other hand, CD is characterized by augmented inflammatory responses. Therefore, anti-inflammatory treatments such as Azathioprine are widely used to reduce CD symptoms [Citation45]. We found that prescribing anti-inflammatory drugs such as Azathioprine could decrease gp91phox expression in the patients’ group to a normal level. Also, the expression of sod2, nrf2 and its downstream genes including sod1 and cat decreased following Azathioprine treatment. It has been demonstrated that oxidative stress results in abnormal immune responses and over-expression of pro-inflammatory cytokines including TNF-α, interleukin (IL)-1 and IL-6 which have essential roles in the progress and maintenance of CD pathogenesis [Citation47]. Further investigations have also shown inflammatory cytokines promote over-expression of gp91phox through the NF-κB pathway as well as inflammasome signaling [Citation48]. According to a study by Abais et al., NRF2 has a protective effect against inflammatory cytokines in a mouse model of chronic colitis [Citation49]. In line with our research, long-term prescription of anti-inflammatory drugs such as Azathioprine has been shown to have an adverse effect on NRF2 expression and it is associated with increased oxidative stress [Citation50].
Conclusion
The results of this study showed that patients with CD tend to show increased expression of gp91phox, sod1 and cat in the NRF2 dependent pathway. Also, we showed immunosuppressant drugs such as Azathioprine may reduce oxidative stress-related genes in patients with CD, most likely through interrupting DNA and RNA biosynthesis and decreasing pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6. As a limitation, we were unable to assess the enzyme activities and protein levels. So, as a suggestion, further studies in this field may contribute to a better understanding of underlying mechanisms of CD pathogenesis.
Acknowledgments
This research was supported by Tehran University of Medical Sciences (grant no: 98-01-27-41797). We thank all patients and healthy subjects for their participation in this study. The authors declare that there is no conflict of interests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Boyapati R, Satsangi J, Ho GT. Pathogenesis of Crohn's disease. F1000Prime Rep. 2015;7:44.
- Wójcik B, Loga K, Włodarczyk M, et al. Extraintestinal manifestations of Crohn's disease. Prz Gastroenterol. 2016;11(3):218–221.
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778.
- Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13(4):481–489.
- Younis N, Zarif R, Mahfouz R. Inflammatory bowel disease: between genetics and microbiota. Mol Biol Rep. 2020;47(4):3053–3063.
- Deretic V. Links between autophagy, innate immunity, inflammation and Crohn's disease. Dig Dis. 2009;27(3):246–251.
- Lee JY, Wasinger VC, Yau YY, et al. Molecular pathophysiology of epithelial barrier dysfunction in inflammatory bowel diseases. Proteomes. 2018;6(2):17.
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306.
- Pascal V, Pozuelo M, Borruel N, et al. A microbial signature for Crohn's disease. Gut. 2017;66(5):813–822.
- Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94(1):174–181.
- Fais S, Capobianchi MR, Pallone F, et al. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn's disease. kinetics of in vitro response to interferon gamma inducers. Gut. 1991;32(4):403–407.
- Hernández-Espinosa DR, Massieu L, Montiel T, et al. Role of NADPH oxidase-2 in the progression of the inflammatory response secondary to striatum excitotoxic damage. J Neuroinflammation. 2019;16(1):91.
- Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59(9):1428–1459.
- Yang Y, Bazhin AV, Werner J, et al. Reactive oxygen species in the immune system. Int Rev Immunol. 2013;32(3):249–270.
- De Rasmo D, Ferretta A, Russo S, et al. PBMC of multiple sclerosis patients show deregulation of OPA1 processing associated with increased ROS and PHB2 protein levels. Biomedicines. 2020;8(4):85.
- Denson LA, Jurickova I, Karns R, et al. Clinical and genomic correlates of neutrophil Reactive oxygen species production in pediatric patients With Crohn's disease. Gastroenterology. 2018;154(8):2097–2110.
- Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19.
- Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal. 2008;10(12):2023–2033.
- Johnson DA, Amirahmadi S, Ward C, et al. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci Official J Soc Toxicol. 2010;114(2):237–246.
- Suzuki T, Murakami S, Biswal SS, et al. Systemic activation of NRF2 alleviates lethal autoimmune inflammation in scurfy mice. Mol Cell Biol. 2017;37(15):e00063-17.
- Sireesh D, Dhamodharan U, Ezhilarasi K, et al. Association of NF-E2 related factor 2 (Nrf2) and inflammatory cytokines in recent onset type 2 diabetes mellitus. Sci Rep. 2018;8(1):5126.
- Pryor RJ, Wittwer CT. Real-time polymerase chain reaction and melting curve analysis. Meth Mol Biol. 2006;336:19–32.
- Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126.
- Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167.
- Luceri C, Bigagli E, Agostiniani S, et al. Analysis of oxidative stress-related markers in Crohn's disease patients at surgery and correlations with clinical findings. Antioxidants. 2019;8(9):378.
- Bourgonje AR, von Martels JZH, Bulthuis MLC, et al. Crohn's disease in clinical remission Is marked by Systemic oxidative stress. Front Physiol. 2019;10:499.
- Alzoghaibi MA. Concepts of oxidative stress and antioxidant defense in Crohn's disease. World J Gastroenterol. 2013;19(39):6540–6547.
- Di Dalmazi G, Hirshberg J, Lyle D, et al. Reactive oxygen species in organ-specific autoimmunity. Auto Immun Highlights. 2016;7(1):11.
- Bao S, Carr ED, Xu YH, et al. Gp91(phox) contributes to the development of experimental inflammatory bowel disease. Immunol Cell Biol. 2011;89(8):853–860.
- Regmi SC, Park SY, Ku SK, et al. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radic Biol Med. 2014;69:377–389.
- Guan G, Lan S. Implications of antioxidant systems in inflammatory bowel disease. Biomed Res Int. 2018;2018:1290179.
- Wang Y, Branicky R, Noë A, et al. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217(6):1915–1928.
- Fukui M, Zhu BT. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic Biol Med. 2010;48(6):821–830.
- Dincer Y, Erzin Y, Himmetoglu S, et al. Oxidative DNA damage and antioxidant activity in patients with inflammatory bowel disease. Dig Dis Sci. 2007;52(7):1636–1641.
- Iborra M, Moret I, Rausell F, et al. Role of oxidative stress and antioxidant enzymes in Crohn's disease. Biochem Soc Trans. 2011;39(4):1102–1106.
- Verspaget HW, Peña AS, Weterman IT, et al. Diminished neutrophil function in Crohn's disease and ulcerative colitis identified by decreased oxidative metabolism and low superoxide dismutase content. Gut. 1988;29(2):223–228.
- Nazli A, Yang P-C, Jury J, et al. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol. 2004;164(3):947–957.
- Söderholm JD, Yang PC, Ceponis P, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123(4):1099–1108.
- Rodenburg W, Keijer J, Kramer E, et al. Impaired barrier function by dietary fructo-oligosaccharides (FOS) in rats is accompanied by increased colonic mitochondrial gene expression. BMC Genomics. 2008;9:144.
- Novak EA, Mollen KP. Mitochondrial dysfunction in inflammatory bowel disease. Front Cell Dev Biol. 2015;3:62,
- Mitsuishi Y, Motohashi H, Yamamoto M. The Keap1–Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol. 2012;2:200.
- Itoh K, Ye P, Matsumiya T, et al. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J Clin Biochem Nutr. 2015;56(2):91–97.
- Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98(3):1169–1203.
- Kasai S, Shimizu S, Tatara Y, et al. Regulation of Nrf2 by mitochondrial Reactive oxygen species in physiology and pathology. Biomoleules. 2020;10(2):320.
- Wu J, Gao Y, Yang C, et al. Low-dose azathioprine is effective in maintaining remission among Chinese patients with Crohn's disease. J Transl Med. 2013;11:235.
- Timmer A, Patton PH, Chande N, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;2016(5):Cd000478.
- Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16(1):26–42.
- Gerstgrasser A, Melhem H, Leonardi I, et al. Cell-specific activation of the Nrf2 antioxidant pathway increases mucosal inflammation in acute but Not in chronic colitis. J Crohns Colitis. 2017;11(4):485–499.
- Abais JM, Xia M, Zhang Y, et al. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–1129.
- Kalra S, Knatko EV, Zhang Y, et al. Highly potent activation of Nrf2 by topical tricyclic bis(cyano enone): implications for protection against UV radiation during thiopurine therapy. Cancer Prevent Res. 2012;5(7):973–981.
