ABSTRACT
Objectives
Ulcerative colitis (UC), an inflammatory bowel disease, affects mucosal lining of colon leading to inflammation and ulcers. Sulforaphane is a natural compound obtained from cruciferous vegetables. We aimed to investigate potential therapeutic effects of sulforaphane in experimentally induced UC in rats through affection antioxidant activity, mitochondrial biogenesis and DNA polymerization.
Methods
UC was induced in rats via an intracolonic single administration of 2 ml of 4% acetic acid. UC rats were treated with 15 mg/kg sulforaphane. Samples of colon were used to investigate gene expression and protein levels of peroxisome proliferator-activated receptor-gamma coactivator (PGC-1), mitochondrial transcription factor A (TFAM), mammalian target of rapamycin (mTOR), cyclin D1, nuclear factor erythroid 2-related factor-2 (Nrf2), heme Oxygenase-1 (HO-1) and proliferating cell nuclear antigen (PCNA).
Results
UC showed dark distorted Goblet cell nucleus with disarranged mucus granules and no distinct brush border with atypical microvilli. All morphological changes were improved by treating with sulforaphane. Finally, treatment with sulforaphane significantly increased expression of PGC-1, TFAM, Nrf2 and HO-1 associated with reduction in expression of mTOR, cyclin D1 and PCNA.
Conclusion
Sulforaphane could cure UC in rats. The protective activity can be explained by enhancing antioxidant activity, elevating mitochondrial biogenesis and inhibiting DNA polymerization.
1. Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease. The prevalence of UC is 5.50–24.30 cases per 100,000 population. It can be correlated to many factors including heredity, genetic abnormalities, geographical environment, altered dietary patterns, autoimmunity, intestinal barrier dysfunction, smoking, microbiota dysbiosis, or abnormal host immune reactions [Citation1]. UC is characterized by repeated periods of relapse and remission. Pathogenesis of UC begins with inflammation of colon mucosa followed by ulceration. Only 10–20% of the patients have aggressive disease. About 70–80% of the patients suffer from relapse and 50% of the patients require hospitalization [Citation2]. Treatment of UC involves conserving stable remission, to reduce relapses and prevent the development of colitis associated cancer via targeting the immune response and proinflammatory factors [Citation3].
mTOR, a serine/threonine kinase, regulates cellular energy, metabolism, and differentiation of T cells. The signaling pathway of mTOR has a key role in mediating many cellular processes such as necrosis, apoptosis, and inflammation leading to the incidence and development of many diseases. It is linked to mitochondrial biogenesis [Citation4]. Mitochondrial biogenesis is a term given to any process that elevated mitochondrial number and size. It is mediated by any physiologic stimuli such as dietary modifications, physical exercise, and temperature. It involves the synthesis of new mitochondrial DNA, proteins, and membrane, as the new mitochondria was formed by the fission of preexisting mitochondria instead of de novo formation. It is regulated by peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC1α), which is a transcription factor that controls the production of mitochondrial proteins [Citation5].
Sulforaphane is 1-isothiocyanato-4-(methylsulfinyl)butane. It belongs to the isothiocyanate family that is quite present in many cruciferous vegetables such as broccoli, Northern carrot, and kale [Citation6]. Sulforaphane possesses many biological activities including antimicrobial, antioxidant, anti-inflammatory, and immunomodulatory effects [Citation7]. Sulforaphane was reported to have many therapeutic effects as protection against gastric ulcers [Citation8], cardiovascular diseases [Citation9], chronic kidney disease [Citation10], immune regulation functions [Citation11], aging, and neurodegenerative diseases [Citation12]. The metabolism of sulforaphane takes place through glutathione conjugation producing sulforaphane-N-acetylcysteine [Citation13]. It affects the structure of the intestinal microbial community and attenuates colitis by alylhydrogen receptor (AHR) as it reversed UC-induced dysbiosis [Citation1]. Sulforaphane is a safe compound with an LD50 value of 212.67 mg/kg in reference [Citation14].
Sulforaphane was reported previously to protect against colitis by reducing the expression of inflammatory biomarkers inside intestinal mucosa and overexpression of Nrf2 dependent genes [Citation6], reduction of IL-6 synthesis [Citation15], altering gut bacterial compositions [Citation1] or activating AMPK [Citation16]. However, no previous study investigated the effect of sulforaphane on mitochondrial biogenesis and DNA polymerization. Therefore, we conducted this study to investigate the therapeutic effects of sulforaphane in experimentally induce UC in rats by investigating its effect on the colon cellular mitochondrial biogenesis and DNA polymerization.
2. Materials and methods
2.1. Animals and treatment outlines
Thirty-six Sprague Dawley rats weighed 180–200 g was selected. Rats were kept in standard conditions of temperature and regular 12 h light/12 h dark cycle. All methods were carried out in accordance with guidelines and regulations for working with experimental animals and the work protocol was approved by the local ethical committee in the Faculty of Pharmacy, Mansoura University. All methods are reported in accordance with ARRIVE guidelines for the reporting of animal experiments. Rats were classified into three groups with 12 rats each.
2.1.1. Control group
Rats were fasted for 12 h overnight before starting the procedure. The rats underwent anesthesia using an intraperitoneal injection of 30 mg/kg of 3% pentobarbital sodium. Rats had an intracolonic single administration of 2 ml of saline using a soft pediatric lubricated catheter under ether anesthesia. Rats were kept in a horizontal position for 2 min to prevent draining of saline.
2.1.2. UC group
Rats were fasted for 12 h overnight before starting the procedure. The rats underwent anesthesia using an intraperitoneal injection of 30 mg/kg of 3% pentobarbital sodium. Colitis was induced in colon tissues via an intracolonic single administration of 2 ml of 4% acetic acid through a soft pediatric lubricated catheter under ether anesthesia. Rats were kept in a horizontal position after acetic acid administration for 2 min to prevent draining of acetic acid.
2.1.3. UC treated with sulforaphane
After induction of ulcerative colitis in rats, they were given 15 mg/kg sulforaphane (Sigma Aldrich Chemicals Co., St Louise, MO, U.S.A.) by oral gavage daily for two weeks.
Only one previous study illustrated the role of sulforaphane in treating UC in mice using a dose of 20 mg/kg [Citation1]. In addition, there was no previous study that used sulforaphane in treating UC in rats. Therefore, A set of preliminary studies were performed testing three different concentrations of sulforaphane, 10, 15, and 20 mg/kg. The dose of 15 mg/kg is selected as it was found to produce the best results.
2.2. Sample collection
Blood samples were collected from the trunk followed by centrifugation at 3000 rpm for 5 min. The separated serum samples were stored at −80°C. The whole colon was separated, weight, and measured. A piece of the colon was fixed in 10% buffered formalin for subsequent morphologic analysis. Another part was homogenized in a 10-fold volume of ice-cold 0.01 M, pH 7.4 sodium potassium phosphate buffer supplemented with 1.15% KCl. The separated supernatant was stored at −80°C.
2.3. Morphologic analysis and immunohistochemistry
The paraffin-embedded samples were cut into 5 µm sections using a rotary microtome and then transferred to a 38.5°C warm water bath to be picked up on the slides from the surface of the water. The slides were oven dried at 42°C overnight. At the time of staining, sections were deparaffinized using an ascending concentration of ethanol in xylene. The sections were then rinsed in tris buffer, pH 7.6. Sections were stained with Masson trichrome. Masson trichrome is greatly used to investigate muscular pathologies such as muscular dystrophy. In addition, it can be used for the calculation of the fibrotic area, which is stained blue. Sections were investigated under the microscope in a masked manner. The fibrotic area was calculated using high field powers in 10 different areas of each rat. The results of the fibrotic figure are presented as the mean ± SE.
For immunohistochemistry, sections were incubated with monoclonal proliferating cell nuclear antigen (PCNA) (Sigma Aldrich Chemicals Co.) at 4°C. Sections were then incubated with horseradish peroxidase conjugated antibody. Next, 2% of 3,3′-diaminobenzidine in Tris-buffer was used as a chromogen. Hematoxylin was used as a counter stain. The score of IHC in colon tissues was used to indicate 0 (no positive cells per high power field), 1 or infrequent (small number of positive cells), 2 or common (moderate number of positive cells), and 3 or widespread (high numbers of positive cells). The investigations were done using a digital camera-aided computer system (Nikon Digital Camera, Japan).
2.4. Specimen preparation for transmission electron microscopy
Samples measuring more than 1 mm3 were separated and fixed using glutaraldehyde at 4°C for 4 h. Next, the samples were dehydrated using a graded series of ethanol and propylene oxide. They were then embedded in epoxy resin. Ultrathin sections were observed at 160 kV using a JEOL JEM-2100 at Electron microscope Unit, Mansoura University, Egypt.
2.5. Measurement of oxidative stress and antioxidant activities
Conlon lysate levels of nitric oxide (NO) at wavelength 540 nm, malondialdehyde (MDA) at wavelength 534 nm, reduced glutathione at wavelength 405 nm and glutathione peroxidase at wavelength 340 nm were assessed using commercially available kits (BioDiagnostic Company, Giza, Egypt) using BioTek spectrophotometer, Highland, VT, U.S.A.
2.6. Enzyme-linked immunosorbent (ELISA) assay
Commercially available ELISA kits were used for the assessment of mitochondrial transcription factor A (TFAM), peroxisome proliferator-activated receptor-gamma coactivator (PGC-1), mammalian target of rapamycin (mTOR), Cyclin D1, nuclear factor erythroid 2-related factor 2 (Nrf2) and Heme Oxygenase-1 (HO-1, MyBioSource, Inc., San Diego, CA, U.S.A.) according to manufacturer’s instructions using Spectro UV-VIS Double Beam PC Scanning Spectrophotometer (Labomed Inc., Los Angeles, CA, U.S.A.).
2.7. Quantitative real-time polymerase chain reaction (RT-PCR)
RNeasy Mini kit (Qiagen, U.S.A.) was used to separate the RNA content of rat hepatic cells. Maxima® SYBR Green/Fluorescein Master Mix (Fermentas, U.S.A.) was used to evaluate the total RNA amount. One microgram of RNA was reverse transcribed into cDNA using QuantiTect® Reverse Transcription Kit (Qiagen, U.S.A.). PGC-1, TFAM, mTOR, cyclin D1, Nrf2, HO-1, and PCNA mRNA levels in rat hepatic lysate were determined using Maxima® SYBR Green/Fluorescein qPCR Master Mix by Rotor-Gene Q (Qiagen, U.S.A.). Moreover, rat β-actin was used as a housekeeping gene and internal reference control. The work was done using Applied Biosystem StepOne Plus, Foster City, CA, U.S.A. The gene specific PCR primers used were summarized in .
Table 1. The primers set used for detection of gene expression in rats.
2.8. Statistical analysis
For the representation of quantitative variables, mean ± standard error was used. For evaluation of the least number of rats needed in each group, a SOLO power analysis was used as significance was considered at P ≤ .05 and statistical power at 0.90. For assessment of normality of sample distribution, Kolmogorov–Smirnov (K–S) test was used. For comparison between groups, a one-way analysis of variance (ANOVA) was used, followed by a post hoc Bonferroni correction test when the difference is significant. Statistical analyses were done using SPSS version 20 (Chicago, IL, U.S.A.). Statistical significance was predefined as P ≤ .05.
3. Results
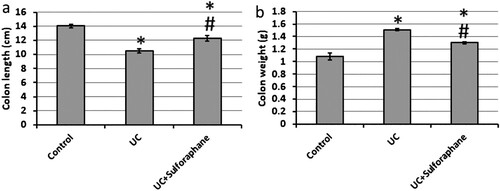
3.1. Effect of sulforaphane on UC-induced alteration in colon length and weight
Investigation of colons from rats with UC revealed a significant reduction in colon length as well as a significant increase in colon weight as compared with the control group. The use of sulforaphane in treating UC rats reversed these effects to be near the control group ().
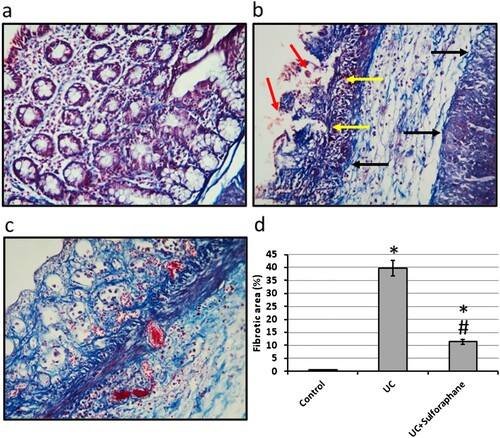
3.2. Effect of sulforaphane on UC -induced morphological changes
Microscopic images of colon sections of rats stained with Masson trichrome in the control group showed normal intestinal glands and minimal bluish stained fibers. Examination of the UC group revealed damaged intestinal glands, severe hemorrhage, and extensive fibrosis. Treatment of UC rats with sulforaphane ameliorated intestinal gland damage and hemorrhage. In addition, treatment of UC with sulforaphane significantly reduced the fibrotic area as compared with the UC group ().
Figure 2. Colon sections stained with Masson trichrome in control group (a), ulcerative colitis (UC, b) and UC treated with 15 mg/kg sulforaphane (c). Examination of UC group revealed damaged intestinal glands (yellow arrows), severe hemorrhage (red arrows) and extensive fibrosis (black arrows). The fibrotic area was calculated for each group (d). The micro-images represented the results of examining three rats in each group with examination of 10 fields in each rat. *Significant difference as compared with control group at p < .05. #Significant difference as compared with UC group at p < .05.
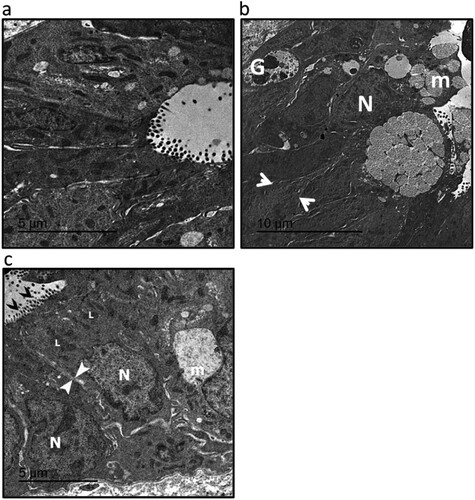
Electron micrographs (transmission electron microscopy) of colon samples from UC showed the dark distorted Goblet cell nucleus (G) with disarranged mucus granules (m). The enterocytes nucleus is not in a basal position (N). The cells are separated by a wide intercellular space (between white arrowheads). While investigating samples from UC rats treated with sulforaphane showed Goblet cells with disarranged mucus granules (m) and areas of distinct brush border with typical microvilli (black arrows heads). The enterocytes nucleus is in a basal position (N) with less numerous lysosomes (L) and no cytoplasmic vacuoles. The cells are separated by a narrow intercellular space (between white arrowheads) ().
Figure 3. Electromicrographs (transmission electron microscopy) of colon samples from the control group (a), ulcerative colitis (UC, b) and UC treated with 15 mg/kg sulforaphane (c). Images represented Goblet cell nucleus (G), mucus granules (m), typical microvilli (black arrows heads), enterocytes nucleus in a basal position (N), lysosomes (L) and intercellular space (between white arrow heads). Scale bar is 5 µm. The micro-images represented the results of examining three rats in each group with examination of 10 fields in each rat.
3.3. Effect of sulforaphane on UC-induced mitochondrial biogenesis
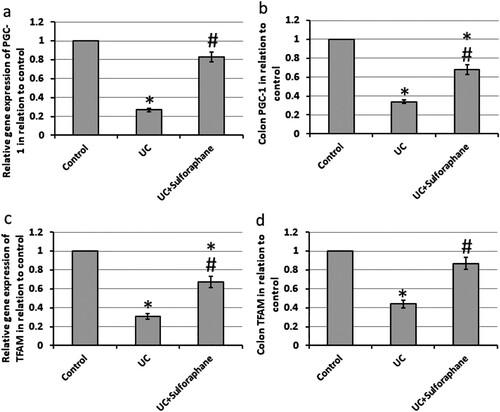
UC results in a 73% and 69% decrease in the gene expression of PGC-1 and TFAM associated with 64% and 56% reduction in the protein levels of both compounds, respectively, as compared with the control group. Treatment of UC rats with sulforaphane significantly increased both gene expression and protein levels of PGC-1 and TFAM as compared with UC rats ().
Figure 4. Effect of ulcerative colitis (UC) and 15 mg/kg sulforaphane on gene expression of proliferator-activated receptor-gamma coactivator (PGC-1, a) and mitochondrial transcription factor A (TFAM, c) as well colon levels of PGC-1 (b) and TFAM (d). *Significant difference as compared with control group at p < .05. #Significant difference as compared with UC group at p < .05.
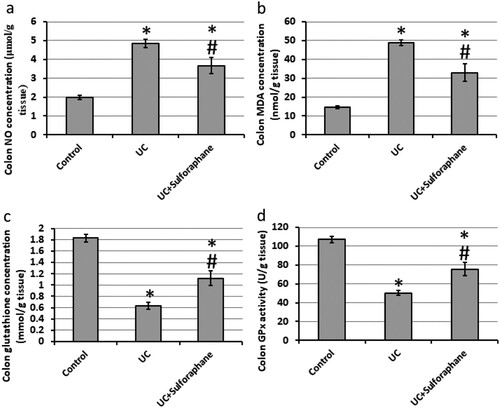
3.4. Effect of sulforaphane on UC-induced activation of oxidative stress
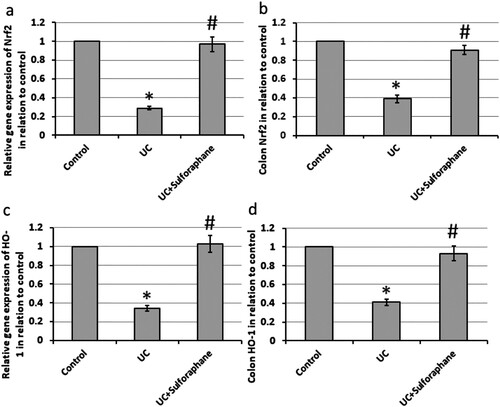
Induction of UC in rats leads to 71% and 66% reduction in the gene expression of Nrf2 and HO-1, respectively, associated with 61% and 59% reduction in protein levels of Nrf2 and HO-1, respectively, in the colon of rats as compared with the control rats. Treatment of UC rats with sulforaphane reversed these effects ().
Figure 5. Effect of ulcerative colitis (UC) and 15 mg/kg sulforaphane on gene expression of nuclear factor erythroid 2-related factor 2 (Nrf2, a) and Heme Oxygenase-1 (HO-1, c) as well colon levels of Nrf2 (b) and HO-1 (d). *Significant difference as compared with control group at p < .05.
Rats with UC showed a significant increase in the levels of NO (4.86 μmol/g) and MDA (48.9 nmol/g) as compared with the control group, 1.98 μmol/g, and 14.67 nmol/g, respectively. In addition, UC results in a significant reduction in glutathione peroxidase activity (50.47 U/g) and reduced glutathione concentration (0.63 mmol/g) as compared with the control group, 107.07 U/g, and 1.83 mmol/g, respectively. Treatment of UC rats with sulforaphane significantly decrease the levels of NO (3.67 μmol/g) and MDA (33.0 nmol/g) associated with a significant increase in glutathione peroxidase activity (75.57 U/g) and reduced glutathione (1.12 mmol/g) as compared with the UC group ().
3.5. Effect of sulforaphane on UC-induced DNA polymerization
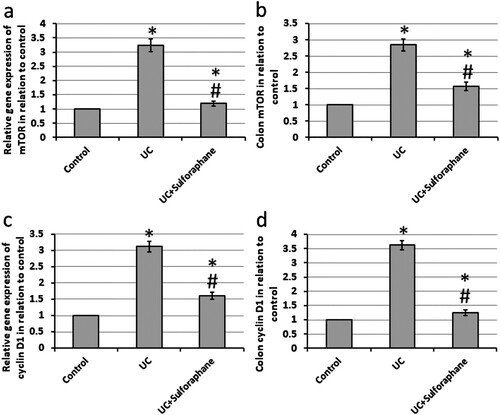
UC results in a 3.24- and 3.12-fold increase in the gene expression of mTOR and cyclin D1 associated with a 2.84- and 3.62-fold increase in the protein levels of both compounds, respectively, as compared with the control group. Treatment of UC rats with sulforaphane significantly reduced both gene expression and protein levels of mTOR and cyclin D1 as compared with UC rats ().
Figure 7. Effect of ulcerative colitis (UC) and 15 mg/kg sulforaphane on gene expression of mammalian target of rapamycin (mTOR, a) and cyclin D1 (c) as well colon levels of mTOR (b) and cyclin D1 (d). *Significant difference as compared with control group at p < .05. #Significant difference as compared with UC group at p < .05.
3.6. Effect of sulforaphane on UC-induced expression of PCNA
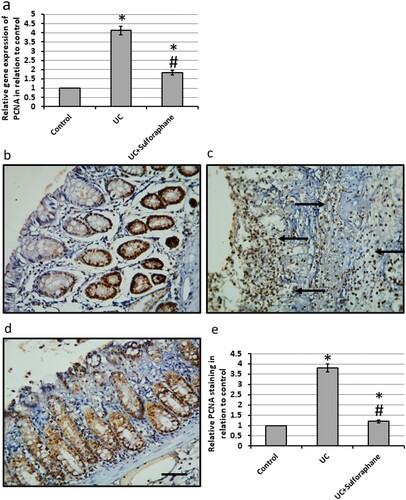
UC caused a 4.23-fold increase in the gene expression of PCNA as compared with the control group. In parallel, investigation of colon sections stained with anti-PCNA antibodies revealed intense reaction and immune staining of the colon tissues. Treatment of UC rats with sulforaphane significantly reduced the expression of PCNA associated with a reduction in the immune staining of colon sections stained with anti-PCNA antibodies as compared with UC rats ().
Figure 8. Effect of ulcerative colitis (UC) and 15 mg/kg sulforaphane on gene expression of proliferating cell nuclear antigen (PCNA, a) as well colon sections stained with anti-PCNA antibodies in control group (b), UC group (c) and UC group treated with sulforaphane (d). Immunohistochemistry score of positive staining (e). *Significant difference as compared with control group at p < .05. #Significant difference as compared with UC group at p < .05. The micro-images represented the results of examining three rats in each group with examination of 10 fields in each rat.
4. Discussion
After induction of UC in rats, treatment with sulforaphane resulted in protective effects as indicated by restoring the normal colon length and weight. Moreover, the pathological investigations of micro-images of colon sections stained with Masson trichrome revealed that sulforaphane ameliorated UC-induced intestinal glands damage, severe hemorrhage, and inflammatory cell infiltration in the mucosa and the submucosa associated with a reduction in the fibrotic area as compared with UC rats. Finally, investigation of colon sections using an electron microscope revealed that sulforaphane attenuated disarranged mucus granules, dark distorted Goblet cell nucleus, and decreased numerous lysosomes. Only one previous study reported the therapeutic effects of sulforaphane in UC through alteration of microbiota and production of anti-inflammatory cytokines [Citation1].
PGC-1 is a transcriptional co-activators of many genes that are involved in energy management, mitochondrial biogenesis, respiration, glucose homeostasis, and many physiological processes inside the body such as aging and stress response. It could protect the body against oxidative stress-related diseases [Citation17]. It promotes mitochondrial biogenesis through activation of both nuclear transcription factors and estrogen-related receptor-α leading to enhancement of the expression of the genes that help in encoding mitochondrial proteins. The overexpression of the PGC-1 gene was linked with many health benefits in many diseases such as muscular and neurodegenerative disorders [Citation18]. In addition, some recent studies reported the role of overexpression of TFAM and PGC-1 in reducing the generation of ROS in mitochondria as well as improving mitochondrial respiratory function [Citation19]. Moreover, TFAM is a nuclear gene essential for enhancing the replication of the mitochondrial genome making TFAM a pivotal agent in the process of energy production via oxidative phosphorylation [Citation20]. TFAM is essential for the differentiation of the epidermis [Citation21]. In the intestine, mutations of TFAM have been observed in colon cancers [Citation22]. PGC-1 and TFAM were reported previously to have a role in inflammatory bowel diseases such as crowns disease and ulcerative colitis [Citation23]. In addition, tissue damage in UC is accompanied by the arrest of mitochondrial respiration, loss of mitochondrial DNA, and the expression of mitochondrial proteins [Citation24]. We found that sulforaphane protects against UC by increasing the expression of PGC-1 and TNFA. Sulforaphane was reported previously to protect intestinal epithelial cells against LPS-induced intestinal changes via direct activation of the AMPK/SIRT1/PGC-1ɑ pathway [Citation25] and to enhance the utilization of lipids in the HHL-5 cells and in high fat diet rats by a direct effect on both PGC-1 and HTFM [Citation26] or indirectly modulate the activity of mitochondria through antioxidant activity [Citation27]. However, no previous study illustrated the role of sulforaphane in elevating the expression of PGC-1 and TNFA in UC.
The process of initiation and maintenance of inflammation in UC depends on the imbalance between ROS and antioxidant defense inside the colon. The flow of both neutrophils and monocytes that results from inflammation can produce more ROS through activation of enzymes of the respiratory burst as well as enzymes produced during prostaglandin and leukotriene metabolism. The resulted oxidative stress has a role in mediating intestinal damage in many inflammatory bowel diseases [Citation28]. The resulted fatty acid radicals and lipid hydroperoxides could attack cell membrane and destroy double bonds in polyunsaturated fatty acids leading to more oxidative damage. It has been proved that serum MDA levels are increased in patients with UC [Citation29]. In addition, nitric oxide is an inhibitor of platelet aggregation and neutrophil chemotaxis as well as it helps in the relaxation of both blood vessels and smooth muscles [Citation30]. It is linked to inflammation of the large intestine in UC patients and is associated with a bad diagnosis of patients [Citation31]. Nitric oxide was found to be overexpressed in patients with UC, especially in the colonic mucosa leading to pathological changes in tissues, DNA damage, production of inflammatory cytokines, inhibition of the repair process, and elevating the risk of conversion of UC to colon cancer [Citation30,Citation31]. We found that UC results in increased levels of MDA that were ameliorated by treating the mice with sulforaphane.
One of the elements of the antioxidant system in the body is Nrf2. When the body suffers from oxidative stress, Nrf2 is activated and translocated inside the nucleus, leading to the expression of downstream antioxidant enzymes [Citation32]. In addition, the overexpression of Nrf2 was previously reported to improve UC [Citation33]. One of the downstream of Nrf-2 is HO-1, which is one of the antioxidant defense proteins [Citation34]. It was reported previously to prevent colon tissue oxidative damage [Citation35]. Moreover, the epithelium tissue in the colon contains many antioxidant systems, for example, antioxidant enzymes and low molecular-weight molecules represented by reduced glutathione, ascorbic acid, and vitamin E [Citation36]. Reduced glutathione is a significant component of antioxidant defenses of most tissues. It is a thiol-containing tripeptide. It has a high reduction potential, and it promotes the formation of the reduced forms of ascorbate and α-tocopherol. Depletion of tissue glutathione has been shown to be associated with a marked cellular degeneration of colon epithelium. Glutathione of gastrointestinal mucosa is derived from the endogenous synthesis, inter-organ transport, and the absorption of glutathione from diet and biliary secretions [Citation37]. Luminal glutathione makes a large contribution to mucosal levels throughout the small intestine; however, the bioavailability of luminal glutathione in the colon would appear to be limited and potentially dependent on factors such as intestinal transit time and metabolic activity of luminal flora. The fact that the glutathione synthetic rate of the colon is lower than that of many tissues, including the duodenum and small intestine, further suggests that the colon could be particularly vulnerable to abnormalities in glutathione metabolism [Citation38]. We found that treating UC rats with sulforaphane results in increased levels of Nrf2, HO-1, reduced glutathione, and glutathione peroxidase. Sulforaphane was reported previously to possess antioxidant activity in many animal models, however, there was no previous study that assessed the effect of sulforaphane on UC-induced reduction in the antioxidant activity.
mTOR is a serine/threonine kinase with the ability to regulate cellular metabolism. It regulates the differentiation of T cells. mTOR signaling pathway plays a vital role in mediating numerous processes, including cell proliferation, apoptosis, necrosis, and inflammation [Citation4]. mTOR is considered as a central regulator of both cell growth and proliferation. It is regulated by cellular energy. The regulation of energy metabolism is mediated by several related signaling pathways, wherein mTOR signaling pathways jointly constitute a switch of anabolic and catabolic processes in cells [Citation39]. Sulforaphane was previously reported to inhibit mTOR in bronchial carcinoid [Citation40], esophageal squamous cell carcinoma [Citation41], bladder cancer cells [Citation42], and endometrial cancer [Citation43], lipopolysaccharide-induced spatial learning and memory dysfunction [Citation44] and rotenone-induced neurotoxicity [Citation45]. However, no previous study illustrated the role of sulforaphane in reducing mTOR expression in UC.
Cyclin D1 gene is a nuclear protein that plays a role in the transition from G1 to S phase in the cell cycle. Cyclins are involved in all phases of the cycle by complexing with Cyclin-dependent kinases. The cell enters the S phase by providing the transcription of the genes required for entry into the S phase and the synthesis of DNA occurs. Cyclin D1 is a downstream cell proliferation related gene of STAT3 [Citation46]. Cyclin D1 was reported to be overexpressed in UC [Citation46–48]. Overexpression of cyclin D1 protein is associated with inflammation and cell proliferation that takes place in active UC [Citation49]. Sulforaphane was also reported to inhibit cyclin D1 expression in human colon carcinoma cells [Citation50] and non-small cell lung cancer cell lines [Citation51].
Finally, we examined the effect of sulforaphane on PCNA, which is a good marker of proliferation activity of intestinal mucosal epithelial cells and is considered as an oncogenic transcription factor. It is a protein present inside the nucleus. It works as a cofactor for DNA polymerase and DNA synthesis. It enhances genomic stability, between polymerase and DNA [Citation52]. It has been reported to play crucial role during UC [Citation53]. We found a significant increase in the gene expression of PCNA associated with an increase in immunostaining of PCNA in micro-images, which is consistent with previous studies [Citation53,Citation54]. We found that the treatment of UC rats with sulforaphane significantly reduced the expression of PCNA. However, no previous study illustrated the role of sulforaphane in reducing the expression of PCNA.
5. Conclusion
Sulforaphane protects against experimentally induced UC in rats. It restores the normal weight and length of the colon. In addition, the pathological investigations of colon sections revealed the ability of sulforaphane to ameliorate UC-induced intestinal glands damage, dark distorted Goblet cell nucleus, severe hemorrhage, inflammatory cell infiltration and decreased numerous lysosomes. The results illustrated that sulforaphane protects against UC through different mechanisms such as enhancing antioxidant activity, elevating mitochondrial biogenesis, and inhibiting DNA polymerization. We believe that our results can be readily translated to clinical use for several reasons. Sulforaphane is a safe natural product as illustrated by many previous studies as the LD50 value of sulforaphane was reported at 212.67 mg/kg compared with 15 mg/kg in our study. Sulforaphane is given orally to rats in our study which could resemble the ability to use it for protection by eating broccoli, Northern carrot, and kale. Finally, contents of sulforaphane in cabbage ranged from 3.91–52.00 mg/kg, while they were 62.64–982.36 mg/kg in florets of broccoli; 18.11–274.00 mg/kg in stems of broccoli and 6.55–256.46 mg/kg in leaves of broccoli [Citation55]. Therefore, the dose used in this study can be obtained by eating about 19–255 g of cabbage, 1–16 g florets of broccoli, 4–55 g stems of broccoli or 4–152 g of leaves of broccoli.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Zhang Y, Tan L, Li C, et al. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Exp. 2020;10(1):119. DOI:10.1186/s13568-020-01053-z.
- Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018;16(3):343–356 e3. DOI:10.1016/j.cgh.2017.06.016.
- Yao D, Dai W, Dong M, et al. MUC2 and related bacterial factors: therapeutic targets for ulcerative colitis. EBioMedicine. 2021;74:103751. DOI:10.1016/j.ebiom.2021.103751.
- Jiang W, Han YP, Hu M, et al. A study on regulatory mechanism of miR-223 in ulcerative colitis through PI3K/Akt-mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(11):4865–4872.
- Ussakli CH, Ebaee A, Binkley J, et al. Mitochondria and tumor progression in ulcerative colitis. J Natl Cancer Inst. 2013;105(16):1239–1248. DOI:10.1093/jnci/djt167.
- Wagner AE, Will O, Sturm C, et al. DSS-induced acute colitis in C57BL/6 mice is mitigated by sulforaphane pre-treatment. J Nutr Biochem. 2013;24(12):2085–2091. DOI:10.1016/j.jnutbio.2013.07.009.
- Kaiser AE, Baniasadi M, Giansiracusa D, et al. Sulforaphane: a broccoli bioactive phytocompound with cancer preventive potential. Cancers (Basel). 2021;13(19). DOI:10.3390/cancers13194796.
- Zeren S, Bayhan Z, Kocak FE, et al. Gastroprotective effects of sulforaphane and thymoquinone against acetylsalicylic acid-induced gastric ulcer in rats. J Surg Res. 2016;203(2):348–359. DOI:10.1016/j.jss.2016.03.027.
- Bai Y, Wang X, Zhao S, et al. Sulforaphane protects against cardiovascular disease via Nrf2 activation. Oxid Med Cell Longev. 2015;2015:407580.
- Liebman SE, Le TH. Eat your broccoli: oxidative stress, NRF2, and sulforaphane in chronic kidney disease. Nutrients. 2021;13(1). DOI:10.3390/nu13010266.
- Sun CC, Li SJ, Yang CL, et al. Sulforaphane attenuates muscle inflammation in dystrophin-deficient mdx mice via NF-E2-related factor 2 (Nrf2)-mediated inhibition of NF-kappaB signaling pathway. J Biol Chem. 2015;290(29):17784–17795. DOI:10.1074/jbc.M115.655019.
- Santin-Marquez R, Alarcon-Aguilar A, Lopez-Diazguerrero NE, et al. Sulforaphane – role in aging and neurodegeneration. Geroscience. 2019;41(5):655–670. DOI:10.1007/s11357-019-00061-7.
- Kumar A, Sabbioni G. New biomarkers for monitoring the levels of isothiocyanates in humans. Chem Res Toxicol. 2010;23(4):756–765. DOI:10.1021/tx900393t.
- Socala K, Nieoczym D, Kowalczuk-Vasilev E, et al. Increased seizure susceptibility and other toxicity symptoms following acute sulforaphane treatment in mice. Toxicol Appl Pharmacol. 2017;326:43–53. DOI:10.1016/j.taap.2017.04.010.
- Wang Y, Jeffery EH, Miller MJ, et al. Lightly cooked broccoli is as effective as raw broccoli in mitigating dextran sulfate sodium-induced colitis in mice. Nutrients. 2018;10(6).
- Deng Z, Rong Y, Teng Y, et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25(7):1641–1654. DOI:10.1016/j.ymthe.2017.01.025.
- Liang D, Zhuo Y, Guo Z, et al. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie. 2020;170:10–20. DOI:10.1016/j.biochi.2019.12.001.
- Rera M, Bahadorani S, Cho J, et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14(5):623–634. DOI:10.1016/j.cmet.2011.09.013.
- Yao K, Zhang WW, Yao L, et al. Carvedilol promotes mitochondrial biogenesis by regulating the PGC-1/TFAM pathway in human umbilical vein endothelial cells (HUVECs). Biochem Biophys Res Commun. 2016;470(4):961–966. DOI:10.1016/j.bbrc.2016.01.089.
- Shi Y, Dierckx A, Wanrooij PH, et al. Mammalian transcription factor A is a core component of the mitochondrial transcription machinery. Proc Natl Acad Sci U S A. 2012;109(41):16510–16515. DOI:10.1073/pnas.1119738109.
- Hamanaka RB, Glasauer A, Hoover P, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal. 2013;6(261):ra8. DOI:10.1126/scisignal.2003638.
- Guo J, Zheng L, Liu W, et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 2011;71(8):2978–2987. DOI:10.1158/0008-5472.CAN-10-3482.
- Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy. 2020;16(1):3–17. DOI:10.1080/15548627.2019.1603547.
- Reifen R, Levy E, Berkovich Z, et al. Vitamin A exerts its antiinflammatory activities in colitis through preservation of mitochondrial activity. Nutrition. 2015;31(11-12):1402–1407. DOI:10.1016/j.nut.2015.05.011.
- Zhang YJ, Wu Q. Sulforaphane protects intestinal epithelial cells against lipopolysaccharide-induced injury by activating the AMPK/SIRT1/PGC-1a pathway. Bioengineered. 2021;12(1):4349–4360. DOI:10.1080/21655979.2021.1952368.
- Lei P, Tian S, Teng C, et al. Sulforaphane improves lipid metabolism by enhancing mitochondrial function and biogenesis in vivo and in vitro. Mol Nutr Food Res. 2021;65(11):e2170023. DOI:10.1002/mnfr.202170023.
- Negrette-Guzman M, Huerta-Yepez S, Tapia E, et al. Modulation of mitochondrial functions by the indirect antioxidant sulforaphane: a seemingly contradictory dual role and an integrative hypothesis. Free Radic Biol Med. 2013;65:1078–1089. DOI:10.1016/j.freeradbiomed.2013.08.182.
- Keshavarzian A, Banan A, Farhadi A, et al. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. 2003;52(5):720–728. DOI:10.1136/gut.52.5.720.
- Baskol G, Baskol M, Yurci A, et al. Serum paraoxonase 1 activity and malondialdehyde levels in patients with ulcerative colitis. Cell Biochem Funct. 2006;24(3):283–286. DOI:10.1002/cbf.1224.
- Gawronska B, Matowicka-Karna J, Kralisz M, et al. Markers of inflammation and influence of nitric oxide on platelet activation in the course of ulcerative colitis. Oncotarget. 2017;8(40):68108–68114. DOI:10.18632/oncotarget.19202.
- Avdagic N, Zaciragic A, Babic N, et al. Nitric oxide as a potential biomarker in inflammatory bowel disease. Bosn J Basic Med Sci. 2013;13(1):5–9. DOI:10.17305/bjbms.2013.2402.
- Deng L, Guo H, Wang S, et al. The attenuation of chronic ulcerative colitis by (R)-salbutamol in repeated DSS-induced mice. Oxid Med Cell Longev. 2022;2022:9318721.
- Tan Y, Zheng C. Effects of alpinetin on intestinal barrier function, inflammation and oxidative stress in dextran sulfate sodium-induced ulcerative colitis mice. Am J Med Sci. 2018;355(4):377–386. DOI:10.1016/j.amjms.2018.01.002.
- Xu B, Qin Y, Li D, et al. Inhibition of PDE4 protects neurons against oxygen-glucose deprivation-induced endoplasmic reticulum stress through activation of the Nrf-2/HO-1 pathway. Redox Biol. 2020;28:101342. DOI:10.1016/j.redox.2019.101342.
- Mei Y, Wang Z, Zhang Y, et al. FA-97, a new synthetic caffeic acid phenethyl ester derivative, ameliorates DSS-induced colitis against oxidative stress by activating Nrf2/HO-1 pathway. Front Immunol. 2019;10:2969. DOI:10.3389/fimmu.2019.02969.
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15(1):71. DOI:10.1186/s12937-016-0186-5.
- Li Y, Pan X, Yin M, et al. Preventive effect of lycopene in dextran sulfate sodium-induced ulcerative colitis mice through the regulation of TLR4/TRIF/NF-kappaB signaling pathway and tight junctions. J Agric Food Chem. 2021;69(45):13500–13509. DOI:10.1021/acs.jafc.1c05128.
- Holmes EW, Yong SL, Eiznhamer D, et al. Glutathione content of colonic mucosa: evidence for oxidative damage in active ulcerative colitis. Dig Dis Sci. 1998;43(5):1088–1095. DOI:10.1023/A:1018899222258.
- Zeng H, Cohen S, Guy C, et al. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity. 2016;45(3):540–554. DOI:10.1016/j.immuni.2016.08.017.
- Mokhtari RB, Qorri B, Baluch N, et al. Next-generation multimodality of nutrigenomic cancer therapy: sulforaphane in combination with acetazolamide actively target bronchial carcinoid cancer in disabling the PI3K/Akt/mTOR survival pathway and inducing apoptosis. Oncotarget. 2021;12(15):1470–1489. DOI:10.18632/oncotarget.28011.
- Lu Z, Zhang Y, Xu Y, et al. mTOR inhibitor PP242 increases antitumor activity of sulforaphane by blocking Akt/mTOR pathway in esophageal squamous cell carcinoma. Mol Biol Rep. 2022;49(1):451–461. DOI:10.1007/s11033-021-06895-9.
- Justin S, Rutz J, Maxeiner S, et al. Chronic sulforaphane administration inhibits resistance to the mTOR-inhibitor everolimus in bladder cancer cells. Int J Mol Sci. 2020;21(11). DOI:10.3390/ijms21114026.
- Rai R, Gong Essel K, Mangiaracina Benbrook D, et al. Preclinical efficacy and involvement of AKT, mTOR, and ERK kinases in the mechanism of sulforaphane against endometrial cancer. Cancers (Basel). 2020;12(5):1273.
- Gao J, Xiong B, Zhang B, et al. Sulforaphane alleviates lipopolysaccharide-induced spatial learning and memory dysfunction in mice: the role of BDNF-mTOR signaling pathway. Neuroscience. 2018;388:357–366. DOI:10.1016/j.neuroscience.2018.07.052.
- Zhou Q, Chen B, Wang X, et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci Rep. 2016;6:32206. DOI:10.1038/srep32206.
- Yu LZ, Wang HY, Yang SP, et al. Expression of interleukin-22/STAT3 signaling pathway in ulcerative colitis and related carcinogenesis. World J Gastroenterol. 2013;19(17):2638–2649. DOI:10.3748/wjg.v19.i17.2638.
- Li F, Yan H, Jiang L, et al. Cherry polyphenol extract ameliorated dextran sodium sulfate-induced ulcerative colitis in mice by suppressing Wnt/beta-catenin signaling pathway. Foods. 2021;11(1):49.
- Liu X, Wu YL, Liu KL, et al. Effects of resveratrol on ulcerative colitis in mice and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019;35(5):447–453.
- Wong NA, Mayer NJ, Anderson CE, et al. Cyclin D1 and p21 in ulcerative colitis-related inflammation and epithelial neoplasia: a study of aberrant expression and underlying mechanisms. Hum Pathol. 2003;34(6):580–588. DOI:10.1016/S0046-8177(03)00125-4.
- Shen G, Xu C, Chen C, et al. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol. 2006;57(3):317–327. DOI:10.1007/s00280-005-0050-3.
- Zuryn A, Litwiniec A, Safiejko-Mroczka B, et al. The effect of sulforaphane on the cell cycle, apoptosis and expression of cyclin D1 and p21 in the A549 non-small cell lung cancer cell line. Int J Oncol. 2016;48(6):2521–2533. DOI:10.3892/ijo.2016.3444.
- Lu YC, Wang P, Wang J, et al. PCNA and JNK1-Stat3 pathways respectively promotes and inhibits diabetes-associated centrosome amplification by targeting at the ROCK1/14-3-3sigma complex in human colon cancer HCT116 cells. J Cell Physiol. 2019;234(7):11511–11523. DOI:10.1002/jcp.27813.
- Lin L, Sun Y, Wang D, et al. Celastrol ameliorates ulcerative colitis-related colorectal cancer in mice via suppressing inflammatory responses and epithelial-mesenchymal transition. Front Pharmacol. 2015;6:320.
- Lin X, Xu W, Shao M, et al. Shenling Baizhu San supresses colitis associated colorectal cancer through inhibition of epithelial-mesenchymal transition and myeloid-derived suppressor infiltration. BMC Complement Altern Med. 2015;15:126. DOI:10.1186/s12906-015-0649-9.
- Li YLZ, Fang Z, Yang L, et al. Development and verification of sulforaphane extraction method in cabbage (Brassica oleracea L. var. capitata) and broccoli (Brassica oleracea L. var. italica Planch. J Med Plants Res. 2012;6(33):4796–4803.