?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective: Hydroxytyrosol (HT) is a polyphenol with a wide range of biological activities. Excessive drinking can lead to oxidative stress and inflammation in the liver, which usually develop into alcohol liver disease (ALD). At present, there is no specific drug to treat ALD. In this paper, the protection effect of HT on ALD and the underline mechanism were studied.
Methods: HepG2 cells were exposed to ethanol in vitro and C57BL/6J mice were fed with a Lieber-DeCarli ethanol liquid diet in vivo.
Results: triglyceride (TG) level in serum and the expression of fatty acid synthase (FASN) were reduced significantly by the treatment with HT The acetaldehyde dehydrogenase (ALDH) activity was increased, the serum level of malondialdehyde (MDA) was decreased, catalase (CAT) and glutathione (GSH) were increased, suggesting that HT may reduce its oxidative damage to the body by promoting alcohol metabolism. Furthermore, according to the mRNA levels of tnf-α, il-6 and il-1β, HT inhibited ethanol-induced inflammation significantly. The anti-inflammatory mechanism of HT may be related to suppress the STAT3/iNOS pathway.
Dissussion: Our study showed that HT could ameliorate ethanol-induced hepatic steatosis, oxidative stress and inflammation and provide a new candidate for the prevention and treatment of ALD.
Abbreviation:
- ADH, alcohol dehydrogenase
- ALD, alcohol liver disease
- ALDH, acetaldehyde dehydrogenase
- ALT, alanine aminotransferase
- AST, aspartate aminotransferase
- CAT, catalase
- COX2, cyclo-oxygen-ase2
- CYP2E1, cytochrome P450 2E1
- DPPH, 2,2-Diphenyl-1-picrylhydrazyl
- DMSO, Dimethyl sulfoxide
- FASN, fatty acid synthase
- GSH, glutathione
- HT, hydroxytyrosol
- iNOS, inducible nitric oxide Synthas
- LDL, low density lipoprotein
- LPS, lipopolysaccharides
- MDA, malondialdehyde
- NO, nitric oxide
- PPAR-γ, peroxisome proliferators-activated receptor
- ROS, reactive oxygen species
- SREBP-1c, sterol regulatory element-binding protein-1c
- STAT3, signal transducer and activator of transcription 3
- TC, total cholesterol
- TG, triglyceride
1. Introduction
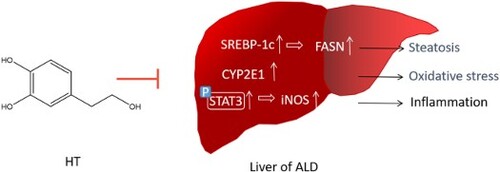
Alcoholic drinks are a kind of important consumer goods in human life. Howerver, excessive drinking can lead to ethanol-induced liver injury, commonly known as alcohol liver disease (ALD) [Citation1]. As a number of disease modifiers exacerbate liver disease progression, 35 percent of problem drinkers develop advanced liver disease [Citation2]. Most of the current ALD treatment drugs are liver-protective products, such as tiopronin, prednison and naltrexone. However, these products still need to be improved in terms of efficacy or safety [Citation3]. Thus, better strategies are urgently needed to prevent ALD and restore liver function concurrently. In the past few years, a lot of studies have been focused on the pathogenesis of ALD, which provide many new ideas for the treatment of ALD. Recent researches show that improving oxidative stress is a new strategy for treating ALD. What’s more, natural compounds are favored by a wide range of scientists due to their safety properties [Citation4–6].
Alcohol is initially metabolized by alcohol dehydrogenase (ADH) to acetaldehyde, which is rapidly metabolized to acetate by ALDH. In liver tissue, the decrease of CAT will lead to alcohol oxidation, which may affect the metabolic rate of ethanol [Citation7]. The microsomal CYP2E1, which stimulates reactive oxygen species (ROS), is also involved in the formation of acetaldehyde [Citation8]. It is generally assumed that promoting ethanol metabolism and reducing acetaldehyde accumulation are beneficial to reduce ALD.
The initial stage of ALD is usually fatty liver, which can develop into alcoholic hepatitis, liver fibrosis and liver cirrhosis [Citation9,Citation10]. The activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum can be used as diagnostic indicators of ALD [Citation11]. Hepatic steatosis, defined as the deposition of fat in hepatocytes, is a common phenomenon in ALD and associated with the progression of fibrosis and leads to cirrhosis and eventual liver failure [Citation12]. The prevention of the formation of fatty liver is essential to inhibit the liver damages induced by ethanol [Citation13]. An increasing number of evidences indicated that enhanced lipid synthesis is associated with a higher expression lipogenic enzymes and cytokines, such as FASN [Citation14]. SREBP-1, a transcription factor related to the control of lipid, glucose and cholesterol metabolism, stimulates expression of FASN [Citation15]. Additionally, the serine and threonine kinase AKT, also known as protein kinase B, is capable of targeting SREBP by enhancing transport of precursor SREBP and reducing degradation of mature SREBP [Citation16].
Inflammation also plays a crucial role in the underlying pathogenesis of ALD. A number of evidences indicated that alcohol exposure led to increased lipopolysaccharides (LPS), which provides the first signal for inflammasome activation [Citation17]. LPS and ROS then induce the production and release of pro-inflammatory cytokines TNF-α and IL-6 [Citation18]. IL-1β is critical to ALD pathogenesis, as it amplifies pro-inflammatory cytokine production, sensitizes hepatocytes to death signals and induces hepatic steatosis by upregulating FASN [Citation19]. STAT3 plays an important role in liver inflammation, which exacerbates hepatitis and liver fibrosis after being activated. Inducible nitric oxide synthas (iNOS), regulated by STAT3, accelerates the hepatitis and liver fibrosis process by producing inflammatory cytokine NO [Citation20].
HT is one of the key polyphenols with strong antioxidant activity in olive oil, which is welcomed by modern people [Citation21]. It has been reported that HT has anti-inflammatory, anti-tumor, antiviral, antibacterial and antifungal properties, and also improves endothelial dysfunction, decreases oxidative stress, and protects neuro- and cardio-problems [Citation22,Citation23]. HT could restrain the progression of fatty acid-induced hepatocellular steatosis and high-fat diet-induced obesity indicating that HT has some influence on lipid metabolism [Citation24,Citation25]. Moreover, previous studies have showed that HT could alleviate hepatic steatosis effectively through the AMPK pathway [Citation26]. Many ALD patients want to improve their health through diet, such as the famous Mediterranean diet characterized by olive oil intake [Citation27]. So, we aim to investigate the protection effect of HT on ALD and the impact of HT on the oxidation/inflammation-related signaling pathway.
2. Materials and methods
2.1. Materials and reagents
Hydroxytyrosol (purity > 98%) was purchased from Chengdu Mansite Biological Chemistry Company (Chengdu, China). DMSO was provided by Solarbio (Beijing, China). Biochemical kits for aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglyceride (TG), malondialdehyde (MDA), glutathione (GSH), catalase (CAT), high density lipoprotein (HDL), low density lipoprotein (LDL) were obtained from Nanjing Jiancheng bioengineering institute (Nanjing, China).
Antibodies for iNOS (131 kDa) and COX2 (69 kDa) were purchased from Abcam (Cambridge, UK). Antibodies for GAPDH (37 kDa), FASN (250 kDa) were purchased from Servicebio (Nanjing, China). Antibodies for SREBP-1c (125 kDa), CYP2E1 (55 kDa) were purchased from Proteintech Group (Wuhan, China). Antibodies for AKT(60 kDa), phospho-AKT (Ser473, 60 kDa), ADH (40 kDa), STAT3 (86/79 kDa) and phospho-STAT3 (Ser727, 86 kDa) were purchased from Cell Signaling Technology (Beverly, MA).
2.2. Free radical scavenging methods
100 μL of DPPH (0.05 mM) prepared by formaldehyde was mixed with 100 μL HT at different concentrations of 12.5, 25, 50, 100 μM. Mixtures were shaken vigorously and incubated for 30 min in the dark and the absorbance (A1) was detected at 517 nm. The inhibition percentage of DPPH+ was calculated by the following formula:
where A0 was the absorbance of methanol solution without DPPH and A2 was of formaldehyde replaced samples to be tested.
ABTS+ stock solution was obtained by mixing ABTS solution and antioxidant in equal proportion and reacting for 12 h at room temperature in the dark. The stock solution was diluted to be ABTS+ working solution by PBS until its absorbance was 0.7 ± 0.02 at 734 nm. 200 μL of ABTS+ working solution was added in 96 well cell plate, then supplemented with 10 μL of different concentrations of HT in each well. The absorbance (A1) was detected at 734 nm after incubated 5 min in the dark. The calculation method of ABTS+ clearance rate is the same as that of DPPH free radical.
2.3. Cell culture and treatment
The HepG2 human hepatocellular cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences. Cells were raised in high-glucose DMEM medium containing 10% fetal bovine serum and 0.01% streptomycin in a humidified atmosphere of 5% CO2 at 37℃. HepG2 cells were treated with ethanol (400 mM) and different concentrations of HT for 48 h, and the cell viability was confirmed by MTT assay.
2.4. Design of animal experiments
All procedures of animal experiment used in this study were carried out strictly to follow Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Protection Committee of Gulou Hospital, Nanjing University (SYXK 2004-0013). All the authors complied with the ARRIVE guidelines experiments. The NIAAA model, invented by National Institute on Alcohol Abuse and Alcoholism, was adopted to stimulate alcoholic fatty liver disease in this study. After one week of acclimatization, 8-week-old male C57BL/6J mice weighing 24-26 g were divided into four groups as follows: control group (Ctrl), ethanol group (EtOH), ethanol plus HT-12.5 mg/kg group (HT), ethanol plus bifendate-100 mg/kg (Positive). Mice in the ethanol group and HT supplement groups were fed a Lieber-Decarli ethanol liquid diet, containing 5% (v/v) ethanol, for 10 days, while the mice in the control group were pair-fed. On the 11th day, mice in the control group were administered maltose and mice in other groups were administered 31.6% ethanol in the early morning. After 12 h fasting, all mice were euthanatized and their blood samples and liver tissues were collected. The whole liver was weighed, cut and fixed in 4% paradormaldehyde or kept in the Ultra-low temperature freeze. Collected blood was centrifuged at 3000 rpm for 20 min, the supernatant fraction was regarded as plasma.
2.5. Measurement of NO secretion
NO, owning active chemical properties, is metabolically converted into NO3− and NO2−. A nitric oxide test kit (Biyuntian, China) was used to reduce NO3− to NO2− using the metal cadmium reduction method, and determine the amount of NO in the cell culture medium by detecting the amount of NO2− using Giress reagent.
2.6. Measurement of GSH/GSSG and CAT levels
The ratio of GSH/GSSG reflects the level of oxidative stress. The content of GSH and GSSG were assayed in cellular protein or serum through GSH/GSSG assay kit. The activity of CAT of cellular protein or serum was assayed with the CAT assay kit, following the manufacturer's protocols.
2.7. Quantitative real-time PCR
Total RNA was extracted from mouse livers or HepG2 cells using TRIzol reagent (Takara, Tokyo, Japan) and was stored at −70°C until used further. Isolated RNA was reverse-transcribed to cDNA using a reagent in accordance with manufacturer’s instructions and then quantitative real-time PCR was performed. The primers are shown in .
Table 1. Sequence of the human primers used in real-time PCR.
2.8. Western blot analysis
Total proteins were extracted from livers or HepG2 cells using ice-cold Western and IP lysates (Beyotime institute of biotechnology, Nanjing, China) containing 1% PMSF and the concentrations of isolated proteins were determined by BCA kits (Beyotime institute of biotechnology, Nanjing, China). 50 μg proteins were loaded and separated by 10% SDS-PAGE and then transferred onto a PVDF membrane. After incubating with blocking buffer for 1 h at room temperature, membranes were incubated with primary antibodies for 12 h at 4°C. Next, membranes were exposed to secondary antibody for 2 h at room temperature and protein bands were visualized by ECL reagent and quantified by ImageJ software.
2.9. Molecular docking analysis
The three-dimensional crystal structure of STAT3 (PDB ID:6DLG) was retrieved from the RCSB Protein Data Bank (https://www1.rcsb.org/structure/6DLG). The downloaded protein was further optimized to remove unwanted protein subunits, heat atoms and water molecules, add missing heavy atoms and formal charges (Autodock). STAT3 protein was taken for further preparation. The hydroxytyrosol ligand in SDF format was downloaded from Pubchem and exported in PDB format. Then the ligand was prepared using the LigPrep module of Autodock so that the ligand was optimized with suitable parameters like using OPLS3 force field, 2D to 3D conversion, generation of all possible ionization states at pH 7.0. Autodock was used for docking. ligand interaction diagram was exported after confirming the optimal structure.
2.10. Statistical analysis
All data were expressed as the mean ± standard deviation (SD) of triplicate experiments performed in a parallel manner. Normal distribution was confirmed by using the Shapiro–Wilk test. Analyses were done using Graph pad software between the control group and multiple-dose groups. The differences between means were determined by students’ t-test and the values were considered statistically significant at *P < 0.05.
3. Results
3.1. HT alleviated ethanol-induced cell injury in HepG2 cells
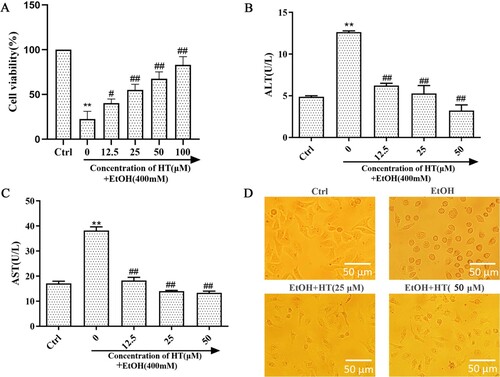
Previously, we determined the applicable concentration of ethanol for the ALD model in vitro via MTT assay (S1). When the ethanol concentration was 400 mM, 2.33% (v/v) in volume ratio, the cell survival rate was nearly 50%. This may be an appropriate concentration to induce significant toxicity without causing too much damage. The previous study showed that HT had a protective effect on cell damage (S2). In order to determine the effect of HT on ethanol-induced cell injury, MTT assay was used to detect cell survival rate on ethanol-induced HepG2 cells. Survival rate of HepG2 cells sharply decreased after ethanol exposure for 48 h ((A)). Treatment of HT significantly increased the survival rate in comparison with the EtOH group. Moreover, HT remarkably decreased the activities of ALT and AST when compared with the EtOH group ((B and C)). To further determine the effect of HT on cell injury induced by ethanol, we observed the status of HepG2 cells in each group ((D)). In Ctrl group, HepG2 cells were polygonal, adherent and grew by static adhesion. After ethanol exposure, cells gradually contracted into spheres and separated from the surrounding cells. Compared with the EtOH group, the cell state obviously improved after HT intervention. These findings implied that HT could ameliorate ethanol-induced liver injury.
Figure 1. Effects of HT on liver injury in ethanol-induced HepG2 cells. HepG2 cells were treated with 400 mM ethanol together with different concentrations of HT. (A) The cell survival rate of HepG2 cells was measured by MTT assay. (B, C) The levels of intracellular ALT and AST were measured. (D) Status of HepG2 cells were observed under the microscope. The data represented as mean ± SD and difference between groups are considered significant at P < 0.05. * Difference to the Ctrl group. # Difference to the EtOH group.
3.2. HT alleviated ethanol-induced hepatic steatosis in HepG2 cells
Ethanol-induced lipid accumulation is a major contributor to the pathogenesis of ALD. Hence, we investigated the protective effect of HT on ethanol-induced hepatic steatosis in HepG2 cells. Levels of TG and TC in the EtOH group were dramatically higher than that in the Ctrl group ((A and B)). When compared with the EtOH group, the HT group showed much lower levels of TG and TC. LDL-C, a lipoprotein particle that carried cholesterol into peripheral tissue cells, was markedly enhanced by ethanol. HT treatment resulted in a decrease in the level of LDL-C ((C)).
Figure 2. Effects of HT on hepatic steatosis in ethanol-induced HepG2 cells. HepG2 cells were treated with 400 mM ethanol together with different concentrations of HT. (A–C) The levels of TG, TC and LDL-C were measured. (D–F) Relative mRNA levels of PPAR-γ, SREBP-1c and FASN by quantitative real-time PCR, HT at a concentration of 50 μM. The data represented as mean ± SD and difference between groups are considered significant at P < 0.05. * Difference to the Ctrl group. # Difference to the EtOH group.
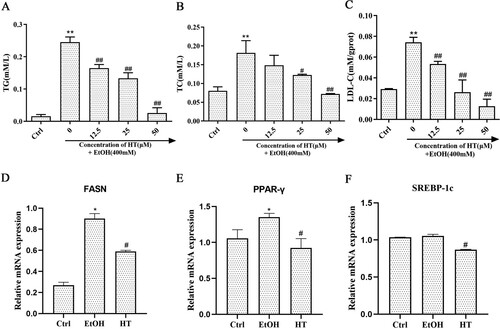
Moreover, we examined the expression of fatty acid oxidation-related genes in HepG2 cells. RT–PCR results showed that mRNA expression of FASN and PPAR-γ significantly increased after ethanol exposure while mRNA expression of SREBP-1c had no significant change ((D–F)). HT decreased the expression of FASN, SREBP-1c and PPAR-γ. Altogether, these results indicated that HT had a protective effect on alleviating ethanol-induced hepatic steatosis in HepG2 cells.
3.3. HT alleviated ethanol-induced oxidative stress and inflammation in HepG2 cells
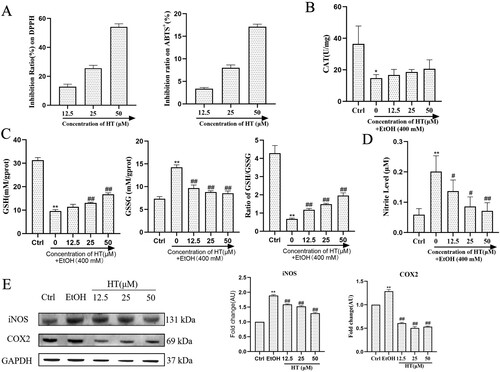
Oxidative stress and inflammation are major contributors in the pathogenesis and progression of ALD, thus we investigated the effect of HT on ethanol-induced oxidative stress and inflammation in HepG2 cells. At first, we measured the scavenging activity of HT on ABTS+ and DPPH radicals. Compared with the EtOH group, HT showed high free radical scavenging activity ((A)). In addition, Ethanol exposure significantly decreased both GSH and CAT levels while HT effectively increased levels of GSH except for the low concentration and improved the activity of CAT to a certain extent ((B and C)). A decrease in the GSH/GSSG ratio is a strong indicator of oxidative stress. As illustrated in (C), HT effectively reversed the increase in GSSG level and the decrease in GSH/GSSG ratio compared to the EtOH group, which indicated the reduction of oxidative stress. Moreover, significant increase in level of NO was detected in the EtOH group compared to the Ctrl group, indicating that ethanol-induced inflammation in HepG2 cells. In comparison with the EtOH group, treatment with HT significantly decreased this parameter ((D)). We then investigated the effect of HT on the expression of iNOS and COX2, which are the important regulators of inflammation. Results showed that ethanol exposure markedly increased protein expression of COX2 and iNOS while HT significantly decreased both expressions of COX2 and iNOS ((E)). Altogether, these findings suggested that HT could ameliorate ethanol-induced oxidative stress and inflammation in ethanol-induced HepG2 cells.
Figure 3. Effects of HT on oxidative stress and inflammation in ethanol-induced HepG2 cells. HepG2 cells were treated with 400 mM ethanol together with different concentrations of HT. (A) The activity of HT on scavenging DPPH and ABTS+ radicals were measured. (B, C) The levels of GSH, GSSG, ratio of GSH/GSSG and CAT activity in HepG2 cells were measured. (D) The level of NO in HepG2 cells was measured. (E) Western blot analysis of COX2 and iNOS. Results were expressed as fold changes to control. The data represented as mean ± SD and difference between groups are considered significant at P < 0.05. * Difference to the Ctrl group. # Difference to the EtOH group.
3.4. HT alleviated ethanol-induced liver injury in chronic-binge ethanol-fed mice
In vivo, we adopted a chronic-binge alcohol feeding mouse model to stimulate alcohol-liver injury. In comparison with the Ctrl group, significant increase in ALT, AST and liver index were observed in the EtOH group. Treatment with HT led to decrease in these parameters ((A and B)).
Figure 4. Effects of HT on liver injury in chronic-binge ethanol-fed mice. (A) The levels of serum ALT and AST were measured. (B) The level of liver index was measured. (C) H&E-stained in liver of mice. (D) Representative images of Tunel staining in mouse liver. The data represented as mean ± SD and difference between groups are considered significant at P < 0.05. * Difference to the Ctrl group. # Difference to the EtOH group.
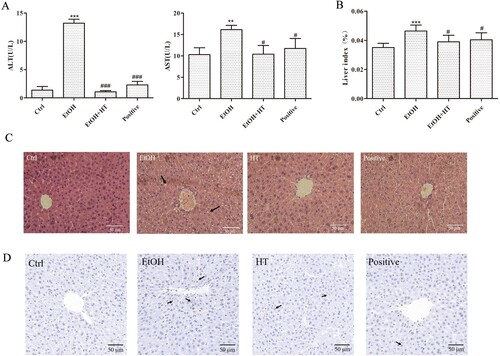
Hepatic histopathological analysis showed that the irregular arrangement of hepatocytes and inflammatory hepatocytes in liver were reversed after HT treatment compared to EtOH ((C)). Hepatic Tunel staining illustrated that the proportion of apoptotic cells increased significantly in the EtOH group, which had been marked with arrows. After HT treatment, the apoptosis rate of cells was greatly reduced, which was close to the normal level ((D)). Taken together, these findings suggested that HT had protective effect against ethanol-induced liver injury, which might contribute to inhibiting progression of ALD.
3.5. HT alleviated hepatic steatosis in chronic-binge ethanol-fed mice
To determine the beneficial effect of HT on ethanol-induced hepatic steatosis in mice, levels of serum TG and TC were measured. While levels of TG and TC notably increased in comparison with the Ctrl group, HT significantly reduce this parameter ((A)). Importantly, there was a significant decrease between mice treated with HT. Besides, HT markedly elevated level of HDL-C, which was regarded as one of preventive factors of arteriosclerosis ((B)). Moreover, as visualized in hepatic Oil-red O staining, ethanol intake induced accumulation of lipid droplets, this change was reversed by the treatment of HT. Among them, HT showed effective protection with the less lipid droplet in hepatocytes ((C)).
Figure 5. Effects of HT on p-AKT/SREBP-1c/FASN pathway in chronic-binge ethanol-fed mice. (A) The levels of serum TG and TC were measured. (B) The level of serum HDL-C was measured. (C) Representative images of oil-red O staining in mouse liver. (D–G)Western blot analysis of AKT, p-AKT, SREBP-1c and FASN. Results were expressed as fold changes to control. The data represented as mean ± SD and difference between groups are considered significant at P < 0.05. * Difference to the Ctrl group. # Difference to the EtOH group.
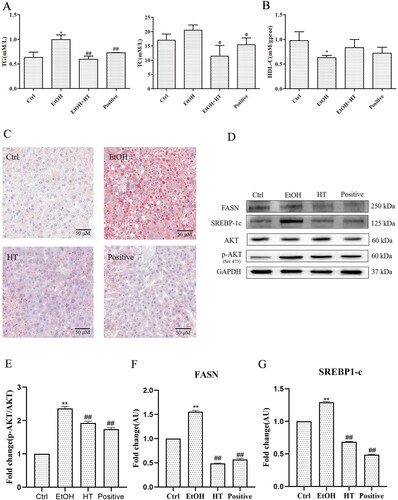
A growing body of evidence has indicated that chronic alcohol consumption promoted hepatic steatosis via SREBP-1c, which plays a crucial role in increasing fatty acid biosynthesis by regulating FASN. Furthermore, it has been reported that AKT could promote SREBP-1 activity. Thus, we performed a Western blot assay to detect the effect of HT on p-AKT/SREBP-1c/FASN signaling pathway. As illustrated in (D), significantly increased expression of p-AKT, SREBP-1c and FASN were detected in the liver of mice in the EtOH group. Treatment with HT reduced the phosphorylation of AKT at the site of Ser473 and the levels of SREBP-1c and FASN significantly in the liver tissue. Combined with the previous results, these findings indicated that HT could inhibit ethanol-induced hepatic steatosis by regulating p-AKT/SREBP-1c/FASN pathway.
3.6. HT alleviated ethanol-induced oxidative stress in chronic-binge ethanol-fed mice
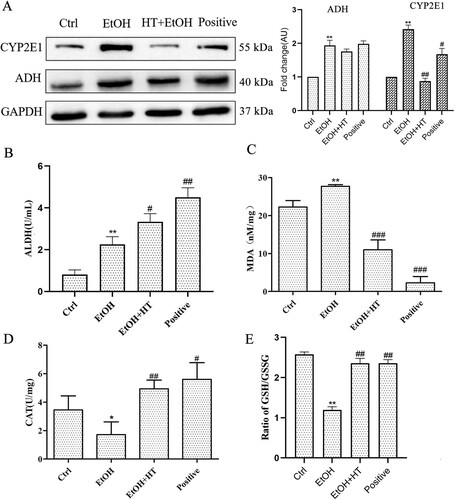
To analyze the efficacy of HT on ethanol-induced oxidative stress, we first assayed protein expression of CYP2E1, an enzyme stimulates ROS production. As shown in (A), ethanol significantly induced hepatic CYP2E1 expression which was remarkably decreased by administration of HT. We also examined the effect of HT on the expression of protein linked to ethanol metabolism. No obvious effect of HT on the protein level ADH was observed. The enzyme activity of ALDH was increased significantly by HT, which accelerated the conversion of unwanted intermediate acetaldehyde to acetic acid ((B)). These data suggested that HT accelerated the metabolism of ethanol, resulting the reduced oxidative damage of acetaldehyde to liver. Moreover, we observed that the generation of MDA, a lipid peroxidation product, was induced by ethanol but inhibited significantly by HT in ethanol-fed mice ((C)). At the same time, serum CAT activity and GSH/GSSG ratio were increased significantly by HT, protecting the liver from oxidative damage indued by ethanol ((D and E)).
Figure 6. Effects HT on hepatic steatosis and oxidative stress in chronic-binge ethanol-fed mice. (A) Western blot analysis of ADH and CYP2E1. Results were expressed as fold changes to control. (B) The level of serum ALDH was measured. (C–E) The levels of serum CAT activity, GSH/GSSG and MDA content were measured. Results were expressed as fold changes to control. The data represented as mean ± SD and difference between groups are considered significant at P < 0.05. * Difference to the Ctrl group. # Difference to the EtOH group.
3.7. HT alleviated ethanol-induced inflammation by regulating STAT3/iNOS pathway in chronic-binge ethanol-fed mice
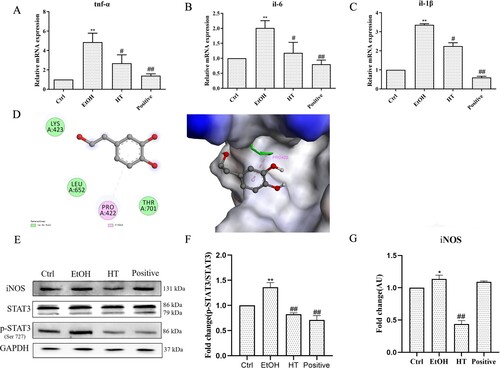
To determine the beneficial effect of the combination of HT on ethanol-induced inflammation in liver, we first measured the expression of pro-inflammatory cytokines including TNF-α, IL-1β and IL-6 in liver tissue. As shown in (A–C), the mRNA levels of tnf-α, il-1β and il-6 were dramatically increased in ethanol-fed mice compared with mice in the Ctrl group. In contrast, these alterations were reversed by treatment of HT. NO, a main inflammatory marker of ALD, is produced by the catalysis of iNOS. The expression of iNOS can be regulated by transcription factor STAT3. Therefore, we detected protein expression of p-STAT3 and iNOS in liver tissue. As shown in (E–G), the protein level of p-STAT3 and iNOS were decreased significantly by the treatment of HT in ethanol-fed mice. The interaction between the STAT3 protein and HT was demonstrated by molecular docking analysis. There is a certain intensity of interaction between STAT3 and HT, and the best induced-fit docking score is −4.651 kcal/mol. Molecular docking showed the HT can interact with STAT3 through van der Waals and pi-Alkyl, and insert into the active domain with PRO422, indicating that HT may decrease the phosphorylation of STAT3 at the site of Ser727 to interfere with the inflammatory signal transduction through targeting STAT3 ((D)). All the results suggested that HT reduced ethanol-induced inflammation by interfering STAT3/iNOS pathway in liver tissue.
Figure 7. Effects of HT on ethanol-induced inflammation in chronic-binge ethanol-fed mice. (A) The effect of HT on the mRNA levels of tnf-α, il-1β and il-6 in the liver tissue was measured by qPCR. (D) The interaction between HT and STAT3 protein with induced-fit docking analysis. (E–G) Western blot analysis of STAT3, p-STAT3 and iNOS. Results were expressed as fold changes to control. Results were expressed as fold changes to control. The data represented as mean ± SD and difference between groups are considered significant at P < 0.05. * Difference to the Ctrl group. # Difference to the EtOH group.
4. Discussion
Although it is well-acknowledged that an economic burden and public health risks are attributed to ALD, managing ALD is currently in a limited pharmacotherapy stage [Citation28]. New therapeutic approaches, therefore, are urgently needed to prevent the progression of ALD. Previous studies have revealed that HT has a protective effect on liver, which has been proved could significantly restrain high-fat diet-induced obesity and hepatocellular steatosis. However, effect of HT on ALD was not investigated. In this paper, we focused on the protective effect and mechanism of HT in ALD, thus providing new options for the treatment of ALD.
The pathogenesis of ALD is associated with hepatic steatosis, oxidative stress, inflammation and regeneration. Hepatic steatosis, the earliest stage of ALD, is characterized by lipid accumulation in hepatocytes and leads to a progression to the advanced disease stage due to oxidative stress and inflammation [Citation29]. We, in the present study, first proved that HT has a protective effect on ALD by alleviating hepatic steatosis in ethanol-induced HepG2 cells and chronic-binge ethanol-fed mice. In this regard, lower levels of TG, TC and LDL-C and higher level of HDL-C in the HT group were observed when compared to EtOH group. Consistent with previous results, H&E, Tunel staining and ORO analysis showed that HT significantly alleviated irregular arrangement of hepatocytes, apoptosis, lipid steatosis with less and smaller lipid droplets in liver. Besides, ethanol-induced alterations in the liver index as well as serum ALT and AST levels were reversed by HT. SREBP-1c, a family of transcription factor, is responsible for the formation of cholesterol, fatty acid and triglycerides. Our results showed that HT, compared with EtOH, significantly reduced expression of SREBP-1c in vivo and in vitro. FASN, regulated by SREBP-1c and directly associated with production of triglycerides, was inhibited by HT. In addition, it has been demonstrated that the reduction of FASN is attributed to deactivation of AKT, which is consistent with our results [Citation30]. Taken together, these results suggested that HT could alleviate ethanol-induced hepatic steatosis by inhibiting p-AKT/SREBP-1c/FASN pathway.
Acetaldehyde, a product of ethanol metabolic, is extremely toxic and carcinogenic to liver and is a major contributor to oxidative stress. To investigate the effect of HT on oxidative damage due to the production of acetaldehyde, we evaluated protein expression of ADH and CYP2E1 in liver of ethanol-fed mice. As expected, HT could significantly blocked expression of CYP2E1 and ADH when compared with the EtOH group. Moreover, higher expression of ALDH, which plays a crucial role in converting acetaldehyde to acetic acid, was observed in the HT group. These results indicated that HT could slow down the production of acetaldehyde and accelerate metabolism of acetaldehyde simultaneously. Consistent with previous results, declined activity of CAT and GSH/GSSG ratio and increased level of MDA were detected after ethanol exposure. Administration of HT, however, effectively prevented the production of MDA and supplemented antioxidant enzymes. Importantly, levels of CAT activity and ratio of GSH/GSSG in HT group were also significantly higher than the EtOH group. Taken together, these results indicated that HT could significantly alleviate ethanol-induced oxidative stress.
The long-term excessive alcohol intake induces accumulation of immune cells and triggers inflammatory response in liver [Citation31]. Activation of STAT3 results in a marked increase in hepatic inflammatory cell infiltration [Citation32]. Downregulation of STAT3 target genes could enhance the hepatic anti-inflammatory function [Citation33]. Our results showed that the phosphorylation of STAT3 was inhibited by HT significantly. We found that HT interacted with STAT3 tightly by van der Waals and pi-Alkyl using molecule docking. As an important protein regulated by STAT3, iNOS usually contributes to liver injury by producing a large amount of NO upon activation. Here, iNOS expression was also decreased significantly by HT treatment in liver tissue. As a result, the mRNA levels of three key inflammatory cytokines (tnf-α, il-1β and il-6) were inhibited significantly by HT in the liver of ethanol-fed mice. Collectively, HT showed well inhibitory activity against ethanol-induced inflammation in the liver tissue through suppressing STAT3/iNOS pathway.
However, it exists several limitations in the present study. CAT is the main enzyme for the decomposition of H2O2, whose increased activity is usually considered as the acceleration of H2O2 decomposition and the alleviation of oxidative stress. However, some peroxidases besides CAT also contributing to the consumption of H2O2, the effect of which on H2O2 in the ALD model should be explored in the following study. What’s more, we use an assay kit to determine the amount of NO in the body by detecting the amount of NO2-. But there is a limitation that the reduction of metallic cadmium may be unstable resulting in the incomplete reduction of NO3− to NO2−. Although this will not affect the experimental conclusions, we will actively improve if there is a more accurate method to detect NO in the body.
5. Conclusion
In conclusion, our study revealed that HT exerted a protective effect against ethanol-induced liver injury by inhibiting hepatic steatosis, oxidative stress and excessive inflammation both in vitro and in vivo. Down-regulating of p-AKT/SREBP-1c/FASN pathway may play an important role in the HT-mediated protection against ethanol-induced hepatic steatosis. Our study also found HT markedly alleviated oxidative stress by inhibiting CYP2E1 while enhancing ALDH2 activity. In addition, we pointed that HT could interfere STAT3/INOS pathway to resist ethanol-induced inflammation by the interaction with STAT3. Collectively, it is plausible to point that the therapy of HT could be a potential therapeutic way for the treatment of ALD.
Ethics approval statement
The study was approved by the Animal Care and Protection Committee of Gulou Hospital, Nanjing University (SYXK 2004-0013) and the related ethical regulations of our university. All methods of the study are reported in accordance with ARRIVE guidelines.
Acknowledgements
We thank Yiwei Zhang and Wenjie Wang (Nanjing Forestry University) for technical assistance. Authors’ contributions: Xianying Fang: Conceptualization, Methodology, Writing - original draft, Validation. Jiamin Cao: Data curation, Methodology, Writing - review & editing. Yuan Dai: Resources. Zhi Tao: Conceptualization, Methodology. Linguo Zhao: Formal analysis, Conceptualization.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that data presented in this study are available in article.
Additional information
Funding
References
- Ran B, Guo CE, Li W, et al. Sea buckthorn (Hippophae rhamnoides L.) fermentation liquid protects against alcoholic liver disease linked to regulation of liver metabolome and the abundance of gut microbiota. J Sci Food Agric. 2021;101(7):2846–2854.
- Fialla AD, Israelsen M, Hamberg O, et al. Nutritional therapy in cirrhosis or alcoholic hepatitis: a systematic review and meta-analysis. Liver Int. 2015;35(9):2072–2078.
- Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124–134.
- Cheng CF, Pan TM. Monascus-fermented red mold dioscorea protects mice against alcohol-induced liver injury, whereas its metabolites ankaflavin and monascin regulate ethanol-induced peroxisome proliferator-activated receptor-γ and sterol regulatory element-binding transcription factor-1 expression in HepG2 cells. J Sci Food Agric. 2018;98(5):1889–1898.
- Singal AK, Bataller R, Ahn J, et al. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113(2):175–194.
- Zhao L, Wang S, Zhang N, et al. The beneficial effects of natural extracts and bioactive compounds on the gut-liver axis: a promising intervention for alcoholic liver disease. Antioxidants (Basel). 2022;11(6):1211.
- Li XX, Jiang ZH, Zhou B, et al. Hepatoprotective effect of gastrodin against alcohol-induced liver injury in mice. J Physiol Biochem. 2019;75(1):29–37.
- Yuan R, Tao X, Liang S, et al. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed Pharmacother. 2018;99:537–542.
- Dunn W, Shah VH. Pathogenesis of alcoholic liver disease. Clin Liver Dis. 2016;20(3):445–456.
- Hosseini N, Shor J, Szabo G. Alcoholic hepatitis: a review. Alcohol Alcohol. 2019;54(4):408–416.
- Cui Y, Ye Q, Wang H, et al. Hepatoprotective potential of aloe vera polysaccharides against chronic alcohol-induced hepatotoxicity in mice. J Sci Food Agric. 2014;94(9):1764–1771.
- Feng R, Chen JH, Liu CH, et al. A combination of Pueraria lobata and Silybum marianum protects against alcoholic liver disease in mice. Phytomedicine. 2019;58:152824.
- Wang GY, Chen SY, Chen YY, et al. Protective effect of rosmarinic acid-rich trichodesma khasianum clarke leaves against ethanol-induced gastric mucosal injury in vitro and in vivo. Phytomedicine. 2021;80:153382.
- Muramatsu M, Kuriyama K, Yuki T, et al. Hepatic lipogenesis and mobilization of peripheral fats in the formation of alcoholic fatty liver. Jpn J Pharmacol. 1981;31(6):931–940.
- Hsieh PF, Jiang WP, Basavaraj P, et al. Cell suspension culture extract of Eriobotrya japonica attenuates growth and induces apoptosis in prostate cancer cells via targeting SREBP-1/FASN-driven metabolism and AR. Phytomedicine. 2021;93:153806.
- Porstmann T, Santos CR, Griffiths B, et al. SREBP activity is regulated by mTORC1 and contributes to akt-dependent cell growth. Cell Metab. 2008;8(3):224–236.
- Kesar V, Odin JA. Toll-like receptors and liver disease. Liver Int. 2014;34(2):184–196.
- Wang W, Zhang Y, Cao J, et al. Extracts of waste from poplar wood processing alleviate experimental dextran sulfate-induced colitis by ameliorating oxidative stress, inhibiting the Th1/Th17 response and inducing apoptosis in inflammatory lymphocytes. Antioxidants (Basel). 2021;10(11):1684.
- Negrin KA, Roth Flach RJ, DiStefano MT, et al. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One. 2014;9(9):e107265.
- Akcora Ö, Vassilios Gabriël A, Ortiz-Perez A, et.al. Pharmacological inhibition of STAT3 pathway ameliorates acute liver injury in vivo via inactivation of inflammatory macrophages and hepatic stellate cells. FASEB Bioadv. 2020;2(2):77–89.
- Tejada S, Pinya S, Del Mar Bibiloni M, et al. Cardioprotective effects of the polyphenol hydroxytyrosol from olive oil. Curr Drug Targets. 2017;18(13):1477–1486.
- Robles-Almazan M, Pulido-Moran M, Moreno-Fernandez J, et al. Hydroxytyrosol: bioavailability, toxicity, and clinical applications. Food Res Int. 2018;105:654–667.
- Bertelli M, Kiani AK, Paolacci S, et al. Hydroxytyrosol: a natural compound with promising pharmacological activities. J Biotechnol. 2020;309:29–33.
- Gori M, Giannitelli SM, Zancla A, et al. Quercetin and hydroxytyrosol as modulators of hepatic steatosis: a NAFLD-on-a-chip study. Biotechnol Bioeng. 2021;118(1):142–152.
- Liu Z, Wang N, Ma Y, et al. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front Microbiol. 2019;10:390.
- Echeverría F, Valenzuela R, Bustamante A, et al. High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism: attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration. Food Funct. 2019;10(9):6170–6183.
- Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34.
- Stickel F, Datz C, Hampe J, et al. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver. 2017;11(2):173–188.
- Ambade A, Lowe P, Kodys K, et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology. 2019;69(3):1105–1121.
- Zhang C, Hu J, Sheng L, et al. Ellagic acid ameliorates AKT-driven hepatic steatosis in mice by suppressing de novo lipogenesis via the AKT/SREBP-1/FASN pathway. Food Funct. 2019;10(6):3410–3420.
- Zamani-Garmsiri F, Hashemnia SMR, Shabani M, et al. Combination of metformin and genistein alleviates non-alcoholic fatty liver disease in high-fat diet-fed mice. J Nutr Biochem. 2021;87:108505.
- Esmail MM, Saeed NM, Michel HE, et.al. The ameliorative effect of niclosamide on bile duct ligation induced liver fibrosis via suppression of NOTCH and Wnt pathways. Toxicol Lett. 2021;347:23–25.
- Xie Y, Li J, Qin H, et al. Paramylon from Euglena gracilis prevents lipopolysaccharide-induced acute liver injury. Front Immunol. 2021;12:797096.