ABSTRACT
Background
Acquired aplastic anemia (AA) is a life-threatening disease associated with an imbalance in Th17/Treg cells. Regulating this balance may be an effective treatment approach for AA. Rhodiola rosea has shown efficacy in AA treatment, but its mechanisms remain unclear.
Purpose
We investigated salidroside's effect (a component of Rhodiola rosea) on Th17/Treg balance in adult AA patients and a mouse model.
Methods
HIF-1α mRNA and protein levels were measured in AA patients' peripheral blood. Flow cytometry, qRT-PCR, and WB analyzed salidroside's impact on T cell differentiation, Th17 cells, Treg cells, STAT3, HIF-1α, and RORγt expression. ELISA measured hematopoietic growth factors in mouse serum.
Results
AA patients exhibited elevated HIF-1α levels. Salidroside improved hematopoietic function, increasing blood cell count and enhancing bone marrow. Salidroside induced SCF, TPO, and IL-3 expression while inhibiting IL-2 in mice. Salidroside reduced STAT3, HIF-1α, RORγt, and IL-17a, while increasing FoxP3 expression, correcting the Th17/Treg imbalance in vitro and in vivo.
Conclusion
Salidroside has potential as a novel AA treatment by correcting the Th17/Treg imbalance through the STAT3/HIF-1α/RORγt pathway.
Introduction
Aplastic anemia (AA) is an immune-mediated disorder characterized by peripheral blood pancytopenia and bone marrow (BM) hypoplasia [Citation1] due to active destruction of hematopoietic cells by effector T lymphocytes [Citation2]. Solomou et al.[Citation3] found that the number of regulatory T cells (Treg cells), which control and suppress autoreactive T cells, was decreased at disease presentation in almost all AA patients examined [Citation4]. T helper (Th)17 cells and Treg cells, which share a common precursor cell type (naïve CD4+ T cells), require a common tumor growth factor (TGF)-β signal for their initial differentiation [Citation5–7]. However, terminally differentiated cells exhibit opposite functions: Th17 cells cause autoimmunity and inflammation, whereas Treg cells inhibit these phenomena to maintain immune homeostasis [Citation8,Citation9]. Thus, the Th17/Treg cell balance is essential for preserving autoimmunity and tolerance. Along with a decreased in Treg cells, the frequency and the total number of Th17 cells are increased in patients with AA, which correlated with disease activity [Citation10]. Considering that the Th17/Treg imbalance is involved in the pathogenesis of AA, targeted inhibition of Th17 cells and upregulation of Treg cells may be effective methods to correct this imbalance and treat AA.
Interleukin (IL)−17 is the most important effector secreted by Th17 cells, and neutralizing anti-IL-17 antibodies have proven to be effective to modulate Th17 immune responses. For example, a single injection of anti-IL-17 antibody reduced the number of Th17 cells in mice with rheumatoid arthritis, and prevented the occurrence of joint inflammation and bone destruction [Citation11]. Multiple injections of anti-IL-17 antibody also effectively reduced autoimmune inflammatory injury and nervous system symptoms in mice with experimental encephalomyelitis [Citation12]. To explore the mechanism of Th17 cells in AA, de Latour et al. [Citation10] depleted Th17 cells using anti-IL-17 injections in a mouse model, which led to increased BM cellularity and reduced bleeding areas on day 10. In addition to using neutralizing antibodies to deplete Th17 cells, the inhibition of Th17 differentiation may also be an effective strategy for AA treatment. Dang et al. [Citation13] discovered that hypoxia inducible factor (HIF)−1α promotes Th17 differentiation by directly inducing retinoic acid receptor-related orphan receptor (ROR)γt transcription and subsequently collaborating with RORγt to regulate downstream Th17 genes. Moreover, HIF-1α inhibits Treg cell differentiation through an active process that targets Foxp3 protein for degradation. Based on the above results, we speculated that inhibiting HIF-1α may inhibit Th17 differentiation and induce Treg generation, thereby correcting the Th17/Treg balance in patients with AA.
Natural products have been a major source of new drugs for centuries. Most of the compounds discovered to inhibit HIF-1α are natural products or synthetic compounds with structures based on natural product leads, as summarized in recent reviews [Citation14–18]. In this study, we focused on Rhodiola rosea and its active compound salidroside [2-(4-hydroxyphenyl)ethyl β-d-glucopyranoside]; see the structure in . Rhodiola rosea is a relatively rare and high-value medicinal plant that grows at high altitudes (up to 2280 m) in the Arctic and mountainous regions of Asia, Europe, and North America [Citation19]. Recent studies have revealed various medicinal properties and biological activities of this plant, including anti-inflammatory [Citation20], anti-aging [Citation21], anti-stress [Citation21], anti-fatigue [Citation19, Citation22], antioxidant [Citation23], anti-viral [Citation24], anti-cancer [Citation24,Citation25], and cancer chemopreventive [Citation21] effects, along with effects of increasing immunity [Citation21, Citation24], enhancing DNA repair [Citation21, Citation26], and modulating adaptation to hypoxia [Citation27] and angiogenesis [Citation28].
Rhodiola rosea is particularly well known for its ability to enhance adaptation to high-altitude and hypoxic conditions. The master regulators of the adaptive response to hypoxia are heterodimeric transcription factors consisting of O2-regulated α subunits (HIF1A/HIF-1α or EPAS1/HIF-2α) and a constitutively expressed ARNT/HIF-1β subunit [Citation29]. Qi et al. [Citation30] reported that an aqueous extract of the Tibetan herb Rhodiola algida var. tangutica downregulated HIF-1α and HIF-2α expression under hypoxic conditions in breast cancer MCF7 cells. In addition, Zhang et al. [Citation31] found that salidroside improved the hypoxic state induced by cobalt chloride and the transdifferentiation of normal rat proximal renal tubular epithelial cells. The mechanism was suggested to involve inhibition of TGF-β1 and HIF-1α expression. These results led us to hypothesize that Rhodiola rosea can theoretically inhibit HIF-1α, thereby inhibiting Th17 differentiation, offering a potential drug candidate for treating AA by regulating the Th17/Treg balance.
Based on the above research results and hypotheses, we aimed to answer the following questions that have not been clarified to date: (1) Is the increased proportion of Th17 cells accompanied by high HIF-1α expression in patients with AA? (2) Does salidroside inhibit HIF-1α expression, thereby inhibiting Th17 differentiation to correct the Th7/Treg imbalance? To address these questions, we evaluated the HIF-1α expression in patients with AA. We further established in vitro and in vivo models of AA to investigate the effects of salidroside on T cell differentiation, and signal transducer and activator of transcription (STAT3), HIF-1α, RORγt, IL-17a and FoxP3 expression. These findings can provide new insight into the mechanism of action of salidroside and a theoretical basis for its potential in treating AA.
Materials and methods
Patients and control subjects
Peripheral blood samples were collected from nine patients with newly diagnosed acquired AA and nine healthy controls. The AA patients were pancytopenia with median white blood cell counts of 2.05 (range 0.52–4.1) × 109/L, median hemoglobin of 73.22 (range 35–112) g/L and median platelet count of 18.56 (range 5–40) × 109/L. Informed consent was obtained in line with the Declaration of Helsinki and the protocols were approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (approval number: K[2020]123). The AA diagnosis was based on the Guidelines for the Diagnosis and Management of Adult Aplastic Anemia [Citation32].
Animals and cells
Healthy female BALB/c mice and DAB/2 mice (18–22 g, 6–8 weeks old) were purchased from Charles River (Beijing, China). Animal studies were conducted in compliance with the Institutional Animal Care and Use Committee of Southern Medical University. Inguinal, brachial, and axillary lymph node cells (LNs) were obtained from DAB/2 mice.
Induction of the BM failure mouse model and treatment grouping
To induce BM failure as an AA model, recipient BALB/c mice were exposed to 5 Gy of total body irradiation, and then the isolated LNs from DAB/2 donor mice were infused into the recipients by tail vein injection at 5.0 × 106 cells per mouse. The AA mice were randomly allocated into three groups (n = 5 per group): (1) AA model group, (2) 50 mg/kg salidroside group, and (3) 100 mg/kg salidroside group. Normal (non-irradiated or infused) mice were used as a control group. Salidroside (C14H20O7, purity 99.79%, Macklin, Shanghai, China) was dissolved in phosphate-buffered saline (PBS) and delivered to the mice orally at 50 mg/kg or 100 mg/kg daily for 15 consecutive days, starting 1 h after the LNs injection. The mice in the AA model group and control group were administered PBS orally instead of salidroside. Blood was collected from the orbital vein of all mice on days 0, 5, 10, and 15 after the LNs infusion. Peripheral blood cell counts were measured using a veterinary blood cell analyzer (Mindray) to observe pancytopenia development. On the 16th day, 24 h after the last dose was administered, the mice were sacrificed to extract BM cells from the femurs. The spleen was collected and lymphocytes were extracted for various analyses specified in each experiment described below.
Effect of salidroside on T cell differentiation in vitro
Peripheral blood lymphocytes from patients with AA were collected and induced to differentiate into Th17 cells in vitro with or without salidroside intervention. Th17-skewing conditions consisted of Iscove’s modified Dulbecco’s medium supplemented with 5% fetal bovine serum, 20 ng/ml IL-6, 2.5 ng/ml TGF-β (Peprotech),and 10 μg/ml neutralizing antibodies against IFNγ, IL-4, and IL-12 (Biolegend). After six days of culture, the cells in each group were prepared with a single-cell suspension for various analyses.
Flow cytometry
Th17 and Treg cells were stained according to the manufacturer’s instructions (MultiSciences) and analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, U.S.A.). CD4, CD25, and Foxp3 expression of Treg cells were examined in peripheral blood mononuclear cells (PBMCs) by 3-color flow cytometry. To detect Th17 cell differentiation, the cells were stimulated for 4 h with 25 ng/ml phorbol myristate acetate and 1 µg/ml ionomycin in the presence of 2 µM monensin. CD3ϵ, CD4, and IL-17A representing Th17 cell expression in PBMCs were detected by 3-color flow cytometry.
Western blot
Total proteins were extracted using RIPA lysis buffer (Thermofisher) from lymphocytes collected from vitro experiments and isolated from mice spleen. Bradford protein assay was used to measure the protein concentration. The proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto polyvinylidene difluoride (PVDF) membranes. After being blocked with 5% nonfat dry milk, the membranes were incubated with primary antibodies against HIF-1α, RORγt, FoxP3 and p-STAT3/STAT3 (all from Abcam, Cambridge, MA), followed by secondary antibody horseradish peroxidase-conjugated goat anti-rabbit IgG. The bands were quantified using Quantity One 4.62 software (Bio-Rad Laboratories, Inc., Hercules, CA). β-actin served as the loading control.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol Reagent (Tiangen, Beijing). cDNA was synthesized using a QuantScript RT Kit (Tiangen). The cDNA was then used as a template in qRT-PCR with FastFire qPCR PreMix (Tiangen) on an ABI StepOne Real-Time PCR system (Thermo Fisher). HIF-1α, FoxP3, IL-17A, and RORγt mRNA levels were determined by the 2–ΔΔCt method and normalized to the level of the β-actin gene. The primer sequences are shown in .
Table 1. The primer sequences of mouse (m) and human (h) origins used in qRT-PCR.
Serum preparation and ELISA assays
Blood samples were collected from the orbital venous plexus while the mice were in diestrus. After standing at room temperature for at least 1 h, serum from samples was harvested by centrifugation at 4000 rpm for 10 min. Serum SCF, TPO, IL-2 and IL-3 in mice models after 15 days of treatment were detected by ELISA kits (MEIMIAN, China) according to the manufacturer’s instructions.
BM morphology and pathology
The BM cells were removed from the right femurs of the mice and smeared. Cell morphology was observed under a microscope after staining with Wright’s Giemsa stain. The left femurs of the mice were fixed, decalcified, paraffin-embedded, sliced in semi-thin sections, and stained with hematoxylin–eosin. The degree of myeloproliferative, hematopoietic tissue capacity, and sinusoid structure of the BM were observed under a microscope.
Statistical analysis
Statistical analysis was performed using Stata13.0 and GraphPad 6.00 software. All experiments were independently repeated three times and performed in triplicate. Continuous data are reported as mean ± standard deviation (SD) and were determined using the two-tailed t-test or one-way analysis (ANOVA). The Bonferroni method was used for pairwise comparisons of multiple groups compared with ANOVA. Statistical significance was set at P < 0.05.
Results
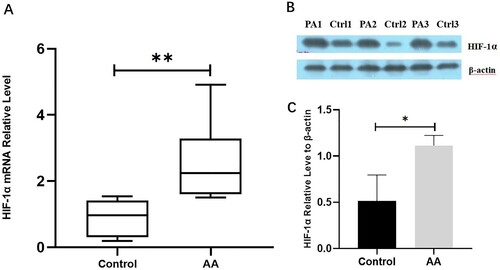
Increased HIF-1α in CD4+ T cells from patients with acquired AA
All AA patients examined (n = 9) had significantly increased relative HIF-1α mRNA compared with those of the healthy controls (A). We selected three patients with AA to test HIF-1α protein levels. The HIF-1α protein level (expressed relative to the level of the control β-actin) was also higher than that of the controls (B and C).
Figure 2. HIF-1α expression increased in patients with AA. (A) Relative HIF-1α mRNA levels in AA patients were higher than those in the controls (P < 0.01). (B) HIF-1α protein expression for three patients with AA increased relative to that of controls. (C) HIF-1α protein levels relative to β-actin were higher than in AA patients than those of healthy controls (P < 0.05).
Salidroside accelerated hematopoietic recovery from BM failure in mice
Mice treated with salidroside recovered faster from BM failure on day 10 compared with the control group (treated with PBS), as determined by comparison of the cell counts in the blood. Total white cell, hemoglobin, and platelet counts were all higher in the salidroside group than in the AA model group, reaching statistical significance for white cells (P = 0.005) and platelets (P = 0.028) in the 50 mg/kg salidroside group, and for white cells (P = 0.000), hemoglobin (P = 0.046), and platelets (P = 0.005) in the 100 mg/kg salidroside group. The leukocyte count in the 100 mg/kg salidroside group was significantly higher than that in the 50 mg/kg salidroside group (P = 0.005). There were no significant differences in hemoglobin and platelet counts between the two groups (P > 0.05). However, compared with those of the control group, the blood cell counts of the two salidroside groups were significantly lower on the 10th day (all P < 0.05; A–C).
Figure 3. Blood counts, bone marrow smear, and bone marrow pathological changes after salidroside treatment in the AA mice model. (A) White blood cell counts at different time points after salidroside administration. (B) Hemoglobin at different time points after salidroside administration. (C) Platelet count at different time points after salidroside administration. #, P < 0.05 compared with the control group; *, P < 0.05 compared with the AA group. (D) Blood cell smears in each group after the 15 days of administration (150×). (E) Bone marrow pathology in each group after the 15 days of administration (400×).
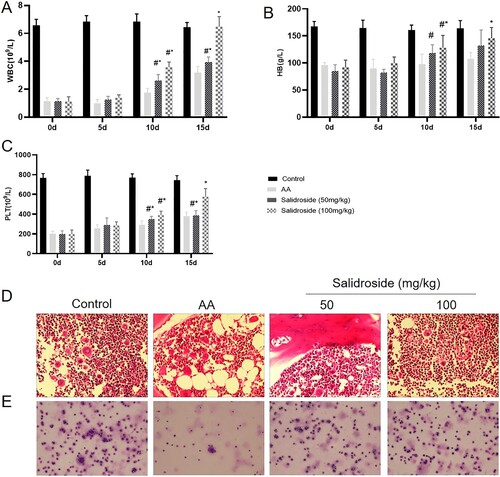
On the 15th day, the blood counts of the mice in the salidroside groups increased. White cells in the 50 mg/kg salidroside group were significantly higher than those in the AA model group (P = 0.016). In contrast, total white cell, hemoglobin, and platelet counts were higher in the 100 mg/kg salidroside group than in the AA model group (all P < 0.05). The leukocyte and platelet counts in the 100 mg/kg salidroside group were significantly higher than those in the 50 mg/kg salidroside group (both P < 0.01). Compared with those of the control group, leukocyte and platelet counts were lower in the 50 mg/kg salidroside group (both P < 0.01), and there were no significant differences in hemoglobin levels among groups. The leukocytes and hemoglobin in the 100 mg/kg salidroside group recovered from BM failure, and there was no significant difference from those of the control group. However, the platelet count remained lower than that in the control group (P = 0.007) (A–C).
In addition, on day 15, the BM smear and histological examination of mice with BM failure revealed increased BM cellularity and decreased adipose tissue after treatment with salidroside (D and E).
Salidroside inhibits HIF-1α and regulates the Th17/Treg balance
To investigate whether salidroside could regulate the balance between Th17 and Treg cells, we first determined the Th17 and Treg cell proportions in the in vitro cell experiment using flow cytometry. As shown in (A and B), salidroside (50 and 100 μM) could inhibit the differentiation of Th17 cells compared to that of induced cultured Th17 cells (P = 0.011 and P = 0.000, respectively). After salidroside treatment (50 and 100 μM), the Th17 cell proportion was close to that detected in the normal culture group (P > 0.05), indicating that the Th17-induced medium effect on T cells could be offset by salidroside. In the Th17-inducing medium system, an increased Treg cell proportion was observed in the salidroside 100 μM group (P = 0.007) (A and B). However, the other groups did not show significant differences, possibly due to the use of Th17 induction medium. These results suggested that salidroside inhibited the differentiation of T lymphocytes into Th17 cells and upregulated Treg cell proportions.
Figure 4. Salidroside inhibits HIF-1α and regulates the Th17/Treg balance in the in vitro cell experiment. (A) The proportion of Th17 and Treg cells in the peripheral blood of AA patients analyzed by flow cytometry. (B) Quantitative analysis of the proportion of Th17 and Treg cells in the peripheral blood of AA patients. *, P < 0.05 compared with the induced cultured Th17 group. (C) Quantitative analysis of the determination of HIF-1α, IL-17a, RORγt, and Foxp3 by qRT-PCR in lymphocytes. *, P < 0.05 compared with the induced cultured Th17 group. (D) Detection of HIF-1α, IL-17a, RORγt, Foxp3, p-JAK2, JAK2, p-STAT3, and STAT3 proteins by WB. (E) Statistical diagram of relative expression levels of HIF-1α, IL-17a, RORγt, Foxp3, p-JAK2, JAK2, p-STAT3, and STAT3 proteins.*, P < 0.05 compared with the induced cultured Th17 group.
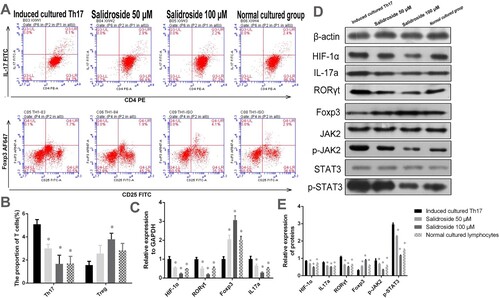
To determine whether HIF-1α plays an important role in the regulation of T cells by salidroside, we analyzed the relative mRNA and protein expression levels of HIF-1α in T cells using qRT-PCR and WB, respectively. The HIF-1α mRNA expression level in the T lymphocytes of the salidroside-treated groups (50 and 100 μM) was significantly lower than that in the induced cultured Th17 group (P = 0.000) (C). In addition, compared with the induced cultured Th17 group, the RORγt and IL-17 mRNA expression levels (as the specific transcription factor receptor and characteristic marker of Th17 cells, respectively) in T cells showed a decreasing trend in the salidroside groups (P < 0.05). The Foxp3 mRNA expression level (as the key transcription factor of Tregs) in T cells increased in the salidroside groups (50 and 100 μM) (P < 0.05), consistent with the increase in Treg cell percentage detected by flow cytometry (C). Similarly, WB results showed that HIF-1α, RORγt, and IL-17a protein expression in T cells was inhibited by salidroside, whereas Foxp3 protein expression was upregulated by salidroside (all P = 0.000, D and E). These results suggested that salidroside could inhibit HIF-1α and RORγt expression and improve the expression of Foxp3, leading to a change in the balance between Th17 and Treg cells.
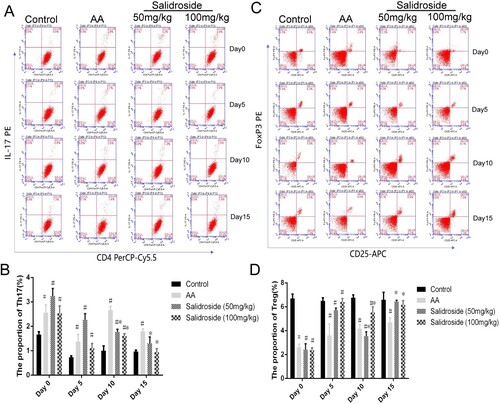
To further determine whether HIF-1α mediated the effects of salidroside on the Th17/Treg balance, we conducted experiments in mice with BM failure. On days 5, 10, and 15 after the intervention, we collected the peripheral blood of the mice for flow cytometry detection. We found that salidroside (50 and 100 mg/kg) could inhibit the proliferation of Th17 cells at days 10 and 15, compared with that of the AA model group (A and B, P < 0.05). In particular, the Th17 cell proportion in the mice approached that of normal mice at day 15. No inhibition of Th17 cells was observed on day 5, and a similar effect was observed in peripheral blood cell counts. The upregulation of Treg cells by salidroside was also observed on days 10 and 15 (C and D; P < 0.05). On day 15, the Treg cell proportion in the salidroside group was close to that in the control group (P > 0.05), suggesting that the effect of salidroside was optimal on day 15.
Figure 5. Proportion of T cells in the peripheral blood of mice treated with salidroside at different times. (A) The proportion of Th17 cells in mice analyzed by flow cytometry. (B) Quantitative analysis of the proportion of Th17 cells. #, P < 0.05 compared with the control group; *, P < 0.05 compared with the AA group. (C) Proportion of Treg cells in mice analyzed by flow cytometry. (D) Quantitative analysis of the proportion Treg cells. #, P < 0.05 compared with the control group; *, P < 0.05 compared with the AA group.
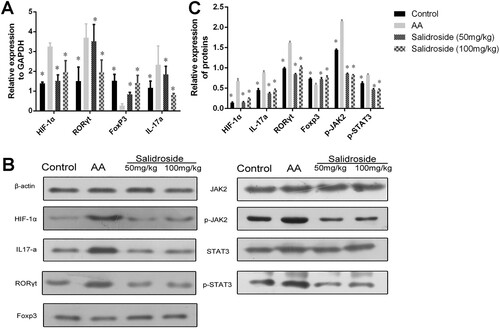
Next, we explored the effect of salidroside on the expression of HIF-1α, RORγt, IL-17a, and Foxp3 in T lymphocytes isolated from the spleens of the mice, using qRT-PCR and WB. As shown in (A), compared with the AA model group, salidroside (50 and 100 mg/kg) significantly inhibited HIF-1α, RORγt, and IL-17a expression (P < 0.05). However, Foxp3 expression increased in the salidroside groups (P < 0.05). High-dose salidroside (100 mg/kg) had a significant effect on RORγt and Foxp3 expression, which did not differ significantly from the levels in the control mice. The same effects of salidroside on HIF-1α, RORγt, IL-17a, and Foxp3 expression were detected at the protein level by WB (B and C).
Figure 6. Determination of related cytokines of the STAT3/HIF-1α/RORγt signaling pathway in mice measured by qRT-PCR and WB. (A) Quantitative analysis of the determination of HIF-1α, IL-17a, RORγt, and Foxp3 mRNA levels by qRT-PCR. *, P < 0.05 compared with the AA group. (B) Detection of HIF-1α, IL-17a, RORγt, Foxp3, p-JAK2, JAK2, p-STAT3, and STAT3 proteins by WB. (C) Quantitative analysis of the relative expression of HIF-1α, IL-17a, RORγt, Foxp3, p-JAK2, JAK2, p-STAT3, and STAT3 proteins.*, P < 0.05 compared with the AA group.
Salidroside inhibits STAT3/HIF-1α/RORγt signaling
Given the observed effects of salidroside on regulation of the Th17/Treg cell balance and downregulation of HIF-1α, we were motivated to further explore whether salidroside works through the STAT3/HIF-1α/RORγt signaling pathways. We detected JAK2, phosphorylated (p)-JAK2, STAT3, and p-STAT3 expression in T lymphocytes from AA patients and mice using WB. The results showed that p-JAK2 and p-STAT3 expression levels decreased significantly in the salidroside groups (P = 0.000, D, E, B and C), with minimal differences in JAK2 and STAT3 expression among the groups. These results suggested that STAT3/HIF-1α/RORγt signaling might be inhibited by salidroside, as evidenced by decreased p-JAK2 and p-STAT3 in cells, leading to Th17 cell reduction.
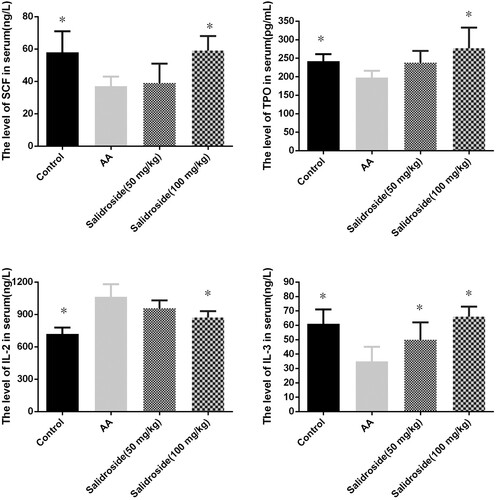
Salidroside promotes the secretion of SCF, TPO, and IL-3, while inhibiting IL-2
In order to investigate the effects of salidroside administration on hematopoietic factors, serum SCF, TPO, IL-2 and IL-3 were measured in each group of mice models. As shown in the , compared with the control group, mice in the AA group had a significant decrease in serum SCF, TPO, and IL-3 (P < 0.05), while IL-2 was significantly elevated (P < 0.05). Compared to the AA group, the serum levels of SCF, TPO, and IL-3 of mice in the 100 mg/kg salidroside group were significantly increased (P < 0.05), while IL-2 was significantly decreased (P < 0.05). The therapeutic effect of salidroside in the 50 mg/kg group is worse than that in the 100 mg/kg group, but it can significantly increase serum IL-3 compared to the AA group (P < 0.05). These results indicate that salidroside may also promote hematopoiesis by regulating hematopoietic factors.
Discussion
Previous studies have confirmed that immune abnormalities in patients with AA include an imbalance of the Th17/Treg cell ratio, which manifests as a decrease in the Treg cell proportion in the peripheral blood and an increase in Th17 cells [Citation4, Citation10]. Dang et al. [Citation13] identified HIF-1α as a major player in developing Th17 cells. Ben-Shoshan et al. [Citation33] demonstrated that hypoxia determines the anti-inflammatory program by driving HIF-1α expression, thereby increasing the number and suppressive properties of CD4 + CD25+ Tregs. Both of these studies thus demonstrated the crucial role of HIF-1α in modulating the Th17/Treg balance. You et al. [Citation34] found that the plasma proteome and metabolome of patients with AA differed from those of healthy donors in pathways involved in T-cell activation, including HIF-1 signaling pathways. These studies led us to suspect the involvement of HIF-1α in AA pathogenesis. Our clinical observations confirmed that HIF-1α gene and protein expression levels are increased in the peripheral blood of AA patients compared with those of healthy controls. Based on a search of the literature, to our knowledge, this is the first report of abnormal HIF-1α expression in AA patients, which may provide a valuable direction for targeting and inhibiting Th17 differentiation and regulating the Th17/Treg balance to effectively treat AA.
For centuries, Rhodiola has been used as a traditional herbal medicine with proven safety and health benefits. In this study, firstly, we used salidroside, the main active component of Rhodiola rosea, which is water-soluble and highly bioavailable via oral administration [Citation21] to treat an AA mouse model mice by gavage. We regularly monitored the blood cell counts to verify the therapeutic effect of salidroside on AA. The blood cell count of the AA mice gradually increased after the 10th day. On the 15th day, white blood cells and hemoglobin recovered, and platelets also increased significantly, although to a lesser extent than those in the healthy control group. As a disease characterized by pancytopenia, the blood cell count increase in AA model mice directly verifies the therapeutic effect of salidroside on AA. This is the first study to report that salidroside can effectively improve AA in a mouse model, based on significant improvement in blood cell counts.
We then explored the possible mechanism of action of salidroside in cell experiments and animal models. The results showed that the HIF-1α mRNA and protein expression levels decreased after salidroside treatment, indicating that salidroside can inhibit HIF-1α. Similarly, a decrease in the RORγt expression and Th17 cells proportion was detected. In contrast, the Treg cell proportion increased. Yang et al. [Citation35] demonstrated that reducing HIF-1α expression in BM mesenchymal stem cells by chronic intermittent hypobaric hypoxia pretreatment had anti-AA effects in a rat model. Our results suggest that salidroside plays a role in treating AA by inhibiting HIF-1α expression, thereby inhibiting RORγt transcription and Th17 cell differentiation to ultimately regulate the Th17/Tregs balance. Whether salidroside inhibits or upregulates HIF-1α expression is controversial, and the results reported in different studies vary depending on cancer or normal physiological functions [Citation21]. For cancer cells, Rhodiola rosea extracts and salidroside were found to inhibit the mTOR pathway [Citation36,Citation37] and angiogenesis [Citation28, Citation38], and downregulated HIF-1α/HIF-2α expression [Citation30, Citation39, Citation40]. Under normal physiological function, Rhodiola rosea extracts and salidroside were reported to activate the mTOR pathway to protect brain neurons from ischemic injury [Citation41], protect against oxidative endothelial injury [Citation42–44], increase erythropoietin mRNA expression by inducing HIF-1α protein accumulation in human embryonic kidney fibroblast (HEK293 T) cells [Citation45], stimulate paracrine function, and promote neovascularization by inhibiting PHD3 and stabilizing HIF-1α proteins in the skeletal muscles [Citation46].
STAT3-mediated pathways promote pathological immune responses [Citation47–49]. Harris et al. [Citation50]. provided in vivo evidence that the fundamental role of STAT3 signaling in autoimmunity is related to its absolute requirement for generating Th17 cell responses. Studies have also suggested that the STAT3/IL-17 signaling pathway is associated with AA occurrence [Citation51,Citation52]. Dang et al. [Citation13] confirmed that HIF-1α expression is upregulated in a STAT3-dependent manner in T cells under Th17-skewing conditions. The above studies suggest that the inhibition of HIF-1α by salidroside in an AA mouse model may act through the STAT3 pathway. Therefore, we further explored whether salidroside functions through the STAT3/HIF-1α/RORγt signal pathways. The results showed that salidroside could inhibit STAT3 phosphorylation (p-STAT3) in both the cell experiments and mouse models. This is consistent with a previous study showing that salidroside inhibited STAT3 nuclear localization, thereby inhibiting the JAK2-STAT3 pathway [Citation53]. These results suggest that STAT3/HIF-1α/RORγt signaling might be inhibited by salidroside, leading to Th17 cell reduction.
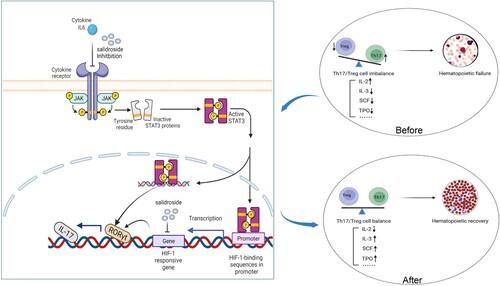
IL-6 is a key factor in inflammation, autoimmunity, and cancer [Citation54], and its effect is mainly exerted through the IL-6-STAT3 pathway [Citation55–58]. IL-6, through the JAK-mediated phosphorylation of STAT3, is required for Th17 cell generation and participates in autoimmune diseases [Citation9, Citation13]. Gupta et al. [Citation59] observed high IL-6 and IL-8 levels in children with AA, which correlated with disease severity, suggesting that these factors play an important role in AA. Several previous studies have confirmed that salidroside can inhibit IL-6 in inflammation, lung and liver injury, Alzheimer’s disease, inflammatory bowel disease, or tumors [Citation53]. Based on our findings and the above literature reports regarding how salidroside inhibits the STAT3/HIF-1α/RORγt pathway, we speculated that there are two mechanisms to explain the observed effects. First, salidroside inhibits IL-6, which inhibits JAK-mediated STAT3 phosphorylation and reduces Th17 cell generation. Second, we cannot rule out the possibility that salidroside or its metabolites may bind to HIF-1α to directly inhibit it, thereby inhibiting RORγt transcription and Th17 cell generation. Using an auto-dock to determine the binding patterns of small molecules to proteins, Yan et al. [Citation60] found that salidroside exhibited strong binding ability to HIF-1α through molecular docking analysis. However, whether salidroside or its metabolites can enter cells and bind to HIF-1α requires further investigation. Based on our findings and the above analysis, the possible mechanism of the salidroside-mediated regulation of Th17/Treg cells in AA is schematically presented in .
Figure 8. Mechanistic model for the multifactorial role of salidroside in modulating the Th17/Treg balance and improve hematopoiesis.
This study found that after treatment with salidroside, the hemoglobin levels of AA model mice returned to normal. Interestingly, on the 15th day, the hemoglobin levels of mice in the 50 and 100 mg/kg salidroside groups returned to normal. In contrast, leukocytes in the 50 mg/kg salidroside group were lower than those in the control group. This suggests that salidroside may promote erythropoiesis recovery through other mechanisms than immune regulation. Qian et al. [Citation61] investigated the effects of salidroside on oxidative stress-induced apoptosis in human erythrocytes. They found that salidroside significantly increased cell survival and prevented human erythrocytes from undergoing erythropoiesis mediated by H2O2.
Furthermore, another study by the same group showed that salidroside promoted erythropoiesis in erythropoietin (EPO)-treated cells, reducing the number of apoptotic cells in TF-1 erythroblasts after H2O2 treatment [Citation62]. EPO, produced by the kidneys and liver in humans, is a glycoprotein hormone that promotes red blood cell formation in the BM [Citation63]. EPO increases blood cell production by resisting erythroid progenitor cell survival, and inducing their differentiation and proliferation [Citation64]. In addition, in a rat model of pulmonary fibrosis injury, Tang et al. [Citation65] found that salidroside could inhibit the TGF-β1/SMAD-2/−3 pathway, which has also been demonstrated by other groups, thereby enabling erythroid maturation using late-stage erythroblast differentiation and thus improving anemia [Citation66,Citation67]. However, whether salidroside promotes erythrocyte recovery by inducing EPO or inhibiting the TGF-β1/SMAD-2/−3 pathway in this AA mouse model requires further investigation.
Given the complexity of the organism and the pleiotropy of the drug, the regulation of hematopoiesis by salidroside may not be explained by a single mechanism. Therefore, we also evaluated the effect of salidroside on several key stem cell growth factors in mice models. The results suggested that salidroside could promote the production of SCF, TPO and IL-3 while inhibiting IL-2. SCF is a cytokine found in haematopoietic stem cells (HSCs) and causes proliferation and differentiation of cells [Citation68]. TPO is vital for the maintenance of HSCs, it can truly be described as a pan-haematopoietic cytokine [Citation69]. In comparison with other hematopoietic growth factors, IL-3 preferentially supports the proliferation and differentiation of progenitors at early stages of hematopoietic development [Citation70]. In addition, IL-3 exerts a wide spectrum of biological activities on various target cell populations, including T cells, B cells, eosinophils, basophils and monocytes [Citation70]. However, IL-2 may have an important association with the marrow failure of AA patients and thus can help in disease development [Citation71]. Macheevsky et al. [Citation72] found that neutralization of IL-2 with a recombinant humanized anti-IL-2 receptor antibody (daccizumab) resulted in a therapeutic response in patients with moderate AA. The above results show that in addition to immune regulation, salidroside can also play a therapeutic role in AA by regulating hematopoietic growth factors. And the specific mechanisms need to be further explored.
In conclusion, this study provides the first evidence that HIF-1α gene and protein expression is elevated in the peripheral blood of patients with AA compared with that in healthy controls. This is also the first report of the use of salidroside in treating an AA mouse model, resulting in significantly improved blood cell counts. Further mechanistic studies showed that salidroside might inhibit the differentiation of Th17 cells through the STAT3/HIF-1α/RORγt pathway to regulate the Th17/Treg balance, thereby exerting a therapeutic effect. In addition, salidroside may regulate hematopoietic growth factors and promote erythroid hematopoiesis in aplastic mice through other mechanisms. Though our research also has some limitations, for example, in animal experiments, we did not set HIF-1α inhibitors as positive drug controls. We still believe that this study will be valuable in providing new insight into the pathogenesis of AA and offering directions for exploring new therapeutic methods.
Submission declaration
We declare that its publication has been approved by all the authors.
Acknowledgements
The authors would like to thank Editage for the language editing provided for this manuscript. Author contributions: Zenghui Liu and Yang Xiao were mainly responsible for project design, completion, article writing, and the acquisition of financial support. Ling Ouyang, Yu Zhan, and Xuekui Gu were mainly responsible for clinical patient enrollment. Wancheng Chen and Lixuan Chen mainly assisted in the completion of experiments, and proofread the article. Defang Xiong was responsible for obtaining and analyzing the pathological sections and images of mice. Bone marrow cell smears and images were obtained and analyzed by Jiduo Liu. Gao Tianqi was responsible for proofreading, formatting, and submission of the article. Xiaozhen Li and Yanqun Zhou assisted in the completion of cell and animal experiments. Ziyuan Lu mainly assisted in letter reply and ELISA testing of supplementary experiments. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–1656.
- Young NS, Maciejewski J. The pathophysiology of acquired aplastic anemia. N Engl J Med. 1997;336(19):1365–1372. doi:10.1056/NEJM199705083361906
- Solomou EE, Rezvani K, Mielke S, et al. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110(5):1603–1606. doi:10.1182/blood-2007-01-066258
- Solomou EE, Rezvani K, Mielke S, et al. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110(5):1603–1606. doi:10.1182/blood-2007-01-066258
- Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238(1):247–262. doi:10.1111/j.1600-065X.2010.00951.x
- Mangan PR, Harrington LE, 'Quinn O, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi:10.1038/nature04754
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi:10.1038/nature04753
- Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci. 2018;19(3):730–744.
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi:10.1016/j.cell.2010.02.021
- de Latour RP, Visconte V, Takaku T, et al. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood. 2010;116(20):4175–4184. doi:10.1182/blood-2010-01-266098
- Koenders MI, Lubberts E, Oppers-Walgreen B, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167(1):141–149. doi:10.1016/S0002-9440(10)62961-6
- Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237(2):123–130. doi:10.1016/j.cellimm.2005.11.002
- Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi:10.1016/j.cell.2011.07.033
- Nagle DG, Zhou YD. Natural product-based inhibitors of hypoxia-inducible factor-1 (HIF-1). Curr Drug Targets. 2006;7(3):355–369. doi:10.2174/138945006776054979
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi:10.1038/nrc1187
- Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2(10):803–811. doi:10.1038/nrd1199
- Powis G, Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol Cancer Ther. 2004;3(5):647–654. doi:10.1158/1535-7163.647.3.5
- Yeo EJ, Chun YS, Park JW. New anticancer strategies targeting HIF-1. Biochem Pharmacol. 2004;68(6):1061–1069. doi:10.1016/j.bcp.2004.02.040
- Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17(7):481–493. doi:10.1016/j.phymed.2010.02.002
- Pu WL, Zhang MY, Bai RY, et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed Pharmacother. 2020;121:109552. doi:10.1016/j.biopha.2019.109552
- Li Y, Pham V, Bui M, et al. Rhodiola rosea L.: an herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr Pharmacol Rep. 2017;3(6):384–395. doi:10.1007/s40495-017-0106-1
- Olsson EM, von Scheele B, Panossian AG. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009;75(2):105–112. doi:10.1055/s-0028-1088346
- Kosakowska O, Baczek K, Przybyl JL, et al. Antioxidant and antibacterial activity of roseroot (Rhodiola rosea L.) dry extracts. Molecules. 2018;23(7):1767–1781.
- Khanna K, Mishra KP, Ganju L, et al. Golden root: a wholesome treat of immunity. Biomed Pharmacother. 2017;87:496–502. doi:10.1016/j.biopha.2016.12.132
- Song D, Zhao M, Feng L, et al. Salidroside attenuates acute lung injury via inhibition of inflammatory cytokine production. Biomed Pharmacother. 2021;142:111949. doi:10.1016/j.biopha.2021.112045
- Li X, Sipple J, Pang Q, et al. Salidroside stimulates DNA repair enzyme parp-1 activity in mouse HSC maintenance. Blood. 2012;119(18):4162–4173.
- Xie N, Fan F, Jiang S, et al. Rhodiola crenulate alleviates hypobaric hypoxia-induced brain injury via adjusting NF-kappaB/NLRP3-mediated inflammation. Phytomedicine. 2022;103:154240.
- Radomska-Lesniewska DM, Skopinski P, Balan BJ, et al. Angiomodulatory properties of Rhodiola spp. and other natural antioxidants. Cent Eur J Immunol. 2015;40(2):249–262.
- Kumar H, Choi DK. Hypoxia inducible factor pathway and physiological adaptation: a cell survival pathway? Mediators Inflamm. 2015;2015:584758. doi:10.1155/2015/520618
- Qi YJ, Cui S, Lu DX, et al. Effects of the aqueous extract of a Tibetan herb, Rhodiola algida var. tangutica on proliferation and HIF-1alpha, HIF-2alpha expression in MCF-7 cells under hypoxic condition in vitro. Cancer Cell Int. 2015;15:81. doi:10.1186/s12935-015-0225-x
- Zhang L, Xie XS, Li FY, et al. Effects of salidroside on tubular epithelial to myofibroblast transition under cobaltous chloride induced hypoxic status. Sichuan Da Xue Xue Bao Yi Xue Ban. 2010;41(1):43–48.
- Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187–207. doi:10.1111/bjh.13853
- Ben-Shoshan J, Maysel-Auslender S, Mor A, et al. Hypoxia controls CD4 + CD25 + regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008;38(9):2412–2418. doi:10.1002/eji.200838318
- You X, Yang Q, Yan K, et al. Multi-omics profiling identifies pathways associated with CD8(+) T-cell activation in severe aplastic anemia. Front Genet. 2021;12:790990.
- Yang J, Zhang L, Wang H, et al. Protective effects of chronic intermittent hypobaric hypoxia pretreatment against aplastic anemia through improving the adhesiveness and stress of mesenchymal stem cells in rats. Stem Cells Int. 2017;2017:5706193.
- Liu Z, Li X, Simoneau AR, et al. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol Carcinog. 2012;51(3):257–267. doi:10.1002/mc.20780
- Fan XJ, Wang Y, Wang L, et al. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3 K/Akt/mTOR pathway. Oncol Rep. 2016;36(6):3559–3567. doi:10.3892/or.2016.5138
- Skopinska-Rozewska E, Malinowski M, Wasiutynski A, et al. The influence of Rhodiola quadrifida 50% hydro-alcoholic extract and salidroside on tumor-induced angiogenesis in mice. Pol J Vet Sci. 2008;11(2):97–104.
- Chen X, Kou Y, Lu Y, et al. Salidroside ameliorated hypoxia-induced tumorigenesis of BxPC-3 cells via downregulating hypoxia-inducible factor (HIF)-1alpha and LOXL2. J Cell Biochem. 2020;121(1):165–173. doi:10.1002/jcb.29000
- Qin Y, Liu HJ, Li M, et al. Salidroside improves the hypoxic tumor microenvironment and reverses the drug resistance of platinum drugs via HIF-1alpha signaling pathway. EBioMedicine. 2018;38:25–36. doi:10.1016/j.ebiom.2018.10.069
- Zhong X, Lin R, Li Z, et al. Effects of Salidroside on cobalt chloride-induced hypoxia damage and mTOR signaling repression in PC12 cells. Biol Pharm Bull. 2014;37(7):1199–1206. doi:10.1248/bpb.b14-00100
- Tang Y, Vater C, Jacobi A, et al. Salidroside exerts angiogenic and cytoprotective effects on human bone marrow-derived endothelial progenitor cells via Akt/mTOR/p70S6 K and MAPK signalling pathways. Br J Pharmacol. 2014;171(9):2440–2456. doi:10.1111/bph.12611
- Xu MC, Shi HM, Wang H, et al. Salidroside protects against hydrogen peroxide-induced injury in HUVECs via the regulation of REDD1 and mTOR activation. Mol Med Rep. 2013;8(1):147–153. doi:10.3892/mmr.2013.1468
- Zheng XT, Wu ZH, Wei Y, et al. Induction of autophagy by salidroside through the AMPK-mTOR pathway protects vascular endothelial cells from oxidative stress-induced apoptosis. Mol Cell Biochem. 2017;425(1-2):125–138. doi:10.1007/s11010-016-2868-x
- Zheng KY, Zhang ZX, Guo AJ, et al. Salidroside stimulates the accumulation of HIF-1alpha protein resulted in the induction of EPO expression: a signaling via blocking the degradation pathway in kidney and liver cells. Eur J Pharmacol. 2012;679(1-3):34–39. doi:10.1016/j.ejphar.2012.01.027
- Zhang J, Kasim V, Xie YD, et al. Inhibition of PHD3 by salidroside promotes neovascularization through cell-cell communications mediated by muscle-secreted angiogenic factors. Sci Rep. 2017;7:43935. doi:10.1038/srep43935
- Krause A, Scaletta N, Ji JD, et al. Rheumatoid arthritis synoviocyte survival is dependent on Stat3. J Immunol. 2002;169(11):6610–6616. doi:10.4049/jimmunol.169.11.6610
- Harada T, Kyttaris V, Li Y, et al. Increased expression of STAT3 in SLE T cells contributes to enhanced chemokine-mediated cell migration. Autoimmunity. 2007;40(1):1–8. doi:10.1080/08916930601095148
- Frisullo G, Angelucci F, Caggiula M, et al. pSTAT1, pSTAT3, and T-bet expression in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J Neurosci Res. 2006;84(5):1027–1036. doi:10.1002/jnr.20995
- Harris TJ, Grosso JF, HR Y, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179(7):4313–4317. doi:10.4049/jimmunol.179.7.4313
- Gu Y, Hu X, Liu C, et al. Interleukin (IL)-17 promotes macrophages to produce IL-8, IL-6 and tumour necrosis factor-alpha in aplastic anaemia. Br J Haematol. 2008;142(1):109–114. doi:10.1111/j.1365-2141.2008.07161.x
- Gao M, Ge M, Huo J, et al. Leptin-mediated proinflammatory bone marrow environment in acquired aplastic anemia. Cytokine. 2022;152:155829.
- Qi Z, Qi S, Ling L, et al. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol. 2016;35:265–271. doi:10.1016/j.intimp.2016.04.004
- Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33(3):127–148. doi:10.1093/intimm/dxaa078
- Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi:10.1016/j.ccr.2009.01.001
- Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi:10.1038/nrclinonc.2018.8
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19(21):2548–2556. doi:10.1038/sj.onc.1203551
- Jenkins BJ, Roberts AW, Greenhill CJ, et al. Pathologic consequences of STAT3 hyperactivation by IL-6 and IL-11 during hematopoiesis and lymphopoiesis. Blood. 2007;109(6):2380–2388. doi:10.1182/blood-2006-08-040352
- Gupta V, Kumar S, Sonowal R, et al. Interleukin-6 and interleukin-8 levels correlate with the severity of aplastic anemia in children. J Pediatr Hematol Oncol. 2017;39(3):214–216. doi:10.1097/MPH.0000000000000724
- Yan X, Liu J, Zhu M, et al. Salidroside orchestrates metabolic reprogramming by regulating the Hif-1alpha signalling pathway in acute mountain sickness. Pharm Biol. 2021;59(1):1540–1550.
- Qian EW, Ge DT, Kong SK. Salidroside protects human erythrocytes against hydrogen peroxide-induced apoptosis. J Nat Prod. 2012;75(4):531–537. doi:10.1021/np200555s
- Qian EW, Ge DT, Kong SK. Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin. J Ethnopharmacol. 2011;133(2):308–314. doi:10.1016/j.jep.2010.09.025
- Dame C, Kirschner KM, Bartz KV, et al. Wilms tumor suppressor, Wt1, is a transcriptional activator of the erythropoietin gene. Blood. 2006;107(11):4282–4290. doi:10.1182/blood-2005-07-2889
- Sasaki R, Masuda S, Nagao M. Erythropoietin: multiple physiological functions and regulation of biosynthesis. Biosci Biotechnol Biochem. 2000;64(9):1775–1793. doi:10.1271/bbb.64.1775
- Tang H, Gao L, Mao J, et al. Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-kappaB and TGF-beta1/smad-2/-3 pathways. Cell Stress Chaperones. 2016;21(2):239–249. doi:10.1007/s12192-015-0654-4
- Suragani RN, Cadena SM, Cawley SM, et al. Transforming growth factor-beta superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med. 2014;20(4):408–414. doi:10.1038/nm.3512
- Verma A, Suragani RN, Aluri S, et al. Biological basis for efficacy of activin receptor ligand traps in myelodysplastic syndromes. J Clin Invest. 2020;130(2):582–589. doi:10.1172/JCI133678
- Khodadi E, Shahrabi S, Shahjahani M, et al. Role of stem cell factor in the placental niche. Cell Tissue Res. 2016;366(3):523–531. doi:10.1007/s00441-016-2429-3
- Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. Br J Haematol. 2014;165(2):259–268. doi:10.1111/bjh.12772
- Yang YC, Clark SC. Human interleukin 3: analysis of the gene and its role in the regulation of hematopoiesis. Int J Cell Cloning. 1990;8(Suppl 1)):121–128, 128–129.
- De R, Dutta A, Dolai TK, et al. Comparative study of bone marrow and blood plasma levels of IL-2 in aplastic anaemia and their relationship with disease severity. Hematology. 2019;24(1):84–88. doi:10.1080/10245332.2018.1512391
- Maciejewski JP, Sloand EM, Nunez O, et al. Recombinant humanized anti-IL-2 receptor antibody (daclizumab) produces responses in patients with moderate aplastic anemia. Blood. 2003;102(10):3584–3586. doi:10.1182/blood-2003-04-1032