ABSTRACT
Fecal microbiota is a significant factor determining the cause, course, and prognosis of Crohn’s disease (CD). However, the factors affecting mucosa-associated microbiota (MAM) remain unclear. This retrospective study examined the differences in ileal MAM between CD patients and healthy controls and investigated the factors affecting MAM in CD patients to clarify potential therapeutic targets. Ileal MAM was obtained using brush forceps during endoscopic examination from 23 healthy controls and 32 CD patients (most were in remission). The samples’ microbiota was profiled using the Illumina MiSeq platform. Compared to controls, CD patients had significantly reduced α-diversity in the ileum and a difference in β-diversity. The abundance of butyric acid-producing bacteria in the ileal MAM was significantly lower in CD patients with a history of abdominal surgery than in those without. Because butyric acid is a major energy source in the intestinal epithelium, its metabolism via β-oxidation increases oxygen consumption in epithelial cells, reducing oxygen concentration in the intestinal lumen and increasing the abundance of obligate anaerobic bacteria. The suppression of obligate anaerobes in CD patients caused an overgrowth of facultative anaerobes. Summarily, reducing the abundance of butyric acid-producing bacteria in the ileal MAM may play an important role in CD pathophysiology.
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) that causes severe mucosal damage with deep and full-thickness inflammation in any part of the digestive system from the mouth to the anus. The most commonly affected areas are the terminal ileum of the small intestine and colon, where chronic inflammation and CD relapse can cause intestinal strictures, fistulas, abscesses, or painful anal lesions, necessitating repeated surgical operations [Citation1].
In Japan, CD mainly affects the younger generation, with the highest incidence occurring in individuals aged 20–24 years [Citation2]. The main symptoms of CD, including frequent diarrhea, abdominal pain, and malabsorption, significantly impact the quality of life of the younger generation, resulting in suboptimal productivity [Citation3] and an increased economic burden. CD incidence is rising globally, particularly in Asian, South American, and African countries [Citation4]. Therefore, identifying this disease’s underlying cause and developing an effective cure is critical.
Over the last 20 years, biological therapy, including anti-tumor necrosis factor-α (TNF-α), anti-interleukin 12/23 p40, and anti-integrin antibodies, has appeared in CD therapy. Although half of the patients with CD achieve prolonged clinical remission using these therapies, concerns exist regarding adverse events, including allergic infusion reactions, infection, and malignancy. Half of these patients also do not respond to these therapies or lose response over time [Citation5]. These biologics, including short-chain fatty acid-producing bacteria, reportedly affect the gut microbiota composition [Citation6].
Although previous studies have identified several potential causes of CD, including mucosal immune dysregulation, genetic susceptibility, the influence of exposomes, and intestinal dysbiosis, the etiology of CD is not yet fully understood [Citation3]. Intestinal epithelium is generally covered with a thick mucus layer that prevents the entry of intra-luminal contents, such as bile acids, toxins, or pathogenic bacteria, into the intestinal mucosa. However, some bacteria, such as mucosa-associated microbiota (MAM), resides in the mucus. The gut microbiota comprises two components as follows: intra-luminal microorganisms and MAM. Most studies on the intestinal microbiota have been conducted using only feces, which is the final discharge of intra-luminal microorganisms. However, MAM is believed to directly impact not only on epithelial and mucosal function but also on the immune system due to its proximity to the epithelium and may be more deeply involved in the pathophysiology of CD [Citation7]. Recently, we reported that patients with CD show lower α- and β-diversity indices than healthy controls. Particularly, among patients with CD, those who were treated with anti-TNF-α therapy showed a significantly lower abundance of butyric acid-producing bacteria genera than those without anti-TNF-α therapy. Therefore, some factors in patients with CD or therapies may affect the composition of MAM. However, the factors affecting MAM have not yet been fully elucidated.
Several CD types, such as ileal-, ileocolonic-, and colonic- type, exhibit distinct epidemiological, clinical, and pathogenetic characteristics [Citation8], including response to therapy [Citation9]. Studies have also shown variations in the microbiome between the ileum- and colon-dominant CD types [Citation10, Citation11]. However, these studies only analyzed the microbiota in feces without considering MAM.
Therefore, this retrospective study aimed to analyze the differences in ileal MAM between patients with CD and healthy controls while investigating the factors that might affect MAM in patients with CD to clarify potential therapeutic targets.
Materials and methods
Patients and sample collection
Overall, 23 healthy controls undergoing routine medical checkups and 32 patients with CD who visited Kawasaki Medical School between February 2016 and November 2021 were investigated in this study. We excluded patients with CD who took antibiotics within 3 months, those who did not consent to sample collection, and those who did not undergo endoscopic examination. We also excluded healthy controls who reported gastrointestinal symptoms, received medication, or had colonoscopy findings of tumor or inflammatory lesions. CD is classified into three types (ileal, ileocolonic, and colonic) based on the dominant location of mucosal inflammation. Additionally, we evaluated MAM based on factors, including ileal stenosis and intestinal surgery history Since this study is retrospective, the information about dietary fiber and antioxidant supplement use could not be acquired in medical records.
Brush samples were collected from the less inflamed mucosa in the terminal ileum (30 cm from the ileocecal valve) using an endoscopic microbiology brush (COOK, Bloomington, IN, USA), following the usual colonoscopy preparation using polyethylene glycol (PEG). Reportedly, no difference exist in the microbial diversity in biopsy samples obtained from the ileum, ascending colon, descending colon, or rectum of patients with CD [Citation7]. This study was approved by the Ethics and Medical Research Committee of the Kawasaki Medical School (IRB no.: 5733-01), and written informed consent was obtained from each participant before enrollment.
DNA extraction and 16S rRNA sequencing
The brush samples were stored in a sterile nucleic acid stabilizer (guanidium thiocyanate, Tris-HCL, and -EDTA). The methods for extraction of DNA, preparation of a library of amplicons encoding the 16S rRNA gene, and sequencing were as previously described [Citation12]. Microbial DNA was extracted and purified from ileal MAM using QuickGene DNA Tissue kit SII (KURABO, Osaka, Japan). Briefly, bacterial cell lysis was achieved by bead beating in the lysis buffer containing guanidium thiocyanate, and genomic DNA was then be purified using silica membrane column. The DNA concentration was quantified using IMPLEN NanoPhotometer (Munchen, Bavaria, Germany). Microbial DNA was used to amplify the V3–V4 region of the 16S rRNA gene using the primer set 341F (5′ – CCTACGGGNGGCWGCAG – 3′) and 805R (5′ – GACTACHVGGGTATCTAATCC – 3′) [Citation13]. PCR was performed using 25 μL reactions (2.5 μL DNA template, 3 μM of each primer, and 12.5 μL 2×KAPA HiFi HotStart ReadyMix (Nippon Genetics Co., Ltd, Japan)). The cycling parameters were as follows: 4 min initial denaturation at 95˚C, 35 cycles of denaturation at 95˚C for 30 s, annealing at 55˚C for 30 s, extension at 72˚C for 30 s, and a final extension at 72˚C for 5 min. Amplicons were purified by NucleoFast96 PCR plate (TaKaRa bio, Shiga, Japan) and then subjected to PCR with unique dual indices primer set. Resulted amplicons were purified using AMPure XP beads (Beckman-Coulter, Brea, CA, USA) and pooled, followed by 285 bp paired-end sequencing on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) with MiSeq Reagent Kit v3.
Bioinformatic analysis
Sequence data were analyzed using QIIME2 (ver. 2021.11) [Citation14]. First, the sequence was denoised using the DADA2 plugin of QIIME2 and converted into amplicon sequence variants (ASVs). Subsequently, we used the SILVA database (138; 99% Operational Taxonomic Units (OTUs) full-length sequences) and the sklearn-classifier to classify the ASVs taxonomically. To ensure the accuracy of the result, we removed singletons and ASVs assigned to mitochondria and chloroplasts. Subsequently, a phylogenetic tree was generated using SATé-enabled phylogenetic placement (SEPP) [Citation15].
α- and β-Diversities
The metrics for alpha diversity were calculated using QIIME2 by setting the sampling depth to 5000. The observed species, Chao1, and Shannon indices were calculated using the phyloseq package in R software. The beta diversity were estimated based on Bray-Curtis distances and tested by principal coordinate analysis (PCoA) using vegan packages in R software. Statistical differences of beta diversity were analyzed by permutational multivariate analysis of variance (PERMANOVA) with 9,999 permutations using QIIME version 1.9.1.
Statistical analysis
Values are presented as mean ± standard deviation (SD), while categorical data are expressed as counts with percentages. The chi-square test was used to analyze categorical data. The Kruskal–Wallis test was used to compare the diversity and relative abundance of bacterial genera among the three groups with different CD types. Welch’s t-test of R software (ver. 4.2.1) was used to compare the two groups. The β-diversity, Bray–Curtis dissimilarity matrices were calculated, and the difference between the groups was assessed using PERMANOVA, which was implemented using vegan package (ver. 2.5.6) in the R software. Significance was considered at p < 0.05. Furthermore, nonparametric analysis was performed when data were not normally distributed.
Results
Patient characteristics
Overall, 32 patients with CD and 23 healthy controls were enrolled, with the control group having a significantly lower mean age (56.2 ± 12.5 years) and percentage of females than the CD group (39.5 ± 17.2 years) ().
Table 1. The characteristics of healthy control and patients with Crohn’s disease.
In the CD group, the mean Crohn’s disease activity index (CDAI) was 100.5, and five patients exhibited ˃150 CDAI, indicating that most of the patients were in remission. The numbers of patients with ileal-, ileocolonic-, and colonic-CD type were 6, 22, and 4, respectively, suggesting that most (87.5%) of the patients had ileum-associated disease. Regarding medication use, 12 (37.5%), 5 (1.6%), and 11 (34.4%) patients were taking 5-aminosalicylates (5-ASA), azathioprine, and probiotics (Clostoridium butyricum Miyairi 588 strain), respectively [Citation16], and 22 (68.8%) received anti-TNF-α or ustekinumab therapy. Eight (25.0%) patients with CD had a history of intestinal surgery, and 12 (41.0%) had stenosis. Ileum brush samples were obtained from all patients.
Comparative analyses of the α- and β- diversity indices of patients with CD and healthy controls
Patients with CD displayed significantly reduced diversity in terms of the Shannon index (p = 0.006) but not the Chao 1 index (p = 0.06), compared with controls in the MAM of the ileum ().
Table 2. The difference of α-diversity index in ileal MAM between healthy control and patients with Crohn’s disease.
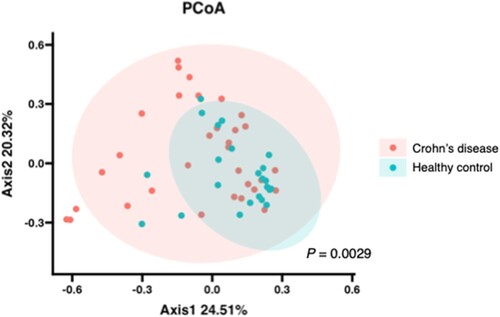
The weighted analysis, which considered the number of leads contained in the OTUs, showed that in the β-diversity analysis, microbial composition was significantly different (p = 0.0029) between the two groups ().
Figure 1. Difference of β-diversity index in MAM between healthy control and patients with Crohn’s disease, Principal coordinate analysis (PCoA) was based on Bray–Curtis dissimilarities in the composition of the ileal MAM. Red and blue dots denote individual samples of patients with Crohn’s disease and healthy controls, respectively. Ellipses indicate 95% confidence intervals. Statistical differences between groups were assessed using PERMANOVA with 9,999 permutations.
Comparison of taxonomic composition at the genus level between patients with CD and healthy controls: a reduced abundance of butyric acid-producing bacteria in patients with CD compared with those in healthy controls
The abundances of g_Clostridium (Lachnospiraceae) and g_Sutterella in the ileum were significantly higher in patients with CD than in healthy controls. In contrast, the abundances of genera Faecalibacterium, Lachnospiraceae (unclassified genera), Blautia, Ruminococcus, Coprococcus, Roseburia, Gemminger, and Butyricicoccus were significantly lower in the ileum of patients with CD than in those of healthy controls (). Among the reduced bacteria, all excluding f_Lachnospiraceae were butyric acid-producing bacteria.
Table 3. Bacterial taxa showing significant difference in relative abundances in ileal MAM between healthy control and patients with Crohn’s disease.
Comparison of taxonomic composition at the genus level among patients with CD according to disease classification: the abundance of some bacteria was significantly different among the three CD types
In the terminal ileum, the abundance of organisms belonging to g_Veillonella, f_Gemellaceae (unclassified genus), f_Actinomycetaceae (unclassified genus), g_cc115 (Erysipelotrichaceae), and f_Christensenellaceae (unclassified genus) was significantly different among the three CD types ().
Table 4. Bacterial taxa showing significant difference in the relative abundances in ileal MAM among types of Crohn’s disease.
Comparison of taxonomic composition at the genus level among patients with CD according to intestinal stenosis: a reduced abundance of Clostridiales in patients with CD with intestinal stenosis
In the terminal ileum, the abundance of organisms belonging to o_Clostridiales (unclassified family/genus) was significantly lower in patients with CD with intestinal stenosis than in those without intestinal stenosis ().
Table 5. Bacterial taxa showing significant difference in relative abundances in ileal MAM between patients with Crohn’s disease with or without stenosis.
Comparison of taxonomic composition at the genus level among patients with CD according to the history of abdominal surgery: a reduced abundance of butyric acid-producing bacteria in CD patients with a history of abdominal surgery
In the terminal ileum, the abundances of organisms belonging to g_Faecalibacterium, g_Dorea, g_Blautia, f_Lachnospiraceae, g_Coprococcus, o_Clostridiales, g_Butyricicoccus, and g_Alistipes were significantly lower in patients with CD with a history of abdominal surgery than in those without (). Bacteria belonging to g_Faecalibacterium, g_Dorea, g_Blautia, g_Coprococcus, and g_Butyricicoccus are butyric acid-producing bacteria ().
Table 6. Bacterial taxa showing significant difference in relative abundances in ileal MAM between patients with Crohn’s disease with or without a history of surgery.
Discussion
Our study revealed a significant reduction in the abundance of butyric acid-producing bacteria in the ileal MAM of patients with CD compared with healthy controls. Additionally, among patients with CD, the reduction in butyric acid-producing bacteria was closely related to the disease location and history of abdominal surgery, suggesting the importance of butyric acid-producing bacteria in CD pathogenesis.
Our findings also showed a significantly lower α-diversity of ileal MAM in patients with CD than in healthy controls and a difference in β-diversity based on the weighted analysis. This suggests that dysbiosis of ‘major species’ occurs in patients with CD and is influenced by CD pathogenesis. This result is consistent with that of a previous report where the microbial composition of patients with CD was different from that of healthy controls determined using feces collected from a cohort of 40 twin pairs [Citation11]. Although they analyzed the intestinal microbiota in the feces of patients with similar genetic backgrounds, the results were similar to ours, where the MAM of individuals with different backgrounds was analyzed. This suggests that genetic background is not greatly involved in CD pathogenesis in Japanese patients. Here, the abundance of butyric acid-producing bacteria was significantly reduced in the MAM of patients with CD.
We also found that among patients with CD, the microbial profiles in the MAM of these patients are significantly different, depending on the predominant disease location. Particularly, the abundance of some bacteria was significantly reduced in ileocolonic CD compared with that in other CD types. A similar result where the reduction in Faecalibacterium and Roseburia was significant in ileal-type CD has been previously reported [Citation11], although the study used feces rather than ileal mucus. Therefore, further investigation should compare the fecal and intestinal mucus. Additionally, the reduction in butyric acid-producing bacteria in the MAM of patients with CD was closely related to the unwanted status of CD, including a history of abdominal surgery, suggesting a protective role for butyric acid in the activity of CD. Butyric acid is usually produced in the intestine through the fermentation of dietary fiber by intestinal bacteria (). It is used to produce energy (adenosine diphosphate to adenosine triphosphate) in the mitochondria of intestinal epithelial cells through the use of oxygen from vessels and maintaining the integrity of epithelial cells, strengthening the function of tight junction protein, and activating regulatory T cells [Citation17–19]. The CO2 produced is released into the intestinal lumen making the intra-luminal environment anoxic, which is important to maintain the normal microbiota ‘eubiosis.’ An anoxic environment is suitable for obligate anaerobes that suppress the growth of facultative anaerobes.
Figure 2. Function of butyric acid-producing bacteria, BA, butyric acid; BA, butyric acid-producing bacteria; ROS, reactive oxygen species.
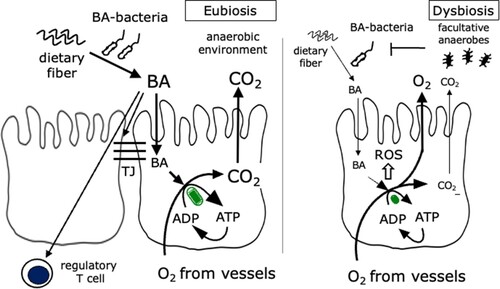
However, a low dietary fiber intake and reduced abundance of butyric acid-producing bacteria result in insufficient oxygen consumption in the mitochondria of intestinal epithelial cells, causing oxidative stress in intestinal cells. Simultaneously, the increased oxygen concentration in the intestinal lumen causes dysbiosis, resulting in the overgrowth of facultative anaerobes and suppressing the adequate growth of obligate anaerobes [Citation20]. Although there is no direct evidence that excessive intra-luminal oxygen results in oxidative stress in the intestinal environment, oxidative stress is considered one of the CD etiologies, and an incomplete oxygen reduction has been shown to correlate with dysbiosis [Citation21]. Oxidative stress-induced damage to the intestinal mucosal layer has been shown to result in bacterial invasion of the mucosa, stimulating the immune response and contributing to disease progression [Citation22]. Evidence has shown that dietary antioxidants can reduce oxidative stress-induced inflammation in the intestinal epithelium and dysbiosis [Citation23]. Here, we found a reduction in the butyric acid-producing bacterial population in the MAM of patients with CD, which might increase intra-ruminal reactive oxygen species production.
This study had some limitations, including a small sample size and significant differences in sex and age between patients with CD and healthy control groups. However, the study showed that the abundance of butyric acid-producing bacteria among patients with CD is significantly lower in the MAM of those with a history of intestinal surgery. It remains unknown which of these observed changes is the cause or consequence of CD; therefore, further large-scale prospective studies should clarify this issue under sex- and age-matched conditions. In this retrospective study, inflammatory markers, including fecal calprotectin and serum leucine-rich α−2 glycoprotein (LRG), were measured in a few patients with CD, most of whom were in remission. Therefore, the relationship between inflammation and MAM is unclear. As MAM was obtained at one-time point in this study, the causal relationship of MAM change with disease progression or remission is unclear.
Additionally, whether the decreased abundance of butyric acid-producing bacteria in patients with CD was correlated with reduced production of butyric acid in the intestine is unclear. To clarify this issue, we quantitatively measured butyric acid in mucus using high-performance liquid chromatography; however, the amount of butyric acid in the mucus sample was relatively small to be measured. Therefore, biopsy samples of ileal mucosa are currently being collected to quantify the amount of butyric acid to evaluate the impact of butyric acid-producing bacteria in the oxidative damage of the ileal mucosal tissue. Besides, the patients took different drugs, which may have affected their MAM composition. In our previous study using brush samples obtained from patients with CD, anti-TNF-α therapy significantly affected the abundance of anti-inflammatory bacteria [Citation6]. A prospective analysis of MAM in patients with CD under the same therapy should be performed. Finally, no evidence indicates that the lower consumption of oxygen in the intestinal epithelial cells and excessive amounts of intra-luminal oxygen cause oxidative stress in the intestinal epithelium and changes in MAM composition. Therefore, we are currently investigating whether the reduction in oxidative stress by the administration of intestine-selective redox nanoparticles [Citation24] can cure intestinal dysbiosis using an animal model.
Conclusively, the study suggests that the reduction in butyric acid-producing bacteria is closely related to the history of abdominal surgery in patients with CD. Therefore, reducing intestinal butyric acid or oxidative stress in the intestine may be a potential treatment target for patients with CD.
Author contributions
Osamu Handa and Akiko Shiotani substantially contributed to the conception, design acquisition of data, analysis, and interpretation of data for the work. Motoyasu Osawa, Hiroshi Matsumoto, and Eiji Umegaki contributed to the sample acquisition and analysis. Tingting Gu greatly contributed to the sample analysis. Osamu Handa was responsible for drafting the manuscript, while Akiko Shiotani, Hiroto Miura, Ryo Inoue, and Yuji Naito critically revised it for important intellectual content. All authors approved the final version of the manuscript for publication and agreed to be accountable for all aspects of the work. They also ensure that questions about the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
Y.N. received scholarship funds from Taiyo Kagaku Co. Ltd. and EA Pharma. Co. Ltd., a collaborative research fund from Taiyo Kagaku Co., Ltd., and lecture fees from Mylan EPD Co. and Takeda Pharma. Co. Ltd., and Mochida Pharma. Co. Ltd., EA Pharma. Co., Ltd., Otsuka Pharma. Co. Ltd., and Miyarisan Pharma. Co. Ltd. This study was partially supported by these funds. Neither the funding agency nor any outside organization participated in the study design or had any competing interests. All companies approved the final version of the manuscript. All authors, except Y.N., received no financial support or otherwise from any organization that may have an interest in the submitted work. No other relationships or activities appear to have influenced the submitted work.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Osamu Handa, upon reasonable request.
Additional information
Funding
References
- Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn's disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol. 2021;19(10):2031–2045.e11. doi:10.1016/j.cgh.2020.10.039.
- Matsuoka K, Fujii T, Okamoto R, et al. Characteristics of adult patients newly diagnosed with Crohn's disease: interim analysis of the nation-wide inception cohort registry study of patients with Crohn's disease in Japan (iCREST-CD). J Gastroenterol. 2022;57(11):867–878. doi:10.1007/s00535-022-01907-2.
- Busch K, da Silva SA, Holton M, et al. Sick leave and disability pension in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2014;8(11):1362–77. doi:10.1016/j.crohns.2014.06.006.
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56–66. doi:10.1038/s41575-020-00360-x.
- Ko Y, Paramsothy S, Yau Y, et al. Superior treatment persistence with ustekinumab in Crohn’s disease and vedolizumab in ulcerative colitis compared with anti-TNF biological agents: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study. Aliment Pharmacol Ther. 2021;54(3):292–301. doi:10.1111/apt.16436.
- Fukushima S, Shiotani A, Matsumoto H, et al. Comparison of mucosa-associated microbiota in Crohn’s disease patients with and without anti-tumor necrosis factor-α therapy. J Clin Biochem Nutr. 2022;70(2):182–188. doi:10.3164/jcbn.21-41.
- He C, Wang H, Liao WD, et al. Characteristics of mucosa-associated gut microbiota during treatment in Crohn's disease. World J Gastroenterol. 2019;25(18):2204–2216. doi:10.3748/wjg.v25.i18.2204.
- Dulai PS, Singh S, Vande Casteele N, et al. Should We divide Crohn's disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol. 2019;17(13):2634–2643. doi:10.1016/j.cgh.2019.04.040.
- Takenaka K, Fujii T, Suzuki K, et al. Small bowel healing detected by endoscopy in patients with crohn's disease after treatment with antibodies against tumor necrosis factor. Clin Gastroenterol Hepatol. 2020;18(7):1545–1552. doi:10.1016/j.cgh.2019.08.024.
- Dicksved J, Halfvarson J, Rosenquist M, et al. Molecular analysis of the gut microbiota of identical twins with Crohn's disease. ISME J. 2008;2(7):716–27. doi:10.1038/ismej.2008.37.
- Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139(6):1844–1854.e1. doi:10.1053/j.gastro.2010.08.049.
- Hayashi A, Mikami Y, Miyamoto K, et al. Intestinal dysbiosis and biotin deprivation induce alopecia through overgrowth of lactobacillus murinus in mice. Cell Rep. 2017;20(7):1513–1524. doi:10.1016/j.celrep.2017.07.057.
- Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1), doi:10.1093/nar/gks808.
- Gao L, Huang Y, Liu Y, et al. Bacterial community structure and potential microbial coexistence mechanism associated with three halophytes adapting to the extremely hypersaline environment. Microorganisms. 2022 May 30;10(6).
- Janssen S, McDonald D, Gonzalez A, et al. Phylogenetic Placement of exact amplicon sequences improves associations with clinical information. mSystems. 2018;3(3).
- Mukai R, Handa O, Suyama Y, et al. Effectiveness of including probiotics to Helicobacter pylori eradication therapies. J Clin Biochem Nutr. 2020;67(1):102–104. doi:10.3164/jcbn.20-37.
- Litvak Y, Byndloss MX, Baumler AJ. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362(6418). doi:10.1126/science.aat9076.
- Wang HB, Wang PY, Wang X, et al. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57(12):3126–35. doi:10.1007/s10620-012-2259-4.
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. doi:10.1038/nature12721.
- Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi:10.1038/s41586-019-1237-9.
- Tomasello G, Mazzola M, Leone A, et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(4):461–466. doi:10.5507/bp.2016.052.
- Bourgonje AR, Feelisch M, Faber KN, et al. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol Med. 2020;26(11):1034–1046. doi:10.1016/j.molmed.2020.06.006.
- Marlow G, Ellett S, Ferguson IR, et al. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn's disease patients. Hum Genomics. 2013;7(1):24), doi:10.1186/1479-7364-7-24.
- Yoshitomi T, Nagasaki Y. Self-Assembling antioxidants for ischemia-reperfusion injuries. Antioxid Redox Signal. 2022;36(1-3):70–80. doi:10.1089/ars.2021.0103.
