ABSTRACT
Introduction: The global threat of a chemical, biological, radiological, or nuclear (CBRN) disaster is an important priority for all government agencies involved in domestic security and public health preparedness. Radiological/nuclear (RN) attacks or accidents have become a larger focus of the United States Food and Drug administration (US FDA) over time because of their increased likeliness. Clinical signs and symptoms of a developing acute radiation syndrome (ARS) are grouped into three sub-syndromes named for the dominant organ system affected, namely the hematopoietic (H-ARS), gastrointestinal (GI-ARS), and neurovascular systems. The availability of safe and effective countermeasures against radiological/nuclear threats currently represents a significant unmet medical need.
Areas covered: This article reviews the development of RN threat medical countermeasures and highlights those specific countermeasures that have been recently patented and approved following the FDA Animal Rule. Patents for such agents from 2015 have been presented.
Expert opinion: Two granulocyte colony-stimulating factor (G-CSF)-based radiation countermeasures (Neupogen® (Amgen, Thousand Oaks, CA) and Neulasta® (Amgen, Thousand Oaks, CA)) have recently been approved by the FDA for treatment of H-ARS and both these agents are radiomitigators, used after radiation exposure. To date, there are no FDA-approved radioprotectors for ARS.
1. Introduction
Uncontrolled, unwanted radiation exposures as a result of radiologic terrorism, military activity, or nuclear accidents present unique challenges to the medical community and to public health authorities. It is widely acknowledged that terrorist organizations have the capability to obtain or to engineer improvised nuclear devices or other types of radiological dispersal devices [Citation1]. Hundreds of nuclear plants worldwide are at risk of a wide range of natural disasters [Citation2]. Accidents or attacks at these locations could expose first responders and surrounding populations to high, potentially lethal doses of ionizing radiation. Radiation injury and illness may occur after exposures to external radiological/nuclear (RN) sources or to internally deposited RN isotopes, as the latter get incorporated into the cells and tissues of the body after ingestion, inhalation, or transdermal absorption [Citation3]. Cells that are generally most sensitive to radiation are those which rapidly divide such as spermatocytes, hematopoietic stem cells, and intestinal crypt cells. At the subcellular level, ionizing radiation damages normal tissue by causing chromosomal aberrations, altering cell-to-cell communication, perturbing essential membrane functions, changing cytokine production, and inducing inflammation, ultimately slowing tissue repair and killing the cells. The extent of ionizing radiation injury varies depending on the distance from the source of the radiation, rate of exposure, the dose of the exposure, and quality of radiation [Citation4]. The LD50 (50% lethal dose) radiation dose (sparsely ionizing, deeply penetrating ionizing radiation) for humans (uncompromised by infection, etc.) is estimated between 3.5 and 4.5 Gy [Citation5–Citation8]. The latter LD50 estimate is based on the assumption that full supportive care has not been provided; however, with increasing levels and qualities of supportive care, the estimated range of the LD50 values increases, namely, 5.0–6.0 Gy with standard regimens of supportive care and 6.0–8.0 Gy with aggressive supportive care including growth factor and blood transfusions [Citation9].
In case of RN exposure, medical care would be focused initially on the treatment of acute radiation syndrome (ARS), also known as acute radiation toxicity or acute radiation sickness. Once the potential clinical complications of suspected ARS are properly attended to, the medical focus will necessarily shift to treatments associated with managing the more chronic, late-arising injuries and disease processes associated with RN exposures. ARS is defined by the Centers for Disease Control and Prevention as ‘an acute illness caused by irradiation of the entire body (or most of the body) by a high dose of penetrating ionizing radiation in a very short period of time (usually a matter of minutes).’ Radiation countermeasures that can lessen or eliminate the impact on health of such types of unwanted RN exposures are urgently needed. The FDA has been and continues to be actively involved in the development of radiation countermeasures. Due to ethical and moral reasons, drugs under development to treat unwanted radiation exposure generally cannot, and should not, be tested for efficacy in healthy human subjects. As a consequence, the FDA developed and implemented an alternative drug approval process involving the use of appropriate animal models to test and evaluate the efficacy of given new medicinals; this new drug approval pathway is commonly referred to as the Animal Rule [Citation10]. This pathway requires efficacy to be demonstrated in one or more animal species that are expected to react with a response predictive for humans, in addition to other criteria [Citation11,Citation12]. Although efforts to produce such medical countermeasures to ARS were initiated more than half a century ago, to date, only granulocyte colony-stimulating factor (G-CSF) (filgrastim, Neupogen) and PEGylated G-CSF (pegfilgrastim, Neulasta) have been approved for hematopoietic acute radiation syndrome (H-ARS) by the FDA () [Citation13–Citation16].
Figure 1. US FDA-approved countermeasures for RN threats following the Animal Rule and other promising agents. In 2002, the FDA issued the Animal Rule to expedite the development of medical countermeasures against CBRN threats. Since then, 2 countermeasures (*) have been approved by the FDA following the Animal Rule.
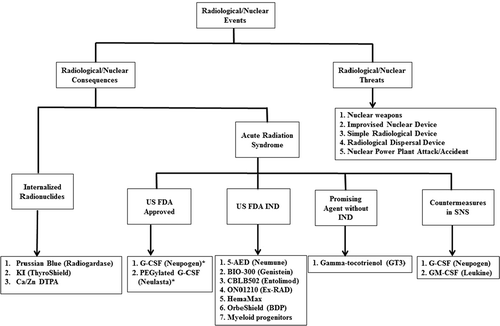
In this second part of our two-part article, we have highlighted only those RN threat countermeasures which have been approved following the FDA Animal Rule [Citation11,Citation12] and agents which have been patented in 2014–2015 for RN threats (). Patent search was executed using the United States Patent and Trademark Office, the European Patent Office (Espacenet), the China Patent and Trademark Office, the Japan Patent Office, and the Canadian Intellectual Property Office websites. We have previously reviewed patents for RN countermeasures during 2011–2014 in recently published articles [Citation13,Citation17].
Table 1. Patents of countermeasures for ionizing radiation.
2. Countermeasures for radiological and nuclear threats
Whole-body or partial-body exposures to intense, high doses of ionizing radiation often result in life-threatening injuries that primarily involve radiosensitive, self-renewing tissues, but most markedly the hematopoietic and gastrointestinal (GI) systems. Low-dose or chronic ionizing radiation exposures, by contrast, are generally considered the domain of ‘late-arising’ neoplastic pathologies (i.e. cancer). Current RN threats can be categorized into five groups: (a) detonation of a sophisticated nuclear weapon (nuclear bomb), (b) detonation of an improvised nuclear device, (c) use of a radiological dispersal device or dirty bomb, (d) use of a simple radiological device, and (e) an attack on a nuclear power plant [Citation18]. ARS is characterized by the differential response of the body’s vital organ systems to various intensities of radiation exposure. There are at least three distinct subsyndromes: hematopoietic, GI, and neurovascular that are all dependent on the total exposure dose, the exposure dose rate, the quality of radiation, and the time and extent of bodily exposure [Citation19–Citation21]. Cutaneous radiation injury is often linked with ARS but is generally not considered a distinct subsyndrome since one can have cutaneous radiation syndrome without manifesting ARS symptoms. Each subsyndrome follows a similar clinical pattern that is divided into three phases: an initial prodromal phase occurring during the first few hours following exposure, a latent phase that shortens with increasing exposure intensity (total dose and dose rate), and a manifest clinical phase.
2.1. ARS
Only two radiomitigators, G-CSF and PEGylated G-CSF, have been approved by the FDA following the agency’s Animal Rule to treat H-ARS. No countermeasures have been FDA approved for the treatment of GI-ARS: radiation-induced neurovascular disorders are largely intractable; therefore, countermeasures for this subsyndrome have not been actively pursued. Radiation countermeasures capable of being administered prior to exposure to protect the population at large from the effects of lethal radiation exposure remain a significant unmet medical need of the US citizenry and have been recognized as a high-priority area by the government [Citation18]. We have limited this article to include agents identified (patents) as potential radiation countermeasures since 2014 (). There are recent, extensive reviews of this subject that list earlier patents [Citation13,Citation14,Citation17,Citation22].
There are several FDA-licensed pharmaceuticals currently available that safely and effectively mitigate the potentially lethal effects of hematopoietic injury following acute ionizing radiation exposure; these agents include, but certainly are not limited to, G-CSF, PEGylated G-CSF, granulocyte-macrophage colony-stimulating factor (GM-CSF, sargramostim, leukine), and a filgrastim biosimilar, tbo-filgrastim that have been used in cases of ARS after radiation accidents [Citation15,Citation23]. These growth factors stimulate bone marrow granulopoiesis and have been used successfully in cancer patients to reduce the incidence, duration, and severity of chemotherapy-induced neutropenia and related complications such as febrile neutropenia. As mentioned above, two of these agents, Neupogen and Neulasta, have been approved by the FDA to treat the severe myelosuppression associated with potentially lethal H-ARS. Seven promising drugs that are relatively new currently have FDA Investigational New Drug (IND) status: 5-androstenediol, BIO 300, CBLB502/Entolimod, Ex-RAD, HemaMax, OrbeShield, and myeloid progenitors [Citation13,Citation22,Citation24]. Neupogen and leukine are already in the Strategic National Stockpile (SNS) [Citation25,Citation26]. Further, γ-tocotrienol has advanced to large animal studies [Citation24]. Amifostine, a well-recognized older, but potent radioprotector, has been approved previously by the FDA for limited clinical use, but not specifically for protection against ARS [Citation22].
2.1.1. G-CSF (Neupogen, filgrastim)
G-CSF has been evaluated as a radiation countermeasure in different strains of mice, canines, nonhuman primates (NHPs), and in the minipig [Citation11,Citation15,Citation27–Citation30]. A majority of these studies have used recombinant G-CSF of human origin because G-CSF is not species specific. The results of these studies suggested that G-CSF consistently enhanced survival and the recovery of blood leukocytes (neutrophils) regardless of radiation source (γ-ray, X-ray, mixed field – neutron and gamma). The demonstrated radiation injury mitigating efficacy of G-CSF was dependent on drug dose, the drug treatment schedule in relation to radiation exposure, duration of the treatment, and the dose of radiation [Citation15]. The estimated dose reduction factors for G-CSF were 1.06 [Citation31], 1.1 [Citation32], or 1.2 [Citation33], depending on G-CSF dose, treatment schedule, route of administration, and strains of mice [Citation34]. G-CSF dose modifying factor has also been determined in canine and NHP models [Citation35,Citation36].
In a Good Laboratory Practice compliant study, critical for FDA approval under the criteria of the Animal Rule, G-CSF significantly increased NHP survival after exposure to 7.5 Gy (LD50/60) of linear accelerator-derived photon radiation compared to vehicle controls [Citation36]. G-CSF also decreased the duration of neutropenia but did not affect the absolute neutrophil count nadir. In this study, G-CSF (10 µg/kg/day) was administered beginning 1 day after irradiation and continued daily until the absolute neutrophil count was >1000/µl for 3 consecutive days.
G-CSF was the first agent to be approved by the FDA with the potential to increase survival in patients (both adult and pediatric) acutely exposed to doses of radiation capable of eliciting H-ARS [Citation23,Citation37]. The FDA approved 10 µg/kg/day of G-CSF as the recommended dose for the H-ARS indication on 30 March 2015. G-CSF has also been used in several radiation accident victims with promising outcomes [Citation15]. Studies showed that maximum efficacy is reached with daily dosing of G-CSF early after irradiation; therefore, a subsequent effort with PEGylated G-CSF focused on simplifying the administration schedule of G-CSF while maintaining an equivalent efficacy profile in lethally irradiated NHPs [Citation23,Citation36].
2.1.2. PEGylated G-CSF (Neulasta/pegfilgrastim)
PEGylated G-CSF is G-CSF molecule with the addition of a 20-kD monomethoxypolyethylene glycol (PEG) molecule to the N-terminal methionine residue [Citation38]. PEGylation of G-CSF results in a lower rate of receptor-mediated clearance compared to G-CSF. Consequently, a single dose of PEGylated G-CSF was shown to be as effective as repeated daily doses of G-CSF in reducing the relative risk of febrile neutropenia, decreasing the duration of neutropenia, and shortening absolute neutrophil recovery time after myelosuppressive chemotherapy [Citation15]. Studies in mice and NHPs have shown the comparable efficacy of one or two doses of PEGylated G-CSF, administered weekly, relative to conventional daily administration of G-CSF in reversing neutropenia and enhancing survival, when administered after total-body irradiation [Citation15,Citation16,Citation39–Citation41]. One study demonstrated that two injections of PEGylated G-CSF, separated by 7 days, stimulate granulopoiesis and significantly enhance neutrophil recovery after total-body irradiation in a NHP low-lethality model of H-ARS [Citation39]. In a recent study, two injections of PEGylated G-CSF significantly enhanced survival of NHPs exposed to mid-lethal (LD50/60 for H-ARS) doses of radiation, which resulted in H-ARS. The survival rate of those treated with PEGylated G-CSF was 91.3% (21/23) compared to 47.8% (11/23) in the control group at 60-day postirradiation. PEGylated G-CSF also significantly decreased the median duration of neutropenia and thrombocytopenia, improved the median time to recovery of absolute neutrophil count and platelet count, increased the mean absolute neutrophil count at nadir, and decreased the incidence of gram-negative bacteremia. PEGylated G-CSF was approved by the FDA on 25 November 2015 for use in improving blood profiles of ARS subjects and for increasing survival in patients acutely exposed to myelosuppressive doses of radiation (H-ARS) in both adults and pediatric patients [Citation23,Citation37].
2.1.3 Recent patents for ARS countermeasures
Several radiation countermeasures are in the initial stages of research and development, with a number of them showing effectiveness against radiation injury when administered either before or after irradiation. A large numbers of patents have been recently approved internationally for agents that have radioprotective or radiomitigative potential for ARS [Citation13,Citation14,Citation17]. A list of such agents is presented in . An ideal drug for FDA approval would work effectively to protect against or treat both H-ARS and GI-ARS. However, since H-ARS and GI-ARS are underpinned by different types of injuries and manifest quite different symptoms, it is appropriate to address them separately during drug development. Some radiation countermeasures are being developed specifically for H-ARS; for example, administration of ET18-0CH3, a phosophocholine, 24 h prior to γ-irradiation appears beneficial to the recovery process [Citation42]. Administration of this agent increases leukocytes and granulocyte levels in blood during the critical period after irradiation and allows animals to survive. An additional cytokine to those already mentioned and that may be administered is interleukin-12 (IL-12, HemaMax). HemaMax has already received FDA IND status. This agent, for which a patent was granted in 2014, has been shown to have radioprotective as well as radiomitigative potential for H-ARS. At low doses (100 ng), IL-12 was able to protect 100% mice against a supralethal radiation dose. This agent has shown consistent efficacy in NHPs [Citation43–Citation45]. IL-12’s efficacy mechanism appears to involve multiple stages of hematopoiesis and includes the induction of IFN-γ [Citation46]. This potential countermeasure has also demonstrated efficacy against lethal combined injury (CI) [Citation47]. Attempts to develop IL-12 as an anticancer immunoadjuvant drug have been complicated by clinical side effects. Novel nitroxide agents have shown radiomitigative potential against H-ARS by scavenging free radicals [Citation48]. Another agent, α-1 antitrypsin (AAT), does not improve survival but allows mice to survive longer than their respective control groups [Citation49]. However, pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), are reduced by AAT while IL-1Rα is upregulated, showing that AAT may be beneficial at the cellular level for recovery after radiation. If this agent is pursued, it may be prudent to combine it with additional countermeasures for optimal efficacy.
Several agents are being developed specifically for GI-ARS [Citation50–Citation53]. Anti-TNF antibody has been tested and proven efficacious to increase survival and to treat GI inflammation [Citation54]. Endogenous production of Smad7, a nuclear regulatory protein, serves to reduce DNA damage and stimulate wound healing [Citation55]. By orally administering Tat-Smad7, damage after high doses of X-ray radiation in the mouse model is minimized (up to 20 Gy). Overexpression of the Smad7 protein does not increase one’s risk of cancer, so this agent may be an attractive candidate for the FDA approval process.
Radiation countermeasures for ARS that might be streamlined for the FDA approval include neutraceuticals, biologicals, and repurposed pharmaceuticals [Citation56,Citation57]. Glyburide is a sulfonylurea that is already approved by the FDA for the treatment of diabetes [Citation58]. It is a blocker of adenosine tripohosphate-sensitive potassium channels and has been shown to regulate apoptosis and mitochondrial permeability. Due to glyburide’s effects on mitochondria and apoptosis, it has been shown to be effective in reducing radiation damage when administered prophylactically, but not following radiation exposure as a radiomitigating agent. Novel biologicals are currently being pursued and include both isolated peptide chains and endogenous proteins. Survival was increased significantly when mice were administered peptide AQGV or the functional peptide of fibroblast growth factor-2 (FGF-P) [Citation59,Citation60]. Peptide AQGV and FGF-P may be viable treatment options after radiation injuries for both H-ARS and GI-ARS, providing an effective treatment at higher radiation doses.
Using a new drug in combination with an already FDA-approved drug may be beneficial to both in terms of improving drug efficacy as well as from a strategic standpoint to move the test drug through the FDA regulatory process. For example, poly-glutamic acid amifostine (GP-A) may be a useful pharmaceutical polymer due to its relationship with amifostine, an FDA-approved radiation countermeasure for limited use [Citation61]. GP-A prolongs the retention time of amifostine within organs and protects DNA from free-radical damage resulting from ultraviolet rays. Neutraceuticals are natural products commonly used globally for the treatment of various diseases. In non-Western cultures, traditional medicine is practiced more commonly. One recently published patent combines jiaogulan (Gynostemma pentaphyllum), hawthorn (Crataegus pinnatifida), and green tea (Camellia sinensis) extracts for a radioprotective efficacy at low doses of X-ray irradiation [Citation62]. There are large numbers of recently isolated agents promising for future developments (). Some agents have shown more promise than others depending on the model being used (single vs. multiple strains of mice).
2.2. Countermeasures for radioisotope-specific toxicities
It needs to be pointed out that there are several, already established, highly efficacious drugs for the prophylaxis and/or treatment of individuals contaminated with selected types of radionuclides; these drugs include potassium iodide (KI; ThyroShield) for the protection of the thyroid gland from radioactive iodine, prussian blue (PB; Radiogardase) for the treatment and reduction of radioactive cesium body burdens, Zn/Ca DTPA (Zn/Ca diethylene triamine pentaacetic acid) for the treatment of plutonium contamination, and Granisetron/Kytril for blocking/minimizing emetic effects of ARS [Citation22]. PB, Zn/Ca DTPA, and Granisetron, all are available in the SNS [Citation22].
3. CI treatments
During a nuclear accident or terrorist attack, the likelihood of a blast following a radioactive explosion is high, which will result in thermal burns, wounding, or hemorrhage in addition to radiation exposure; the victim of such combined exposures would then be classified as having a CI [Citation63–Citation65]. The adverse biological effects induced by CI act synergistically against the host’s survival; wounds or burns in radiation survivors are known to exacerbate ARS, including enteropathy associated with hematopoietic syndrome [Citation7,Citation8]. Radiation exposure is known to compromise the victim’s immune system; opportunistic endogenous bacterial colony can then infect non-sterile wounds or burns, ultimately resulting in sepsis [Citation66]. Polymicrobial septic infections consisting of multiple microorganisms, each having different characteristics, means of defense, and antimicrobial susceptibilities, will accompany injuries from the direct radiation exposure or the CI, especially following LDs of γ-radiation [Citation67,Citation68]. Polymicrobial infections are often especially challenging and limit the effectiveness of conventional therapy.
It has been shown that supportive care alone increases survival chances after ionizing radiation exposure [Citation29]; standard supportive care includes fluid administration, antibiotics, analgesics for pain, antipyretics for fever, and blood product transfusions. CI patients may be administered topical ointments to clean burns and wounds and antibacterial/antimicrobial agents to prevent sepsis. Survival rates increase in CI individuals with supportive care. No drug to date has been approved to specifically treat CI. There are, however, several drugs that appear to increase recovery and promote survival when administered concomitantly with other ionizing radiation injury-specific treatments [Citation7,Citation63,Citation69,Citation70].
Only a few agents have recently been patented to specifically treat after CI. NorLeu3-A (1–7), a heterocyclic compound, increased survival and proliferating epidermal tissue around the burn site in thermal injury-CI model of guinea pigs when administered within 3 days of 2 Gy irradiation and applied daily (doses as low as 10 µg/wound) [Citation71]. This compound also decreased wound healing delay after thermal wound and 6 Gy exposure when administered at 1 mg/ml/day per wound. A group of novel drugs related to 3,4-methylenedioxy-β-methyl-β-nitrostyrene were able to increase the survival rate at 25 days postirradiation exposure when administered 1–2 days before 2–20 Gy irradiation. The drugs in this particular group were not tested for CI directly, instead were tested as radiation and infection treatments separately. They displayed antimicrobial properties toward several bacteria when topically administered to wounds in human volunteers and mice [Citation72].
4. Conclusion
Though G-CSF has been approved by the FDA for H-ARS and has already been procured, along with GM-CSF, for the SNS for use in a RN emergency [Citation23,Citation73], the adverse effects of G-CSF need to be taken into consideration [Citation74–Citation76]. The International Atomic Energy Agency recommends that platelet counts be monitored during G-CSF administration which will be difficult in a mass causality scenario [Citation77]. It is important to note that in a recent study, G-CSF failed to demonstrate radiomitigative efficacy in the NHP model in the absence of supportive care [Citation45]. This observation might be critical in terms of developing medical plans for managing mass casualties arising from unwanted RN exposures and the possible limits of delivering conventional supportive care to the RN injured. It would seem prudent to both acknowledge the effectiveness of recombinant therapies, such as G-CFS and GM-CSF, and to fully recognize the limitations of such therapies, while continuing search for new, still more effective medical countermeasures to RN exposures. Although, the cause for the disparate research findings mentioned above remains unclear, there is still a pressing need to develop radioprotectors which can be administered prior to irradiation. Such agents should demonstrate efficacy in the absence of supportive care as such infrastructure may not be available to all victims under a large-scale mass causality scenario.
5. Expert opinion
The Animal Rule was developed to provide a pathway for the approval of a drug or licensing of biologic products for use in chemical, biological, radiological, or nuclear (CBRN) exposure contingencies without having human efficacy data; the alternative, which is clearly unacceptable, was to leave these products in regulatory ‘limbo’ as INDs and their utility would be significantly weakened by virtue of the fact that a medicinal bearing an IND label would first require informed consent (from the patient) prior to use. The US government has taken quite laudable steps to protect its citizens in the event of a public health emergency by developing a system to procure essential medical countermeasures for CBRN threats. In this regard, promising medical countermeasures do not necessarily require full FDA approval or licensure in order to be produced, stockpiled, and made readily available in the event of a mass casualty scenario. Authorization of these IND-bearing medicinals (commonly referred to as medical countermeasures) under an emergency use provision of the ‘Pandemic and All-Hazards Preparedness Reauthorization Act (PAHPRA) of 2013’ is quite possible, but not without being bureaucratically cumbersome and time-consuming during critical emergency situations [Citation73].
Emergency Use Authorization (EUA) is an important option for medical and public health. It complements the need for timely and useful treatment when the applicable drug has not been approved at all or approved for another specific indication by the FDA [Citation78]. The EUA authority under Section 564 permits the FDA to facilitate availability and use of medical countermeasures required to prepare for and respond to CBRN disasters. PAHPRA improved the current EUA authority to better serve rapid response to public health emergencies. Specifically, by eliminating the need for the original Project BioShield that Department of Health and Human Services secretarial determination in support of an EUA takes the form of an official public health emergency declaration, PAHPRA is designed to facilitate preparation for both actual and potential public health emergencies [Citation73].
There are in fact multiple, highly effective, and essential medical countermeasures that carry only IND status [Citation13,Citation22]. The EUA requires a reasonable database in both the clinic and animal models. G-CSF and GM-CSF were recommended for EUA status due to the overwhelming, consistent safety and efficacious clinical and preclinical database in multiple species. G-CSF and GM-CSF were procured for the SNS much before FDA’s approval of G-CSF for treatments of H-ARS under the PAHPRA [Citation15,Citation73,Citation79].
The Animal Rule requires a comprehensive understanding of the mechanisms of injury, drug efficacy, and efficacy biomarkers. In this context, it is important to identify biomarkers for radiation injury and drug efficacy that can extrapolate animal efficacy results to convert drug doses to those that can be efficacious when used in humans [Citation11,Citation24]. It is important to note that the pharmacokinetic profile across species should be considered to insure appropriate dosing of the drug in animal models. Biomarkers are an important aspect of radiation countermeasure development and can be used as a trigger for intervention as well as in selecting a drug dose and treatment regimen in humans. Biomarkers may also be shown to correlate with the mechanism by which the agent reduces the injury inflicted or with the desired clinical outcome (i.e. reduction in mortality or major morbidity). The human dose of the drug should closely correlate with efficacious doses from the well-controlled animal studies [Citation12,Citation80].
GI-ARS is a major, potentially lethal pathological response that may occur soon after a radiation/nuclear incident [Citation3,Citation81]. Currently, there are no prophylactic countermeasures that are FDA approved for the GI syndrome lethality. Clearly, this deficit represents a major unmet medical need in terms of protecting first responders, military personnel, or remediation workers entering a contaminated area. Both, Neupogen and Neulasta, are effective against H-ARS only and have limited efficacy. Again, there is a significant need to develop safe and effective countermeasures against supralethal doses of radiation causing GI-ARS. The pathophysiology of this syndrome requires depletion of stem cells (stem cell clonogens [SCCs]) within the crypts of the Lieberkuhn, a subset of cells necessary for post-injury regeneration of GI epithelium. Recent studies indicate that depletion of SCCs is not a direct result of DNA damage, but instead coupled to ceramide-induced endothelial cell death (apoptosis) within the mucosal microvascular network. The ceramide generated on the surface of endothelium coalesces to form ceramide-rich platforms and these platforms serve to transmit apoptotic signals [Citation82,Citation83]. Administration of an anti-ceramide monoclonal antibody prevents platform formation on the surface of irradiated endothelial cells of the murine GI tract and, in turn, limits the extent of radiation-induced endothelial apoptosis within the lamina propria of the small intestinal and facilitates recovery of crypt SSCs, thus sparing experimental animals (mice) from early death associated with GI-ARS after supralethal radiation exposures. This antibody represents a new class of therapeutics, with a promising future as an effective countermeasure against GI-ARS mortality [Citation84].
There are several promising radiation countermeasures (other than those listed above) under advanced stages of development for RN threats which have been discussed in recent reviews [Citation13–Citation15,Citation17,Citation22,Citation57,Citation65]. In addition to two approved H-ARS countermeasures, CBLB502 (Entolimod) is at an advanced stage of development. Its pre-EUA dossier is currently under active review with the FDA [Citation85,Citation86]. HemaMax (IL-12) is progressing well with evaluation in large animals [Citation45,Citation46]. γ-Tocotrienol is another promising radiation countermeasure under advanced development [Citation87–Citation89].
Article highlights
Two radiation countermeasures (Neupogen and Neulasta) have been approved by the FDA against H-ARS following the Animal Rule.
Both approved countermeasures are radiomitigators which can be used after radiation exposure and need additional medical supportive care including blood products.
There is no FDA approved radiation countermeasure which can be used prior to radiation exposure as a radioprotector.
There is an unmet need for countermeasures effective against GI-ARS, and treatments effective in the absence of medical supportive care.
Several promising radiation countermeasures demonstrating efficacy against H-ARS and GI-ARS are under development using the Animal Rule and large numbers of newly identified agents have been patented for use as radiation countermeasures.
This box summarizes key points contained in the article.
Declaration of interest
The opinions or assertions contained herein are the professional views of the authors and do not necessarily represent the Armed Forces Radiobiology Research Institute, the Uniformed Services University of the Health Sciences, or the Department of Defense, USA. Mention of specific therapeutic agents does not constitute endorsement by the U.S. Department of Defense, and trade names are used only for the purpose of clarification. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
We apologize to those having contributed substantially to the topics discussed herein that we were unable to cite due to space constraints.
Additional information
Funding
References
- Moulder JE. 2013 Dade W. Moeller lecture: medical countermeasures against radiological terrorism. Health Phys. 2014;107:164–171.
- Bushberg JT, Kroger LA, Hartman MB, et al. Nuclear/radiological terrorism: emergency department management of radiation casualties. J Emerg Med. 2007;32:71–85.
- Hall EJ, Giaccia AJ. Radiobiology for the radiobiologist. 7th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2012.
- Flynn DF, Goans RE. Nuclear terrorism: triage and medical management of radiation and combined-injury casualties. Surg Clin North Am. 2006;86:601–636.
- Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536.
- Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the strategic national stockpile radiation working group. Ann Intern Med. 2004;140:1037–1051.
- DiCarlo AL, Maher C, Hick JL, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–S44.
- Dainiak N, Waselenko JK, Armitage JO, et al. The hematologist and radiation casualties. Hematol Am Soc Hematol Educ Program. 2003;2003:473–496.
- Anno GH, Young RW, Bloom RM, et al. Dose response relationships for acute ionizing-radiation lethality. Health Phys. 2003;84:565–575.
- Allio T. Product development under FDA’s Animal Rule: understanding FDA’s expectations and potential implication for traditional development programs. Therap Inno Regul Sci. Forthcoming 2016. doi: 10.1177/2168479016641717
- Singh VK, Newman VL, Berg AN, et al. Animal models for acute radiation syndrome drug discovery. Expert Opin Drug Discov. 2015;10:497–517.
- Gronvall GK, Trent D, Borio L, et al. The FDA animal efficacy rule and biodefense. Nat Biotechnol. 2007;25:1084–1087.
- Singh VK, Newman VL, Romaine PL, et al. Radiation countermeasure agents: an update (2011-2014). Expert Opin Ther Pat. 2014;24:1229–1255 .
- Singh VK, Romaine PL, Newman VL. Biologics as countermeasures for acute radiation syndrome: where are we now? Expert Opin Biol Ther. 2015;15:465–471.
- Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine. 2015;71:22–37.
- Hankey KG, Farese AM, Blaauw EC, et al. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat Res. 2015;183:643–655 .
- Singh VK, Pollard HB. Patents for Toll-like receptor ligands as radiation countermeasures for acute radiation syndrome. Expert Opin Ther Pat. 2015;25:1085–1092.
- Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123.
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–528.
- Cerveny TJ, MacVittie TJ, Young RW. Acute radiation syndrome in humans. In: Walker RI, Cerveny TJ, editor. Medical consequences of nuclear warfare, textbook of military medicine. Falls Church, VA: TMM Publications, Office of the Surgeon General Publications; 1989. p. 15–36.
- Gusev IA, Guskova AK, Mettler FA. Medical management of radiation accidents. Boca Raton, FL: CRC Press; 2001.
- Singh VK, Romaine PL, Seed TM. Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the strategic national stockpile. Health Phys. 2015;108:607–630.
- Farese AM, MacVittie TJ. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today (Barc). 2015;51:537–548.
- Singh VK, Newman VL, Romaine PL, et al. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev Mol Diagn. 2016;16:65–81.
- Esbitt D. The strategic national stockpile: roles and responsibilities of health care professionals for receiving the stockpile assets. Disaster Manag Response. 2003;1:68–70.
- Need JT, Mothershead JL. Strategic national stockpile program: implications for military medicine. Mil Med. 2006;171:698–702.
- Moroni M, Ngudiankama BF, Christensen C, et al. The Gottingen minipig is a model of the hematopoietic acute radiation syndrome: G-colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total-body gamma-irradiation. Int J Radiat Oncol Biol Phys. 2013;86:986–992.
- Nash RA, Schuening FG, Seidel K, et al. Effect of recombinant canine granulocyte-macrophage colony-stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1994;83:1963–1970.
- MacVittie TJ, Farese AM, Jackson WI 3rd. Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89:546–555.
- Schuening FG, Appelbaum FR, Deeg HJ, et al. Effects of recombinant canine stem cell factor, a c-kit ligand, and recombinant granulocyte colony-stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1993;81:20–26.
- Patchen ML, MacVittie TJ. Granulocyte colony-stimulating factor and amifostine (Ethyol) synergize to enhance hemopoietic reconstitution and increase survival in irradiated animals. Semin Oncol. 1994;21:26–32.
- Sureda A, Kadar E, Valls A, et al. Granulocyte colony-stimulating factor administered as a single intraperitoneal injection modifies the lethal dose (95/30) in irradiated B6D2F1 mice. Haematologica. 1998;83:863–864.
- Hosoi Y, Kurishita A, Ono T, et al. Effect of recombinant human granulocyte colony-stimulating factor on survival in lethally irradiated mice. Acta Oncol. 1992;31:59–63.
- Sureda A, Valls A, Kadar E, et al. A single dose of granulocyte colony-stimulating factor modifies radiation-induced death in B6D2F1 mice. Exp Hematol. 1993;21:1605–1607.
- Mac Vittie TJ, Monroy RL, Vigneulle RM. The relative biological effectiveness of mixed fission-neutron-gamma radiation on the hematopoietic syndrome in the canine: effect of therapy on survival. Radiat Res. 1991;128:S29.
- Farese AM, Cohen MV, Katz BP, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100.
- U.S. Food and Drug Administration. FDA approves neupogen for treatment of patients with radiation-induced myelosuppression following a radiological/nuclear incident; 2015. [cited 2015 Apr 17]. Available from: http://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/AboutMCMi/ucm443245.htm
- Molineux G, Kinstler O, Briddell B, et al. A new form of filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol. 1999;27:1724–1734.
- Farese AM, Cohen MV, Stead RB, et al. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat Res. 2012;178:403–413.
- Chua HL, Plett PA, Sampson CH, et al. Survival efficacy of the PEGylated G-CSFs, Maxy-G34, and Neulasta in a mouse model of lethal H-ARS, and residual bone marrow damage in treated survivors. Health Phys. 2014;106:21–38.
- Kiang JG, Zhai M, Liao PJ, et al. Pegylated G-CSF inhibits blood cell depletion, increases platelets, blocks splenomegaly, and improves survival after whole-body ionizing irradiation but not after irradiation combined with burn. Oxid Med Cell Longev. 2014;2014:481392.
- Richter W, Weber L. Use of tri-substituted glycerol compounds for the treatment of radiation injuries. CA2668923C. 2015.
- Basile LA, Ellefson D, Gluzman-Poltorak Z, et al. HemaMax, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates. PLoS One. 2012;7:e30434.
- Gluzman-Poltorak Z, Mendonca SR, Vainstein V, et al. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J Hematol Oncol. 2014;7:31.
- Gluzman-Poltorak Z, Vainstein V, Basile LA. Recombinant interleukin-12, but not granulocyte-colony stimulating factor, improves survival in lethally irradiated nonhuman primates in the absence of supportive care: evidence for the development of a frontline radiation medical countermeasure. Am J Hematol. 2014;89:868–873.
- Gluzman-Poltorak Z, Vainstein V, Basile LA. Association of hematological nadirs and survival in a nonhuman primate model of hematopoietic syndrome of acute radiation syndrome. Radiat Res. 2015;184:226–230.
- Ellefson D, Galalaher T, Gluzman-Poltorak Z, et al. Mitigation of radiation combined injury (RCI) by interleukin-12. 14th Intl Cong Radiat Resh; 2011; Poland Warsaw.
- Wipf P, Frantz MC. Targeted nitroxide agents. US9216976B2. 2015.
- Crapo JD, Marcondes AMQ, Deeg HJ. Method for ameliorating radiation exposure effects with alpha-1 antitrypsin. US8980574B2. 2015.
- Kantara C, Moya SM, Houchen CW, et al. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab Invest. 2015;95:1222–1233.
- MacVittie TJ, Bennett AW, Farese AM, et al. The effect of radiation dose and variation in neupogen(R) initiation schedule on the mitigation of myelosuppression during the concomitant GI-ARS and H-ARS in a nonhuman primate model of high-dose exposure with marrow sparing. Health Phys. 2015;109:427–439.
- Pejchal J, Sinkorova Z, Tichy A, et al. Attenuation of radiation-induced gastrointestinal damage by epidermal growth factor and bone marrow transplantation in mice. Int J Radiat Biol. 2015;91:703–714.
- Deng W, Kimura Y, Gududuru V, et al. Mitigation of the hematopoietic and gastrointestinal acute radiation syndrome by octadecenyl thiophosphate, a small molecule mimic of lysophosphatidic acid. Radiat Res. 2015;183:465–475.
- Fox BS, Stafford DC. Antibody therapy medium with local activity in the digestive tract. EP2488202B1. 2015.
- Xiaojing W, Farley Y, Qinghong Z. Smad7 therapeutic applications. CN103501803B. 2015.
- Seed TM. Radiation protectants: current status and future prospects. Health Phys. 2005;89:531–545.
- Singh VK, Beattie LA, Seed TM. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 2013;54:973–988.
- Epperly M, Greenberger J, Jiang J, et al. Radioprotective agents. US8883852B2. 2014.
- Benner R, Khan NA, Carlton RM. Use of peptides for the control of radiation injury. CA2645550C. 2014.
- Okunieff P, Zhang L. Fibroblast growth factor (FGF) analogs and uses thereof. EP2310035B1. 2015.
- Wang CH, Chen CH, Chen JY, et al. Pharmaceutical compositions. US8648042B2. 2014.
- Djang AHK. Methods for radiation protection. US8765193B2. 2014.
- DiCarlo AL, Ramakrishnan N, Hatchett RJ. Radiation combined injury: overview of NIAID research. Health Phys. 2010;98:863–867.
- Benjamin GC, McGeary M, McCutchen SR. Assessing medical preparedness to respond to a terrorist nuclear event: workshop report. Washington, DC: The National Academies Press; 2009.
- Singh VK, Ducey EJ, Brown DS, et al. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol. 2012;88:296–310.
- Elliott TB, Bolduc DL, Ledney GD, et al. Combined immunomodulator and antimicrobial therapy eliminates polymicrobial sepsis and modulates cytokine production in combined injured mice. Int J Radiat Biol. 2015;91:690–702.
- Brook I, Elliott TB, Ledney GD, et al. Management of postirradiation infection: lessons learned from animal models. Mil Med. 2004;169:194–197.
- Brook I, Ledney GD. Quinolone therapy in the management of infection after irradiation. Crit Rev Microbiol. 1992;18:235–246.
- Ran XZ, Shi CM, Zheng HE, et al. Experimental research on the management of combined radiation-burn injury in China. Radiat Res. 2011;175:382–389.
- Jacob A, Shah KG, Wu R, et al. Ghrelin as a novel therapy for radiation combined injury. Mol Med. 2010;16:137–143.
- Rodgers KE, diZerega GS. Methods for treating combined radiation and thermal injury. JP5823486. 2015.
- Denisenko PP, Sapronov NS, Tarasenko AA. Nitrostyrene derivatives with antimicrobial action. EP2468275B1. 2015.
- Kels CG. Dispensing medical countermeasures: emergency use authorities and liability protections. Health Secur. 2015;13:139–151.
- van Os R, Robinson S, Sheridan T, et al. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells. 2000;18:120–127.
- Adachi K, Suzuki M, Sugimoto T, et al. Effects of granulocyte colony-stimulating factor (G-CSF) on bleomycin-induced lung injury of varying severity. Toxicol Pathol. 2003;31:665–673.
- Inokuchi R, Manabe H, Ohta F, et al. Granulocyte colony-stimulating factor-producing lung cancer and acute respiratory distress syndrome. Clin Respir J. 2015;9:250–252.
- International Atomic Energy Agency. The radiological accident in gilan; 2002. [cited 2014 Feb 10]. Available from: http://www-pub.iaea.org/books/IAEABooks/6284/The-Radiological-Accident-in-Gilan
- Nightengale SL, Prasher JM, Simonson S. Emergency use authorization (EUA) to enable use of needed products in civilian and military emergencies, United States. Emerging Infect Dis. 2007;13:1046–1055.
- Gronvall G. Biodefense countermeasures: the impact of title IV of the US Pandemic and All-Hazards Preparedness Act. Emerg Health Threats J. 2008;1:e3.
- Aebersold P. FDA experience with medical countermeasures under the Animal Rule. Adv Prev Med. 2012;2012:507571.
- Wang J, Shao L, Hendrickson HP, et al. Total body irradiation in the “hematopoietic” dose range induces substantial intestinal injury in non-human primates. Radiat Res. 2015;184:545–553.
- Rotolo JA, Kolesnick R, Fuks Z. Timing of lethality from gastrointestinal syndrome in mice revisited. Int J Radiat Oncol Biol Phys. 2009;73:6–8.
- Rotolo JA, Mesicek J, Maj J, et al. Regulation of ceramide synthase-mediated crypt epithelium apoptosis by DNA damage repair enzymes. Cancer Res. 2010;70:957–967.
- Rotolo J, Stancevic B, Zhang J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest. 2012;122:1786–1790.
- Krivokrysenko VI, Shakhov AN, Singh VK, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther. 2012;343:497–508.
- Krivokrysenko VI, Toshkov IA, Gleiberman AS, et al. The Toll-like receptor 5 agonist Entolimod mitigates lethal acute radiation syndrome in non-human primates. PLoS One. 2015;10:e0135388.
- Singh VK, Kulkarni S, Fatanmi OO, et al. Radioprotective efficacy of gamma-tocotrienol in nonhuman primates. Radiat Res. 2016;185:285–298.
- Kulkarni S, Singh PK, Ghosh SP, et al. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine. 2013;62:278–285.
- Singh VK, Hauer-Jensen M. Gamma-tocotrienol as a promising countermeasure for acute radiation syndrome: current status. Int J Mol Sci. 2016;17:e663.
