1. Introduction
Preterm births result in higher levels of neonatal morbidity and mortality and have been proven to result in further complications later in the infant’s life [Citation1]. It has also been shown that preterm births are the largest single contributor to the cause of neonatal deaths [Citation2]. Interventions such as dietary changes, habitual changes, and tocolytic agents have all been explored (for review see Goldenberg et al. [Citation3]). Tocolytic agents are compounds that can prevent or interrupt uterine contractions. Current tocolytic agents include β-receptor agonists (ritodrine) [Citation4], progesterone [Citation5,Citation6], calcium channel blockers (nifedipine) [Citation7], and at the forefront of current research, oxytocin receptor (OTR) antagonists.
Ritodrine is an example of a β-receptor agonist which has been used as tocolytic agent with an ability to delay labor onset. Further development of medications to treat preterm birth resulted in the development of calcium channel blockers, namely nifedipine, which had shown an ability to delay labor onset by over 7 days. The nonselective OTR antagonist atosiban (Tractocile™, Ferring Pharmaceuticals) has been proven to be more effective than either activation of β-receptors or inhibiting calcium channels, producing decreased adverse side-effects with the same efficacy [Citation8,Citation9].
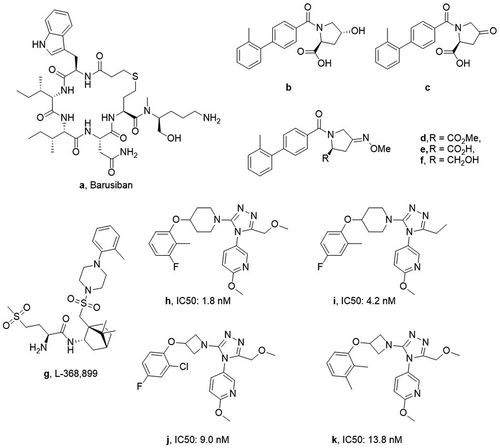
OTRs exist in both the central nervous system (CNS) and in the periphery. In the periphery, OT is responsible for increased cervical contractions and promoting labor. Hence antagonism would prevent these responses and be viable as a strategy toward tocolysis. Efforts have been made to develop more potent and selective OTR antagonists in order to improve on the existing FDA-approved atosiban. Barusiban ()) (Ferring Pharmaceuticals) is the result of these efforts and has resulted in a much more potent OTR antagonist that has increased selectivity over the vasopressin 1a receptor (V1aR).
Research indicates other potential use for these compounds, such as in the area of assistive reproductive technologies (ART). Increased uterine contractions during embryo implantation can lead to a decreased success rate of conception. Administration of an OTR antagonist has been shown to reduce the frequency of uterine contractions and could therefore aid the conception of a child through in-vitro fertilization (IVF). With the decrease in uterine contractions resultant from OTR antagonist administration, higher chances of successful implantation are possible.
Premature ejaculation (PE) has been shown to impact 20–30% of the male population and can lead to mental distress, anxiety, embarrassment, and depression [Citation10,Citation11]. Treatment for this illness could decrease the burden of depressive disorders on a global scale [Citation12]. The involvement of OT in sexual function and behavior has been thoroughly explored in various animal models [Citation4]. OTR antagonists have also been shown to exhibit an effect on intravaginal ejaculatory latency time allowing prolonged activity before ejaculation [Citation13]. The development of non-peptidic OTR antagonists for this purpose has been an area of research for the past 10–15 years [Citation14–Citation19].
In the past 5 years, there have been 4 patent applications from 3 different companies. These patent applications have advanced on previous research in the area by demonstrating an increase in selectivity, potency, or activity. To date there is still only one tocolytic agent based on OTR antagonism and very few treatments for PE.
2. Chemistry
The known tocolytic agent atosiban has poor pharmacokinetic properties [Citation16]. Therefore, Ferring Pharmaceuticals developed the modified peptide, barusiban, which had a high affinity for the OTR and over 20-fold selectivity over the V1aR [Citation20]. Barusiban is a peptide mimetic of atosiban, varying some of the amino acids in order to increase stability and selectivity. The primate studies did not translate into the human studies since a randomized, double-blind, placebo-controlled trial showed no evidence of efficacy in comparison to placebo [Citation21,Citation22]. In an effort to repurpose the molecule, Ferring pharmaceuticals have obtained a patent in 2017 which provides a method by which administration of barusiban, or any OTR antagonist, can aid IVF embryo transplantation [Citation23].
ObsEva, a Swiss clinical-stage biopharmaceutical company has a patent application filed in 2015 which contains an established OTR antagonist as well as its theorized metabolites ((b–f)) [Citation24]. The core structure is a functionalized pyrrolidine with an attached biphenylamide moiety. Through a linear synthetic procedure, outlined in full, all of the metabolites were obtained in good yields, with characterization data claimed to be concordant with the corresponding structure. The lead compound has an O-methyl oxime present which was proved to exist in the Z-isomer exclusively and hence purification techniques were established. Chromatographic purification was found to be the most effective method of isolating the pure isomer. Potential for isomerization was explored and was found not to occur, with testing 6 h after in vivo administration.
Ixchelsis Ltd., an independent biotechnology company, has been developing an OTR antagonist for its potential treatment of PE. Ixchelsis has filed a patent application in the area of treatment of male sexual dysfunction based on the structure of L-368,899 ()) [Citation25]. L-368,899 is a previously reported highly selective OTR antagonist (IC50 6.3 nM for OTR and 148 nM for V1aR) originally designed for the treatment of preterm labor [Citation26]. Ixchelsis is seeking to repurpose this molecule as a potential treatment for sexual dysfunction.
More recently, Ixchelsis Ltd. filed a patent application for a series of triazole derivatives for the purpose of treating sexual dysfunction [Citation27]. These triazole derivatives are based on the known OTR antagonist compound 25 [Citation28] ()) with variations to the 3- and 5-positions on the 1,2,4-triazole core, whilst mostly retaining the methoxypyridine group. The patent reports the detailed synthesis of over 170 compound with yields, and varied spectroscopic data provided for most of the compounds.
3. Biology
Ferring conducted studies that were randomized and placebo-controlled to determine the importance of time of administration of the OTR antagonist, barusiban. Administration of barusiban on the day of cleavage-stage embryo transfer versus blastocyst transfer (day 3 vs. day 5 post-retrieval, respectively) showed a marked difference in the success rate (45% ongoing implantation vs. 27% for placebo).
ObsEva assayed one compound ()) to determine its binding selectivity over the V1aR (Ki = 52 nM for OT and Ki = 120 nM for V1a) and its antagonistic activity (IC50 = 81 nM). These were measured on HEK cells transfected with human OTRs. To determine whether this molecule is suitable for the treatment of preterm labor, they assessed its ability to inhibit the spontaneous uterine contractions in anesthetized late-term pregnant rats. 1(f) was tested in both the pure E- and Z-form, which established the Z-isomer as the active conformer.
Ixchelsis Ltd. determined the potential treatment of PE and effect on erectile function of 1(g) by administration of p-chloroamphetamine to induce ejaculation in anesthetized rats. It was found that 1(g) delayed ejaculation by up to 140% and had no detrimental effect on erectile function. Copulatory efficiency was then measured using parameters such as number and frequency of intromissions (vaginal penetrations), mount frequency, and time taken to remount after ejaculation. It was shown that there were no detrimental effects of 1(g) on copulatory behavior (50 ng/rat intracerebroventricularly icv. and 10 mg/kg subcutaneous injection sc.). No effect was observed by a comparative study with vasotocin, a nonselective OTR antagonist, suggesting the importance of selectivity for these effects to be observed.
Ixchelsis Ltd. used the activity of 4 compounds of the 170 reported in the patent as exemplary compounds. The results were determined by transfecting CHO-cells expressing the OTR with NFAT-β-lactamase and allowed the determination of IC50s. All compounds have been claimed to have an IC50 under 1 μM but the best performing compounds had antagonistic activity between 1 and 15 nM at the OTR (–k)).
4. Expert opinion
Prevention of preterm birth is still very important with the high levels of morbidity and mortality associated with this condition. Despite the seemingly promising target of antagonizing the OTR to decrease uterine contractions to prevent preterm birth, there is still only one drug on the market (atosiban). ObsEva has a compound ()) in clinical trials and is about to begin phase 3. This is more progress than was made with the selective peptide-based barusiban and is hence quite promising.
A significant percentage of men suffer from PE and no pharmacological interventions are currently available. With increasing signs of potential for treatment through the antagonism of the OTR, it seems as though this is an area of research worth pursuing. Ixchelsis currently has a drug in phase 2a clinical trials, being funded by PfizerTM and TVM Life SciencesTM, but no structure has been disclosed.
The patents highlighted in this review have a common theme of repurposing known OTR antagonists. Most interestingly is the developmental prospect of therapeutics for SD via OTR antagonism. With no pharmacological therapies available for PE, this could lead to a brand new drug with the potential to treat a medical issue that effects around 30% of the male population. In addition to this repurposing of known OTR antagonists, it is becoming clearer that the selectivity associated with these known compounds seems to be crucial to their potential therapeutic value. Two of the patents clarified that in comparison to nonselective OTR antagonists, the selective antagonists had a significantly different and positive effect.
It should be noted that the OTR is expressed in both the CNS and periphery, with dissimilar biological consequences. Central OTR activation has been associated with prosocial, anxiolytic, and anti-addictive properties, hence antagonism of these receptors could be seen as detrimental to one’s mental health [Citation29]. Therefore, the pharmacokinetics of the proposed molecules would need to be explored in more depth. The high polarity of compounds 1(a) and 1(g) would suggest preference for peripheral OTRs due to poor blood–brain barrier penetrability. In contrast, the triazole series (1h–k) and the pyrrolidine series (1b–f) possess more lipophilic properties and may have limited selectivity for peripheral OTRs.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269.
- Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? where? why? Lancet. 2005;365(9462):891–900.
- Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;339(5):313–320.
- Yaju Y, Nakayama T. Effectiveness and safety of ritodrine hydrochloride for the treatment of preterm labour: a systematic review. Pharmacoepidemiol Drug Saf. 2006;15(11):813–822.
- Norwitz ER, Caughey AB. Progesterone supplementation and the prevention of preterm birth. Am J Obstet Gynecol. 2011;4(2):60–72.
- van Zijl MD, Bouchra Koullali BW, Mol J, et al. Prevention of preterm delivery: current challenges and future prospects. Int J Womens Health. 2016;8:633–645.
- Read MD, Wellby DE. The use of a calcium antagonist (nifedipine) to suppress preterm labour. BJOG. 1986;93(9):933–937.
- Kashanian M, Akbarian AR, Soltanzadeh M. Atosiban and nifedipin for the treatment of preterm labor. Int J Gynaecol Obstet. 2005;91(1):10–14.
- Moutquin J-M, Sherman D, Cohen H, et al. Double-blind, randomized, controlled trial of atosiban and ritodrine in the treatment of preterm labor: a multicenter effectiveness and safety study. Am J Obstet Gynecol. 2000;182(5):1191–1199.
- Hatzimouratidis K, Amar E, Eardley I, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57(5):804–814.
- Laumann EO, Nicolosi A, Glasser DB, et al. Sexual problems among women and men aged 40–80 y: prevalence and correlates identified in the global study of sexual attitudes and behaviors. Int J Impot Res. 2005;17(1):39–57.
- Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
- McMahon C, Osterloh I, Rosen R, et al. Pd69-01 a phase IIa study to investigate the efficacy and safety of the selective oxytocin receptor antagonist, Ix-01, in men with lifelong premature ejaculation. J Urol. 2017;197(4):e1344.
- Flouret G, Chaloin O, Borovickova L, et al. Design of novel bicyclic analogues derived from a potent oxytocin antagonist. J Pept Sci. 2006;12(6):412–419.
- Liddle J. Glaxo Group Limited. Substituted diketopiperazines and their use as oxytocin antagonists. United States Patent US 8,071,594. 2011.
- Murphy Goodwin T, Millar L, North L, et al. The pharmacokinetics of the oxytocin antagonist atosiban in pregnant women with preterm uterine contractions. Am J Obstet Gynecol. 1995;173(3):913–917.
- Sparks MA, Freidinger RM, Perlow DS, et al. Merck & Co., Inc. Tocolytic oxytocin receptor antagonists. United States Patent 5,726,172. 1998.
- Vrachnis N, Malamas FM, Sifakis S, et al. The oxytocin-oxytocin receptor system and its antagonists as tocolytic agents. Int J Endocrinol. 2011;2011:1–8.
- Williams PD, Bock MG, Evans BE, et al. Nonpeptide oxytocin antagonists: analogs of L-371,257 with improved potency. Bioorg Med Chem Lett. 1999;9(9):1311–1316.
- Nilsson L, Reinheimer T, Steinwall M, et al. Fe 200 440: a selective oxytocin antagonist on the term-pregnant human uterus. BJOG. 2003;110(11):1025–1028.
- Thornton S, Goodwin TM, Greisen G, et al. The effect of barusiban, a selective oxytocin antagonist, in threatened preterm labor at late gestational age: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2009;200(6):627.e1–627.e10.
- Reinheimer TM. Barusiban suppresses oxytocin-induced preterm labour in non-human primates. BMC Pregn Childbirth. 2007;7(1):S15.
- Arce JC, Ferring BV. Oxytocin receptor antagonist therapy in the luteal phase for implantation and pregnancy in women undergoing assisted reproductive technologies. United States Patent US 9579305. 2017..
- Chollet A, Obseva SA. Pyrrolidine derivatives as oxytocin/vasopressin 1a receptors antagonists. United States Patent Application 2015/0073032. 2015.
- Naylor AM, Russell RJ, Street SDA, et al. Treatment of male sexual dysfunction. United States Patent Application US 2015/0072995. 2015.
- Williams PD, Anderson PS, Ball RG, et al. 1-(((7,7-Dimethyl-2(S)-(2(S)-amino-4-(methylsulfonyl)butyramido)bicyclo[2.2.1]heptan-1(S)-Yl)methyl)sulfonyl)-4-(2-methylphenyl)piperazine (L-368,899): an orally bioavailable, non-peptide oxytocin antagonist with potential utility for managing preterm labor. J Med Chem. 1994;37(5):565–571.
- Brown AD, Calabrese AA, Ellis D. Ixchelsis Limited. Substituted triazole derivatives as oxytocin antagonists. United States Patent Application US 2017/0114040. 2017.
- Brown AT, Brown B, Calabrese A, et al. Triazole oxytocin antagonists: identification of an aryloxyazetidine replacement for a biaryl substituent. Bioorg Med Chem Lett. 2010;20(2):516–520.
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683.