Glaucoma [Citation1–Citation8] and macular degeneration [Citation9–Citation11] are among the ophthalmologic diseases increasingly affecting the aging population worldwide. It is estimated that around 76 million patients will suffer of glaucoma in 2020, whereas the prevalence in the population between 40 and 80 years old is expected to increase to 111.8 million in 2040 [Citation2]. Both conditions are neuropathies and are associated with vision loss, eventually leading to blindness. Glaucoma is a multifactorial disease, with the most salient feature being an elevated intraocular pressure (IOP), which still is nowadays the only clinically modifiable risk factor for its development and progression [Citation3–Citation6]. Macular degeneration, also denominated age-related macular degeneration (AMD) is characterized by the loss of vision in the central part of the visual field, involving the central part of the retina where the macula is found [Citation9–Citation11]. Blurred vision and/or visual hallucinations may also occur in AMD patients. These conditions, together with glaucoma, represent the leading causes of blindness in the industrialized countries [Citation1–Citation6,Citation9]. AMD is classified in two main types, the dry or non-neovascular AMD, with a prevalence of 90% among the affected patients [Citation11], and the wet, or neovascular AMD, which involves abnormal neo-vascularization under or nearby the macula [Citation12,Citation13]. The pathogenesis of AMD is poorly understood, but the prevalent hypothesis is nowadays that metabolic and more precisely mitochondrial dysfunctions are the main triggering factors of the disease [Citation14].
On the other hand, glaucoma pathogenesis is much better understood, compared to that of AMD. There are two main categories of this condition: open-angle glaucoma (OAG) and closed-angle glaucoma [Citation1,Citation2]. OAG is the most common form, with a prevalence of about 2% in adults older than 40 years and develops progressively, leading to an elevated IOP of more than 18 mm Hg. In contrast, closed-angle glaucoma occurs unexpectedly showing signs and symptoms such as blurred vision, eye pain, red eyes and similar vision problems [Citation1,Citation2]. IOP is the result of the balance between the production in the ciliary body of aqueous humor (AH), a fluid rich in bicarbonate but containing many other ion and molecules, and its outflow [Citation1–Citation3,Citation6]. Trabecular meshwork (TM) and Shlemm’s canal (SC) are responsible for the drainage of up to 80% of the total AH within the eye, being considered as the conventional outflow pathway, whereas ciliary muscle, supraciliary and suprachoroidal spaces constitute an alternative or unconventional outflow pathway [Citation1–Citation3,Citation6]. Drugs used for the treatment of glaucoma are as a consequence molecules which can interact with the production or the outflow of the AH. Nowadays there are six classes of pharmacological agents clinically used for the management of glaucoma: the β-adrenergic blockers [Citation15], the systemically or topically acting carbonic anhydrase inhibitors (CAIs) [Citation16–Citation19], the α-adrenergic agonists [Citation20], the cholinergic agonists [Citation21], the prostaglandin analogs [Citation22], and the recently introduced in clinical practice Rho kinase inhibitors [Citation8]. It should be mentioned that other targets, such as the melatonin receptors [Citation23], the fatty acid amide hydrolase (FAAH) [Citation3,Citation23]; the adenosine receptors [Citation3,Citation4,Citation21], or nitric oxide, alone [Citation5] or combined with other pharmacologic agents [Citation24,Citation25] were also explored and led to interesting experimental approaches for the management of glaucoma, which however were not yet translated to the clinic.
Considering the wealth of new data in glaucoma and macular degeneration research/management, the current special issue of Expert Opinion on Therapeutic Patents presents a collection of review articles and several patent evaluations which discuss the latest aspects of literature and patent literature in the field.
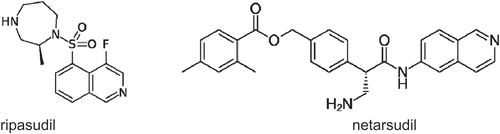
The adrenergic agonists and antagonists, which are among the most used but rather outdated anti-glaucoma drugs, are reviewed by Nocentini and Supuran [Citation26], which present a state of the art review of the field. The carbonic anhydrase (CA) inhibitors (CAIs) are also a rather old class of anti-glaucoma agents [Citation7,Citation27,Citation28]. However, as shown in the review of Supuran et al. [Citation29], the many exciting developments in this field were made possible by a significant number of relevant findings on the various new classes of CAIs reported in the last decade, which differ significantly from the classical sulfonamide inhibitors [Citation30]. The next review articles present the Rho-kinase inhibitors [Citation31], the newest class of anti-glaucoma agents that arrived in clinics in 2017 (ripasudil) and 2019 (netarsudil), respectively (), with their particular pharmacologic and chemical features.
The prostaglandin (PG) analogs, a highly successful class of anti-glaucoma agents, with many representatives widely used clinically, alone or in combination with other agents, were reviewed by Angeli and Supuran [Citation32]. Some new drug targets for innovative such medications are presented exhaustively in the paper by Guglielmi et al. [Citation3]. The next review article of the special issue, by Bua and Supuran [Citation33], presents a fascinating paper on the diagnostic markers for glaucoma, a rather orphan field in the scientific literature but with a wealth of patents claiming various possibilities, based on a variety of diverse biomarkers. The last review article of the special issue deals with the agents used for the prevention and treatment of macular degeneration and macular edema [Citation34]. They include verteporfin, for photodynamic therapy, as well as anti-VEGF agents, the aptamer pegaptanib, the monoclonal antibodies (MAbs) ranibizumab (Lucentis®) and bevacizumab (Avastin®) and the fusion protein aflibercept (Eylea®). All these drugs are effective only for the wet form of AMD, whereas for the dry form there is no treatment available. Macular edema is, on the other hand, treated with nonsteroidal anti-inflammatory drugs and carbonic CAIs [Citation34]. It should be mentioned that only in the last two decades the considerable advances in biomedical fields made possible the advent of these drugs, which completely changed the fate of patients affected by AMD.
The patent evaluation by Capasso and Winum [Citation35] examines an alternative method for the management of macular degeneration (with botulinum toxin), whereas the patent evaluation by Bonardi and Supuran [Citation36] discusses the synthesis of novel Rho-kinase and monoamine transporters inhibitors possessing a benzamide or isoquinoline amide scaffolds, as well as the long term outcome of glaucoma treatment with rho kinase inhibitors.
The most relevant message of this special issue is that the important developments in the ophthalmologic field led to more effective agents for the management of glaucoma and AMD, a condition for which only few years earlier there was no available treatment. Many of the review articles form this special issue also illustrate the present medicinal chemistry and pharmacologic research efforts for finding better such drugs, and this may constitute an important inspiration for scientists active in these fields.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. 2019;210:180–187.
- Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090.
- Guglielmi P, Carradori S, Campestre C, et al., Novel therapies for glaucoma: a patent review (2013-2019). Expert Opin Ther Pat. 2019 Aug 11;29(10):769–780. Article in this special issue. DOI:10.1080/13543776.2019.1653279.
- Mietzner R, Breunig M. Causative glaucoma treatment: promising targets and delivery systems. Drug Discov Today. 2019;24:1606–1613.
- Garhöfer G, Schmetterer L. Nitric oxide: a drug target for glaucoma revisited. Drug Discov Today. 2019;24:1614–1620.
- Carta F, Supuran CT, Scozzafava A. Novel therapies for glaucoma: a patent review 2007-2011. Expert Opin Ther Pat. 2012;22:79–88.
- Masini E, Carta F, Scozzafava A, et al. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat. 2013;23:705–716.
- Schehlein EM, Robin AL. Rho-associated kinase inhibitors: evolving strategies in glaucoma treatment. Drugs. 2019;79:1031–1036.
- Ferris FL 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851.
- Horani M, Mahmood S, Aslam TM. Macular atrophy of the retinal pigment epithelium in patients with neovascular age-related macular degeneration: what is the link? Part I: a review of disease characterization and morphological associations. Ophthalmol Ther. 2019;8:235–249.
- Campbell M, Doyle SL. Current perspectives on established and novel therapies for pathological neovascularization in retinal disease. Biochem Pharmacol. 2019;164:321–325.
- Velez-Montoya R, Oliver SC, Olson JL, et al. Current knowledge and trends in age-related macular degeneration: genetics, epidemiology, and prevention. Retina. 2014;34:423–441.
- Mehta S. Age-related macular degeneration. Prim Care. 2015;42:377–391.
- Léveillard T, Philp NJ, Sennlaub F. Is retinal metabolic dysfunction at the center of the pathogenesis of age-related macular degeneration? Int J Mol Sci. 2019;20:E762.
- Nocentini A, Ceruso M, Bua S, et al. Discovery of β-adrenergic receptors blocker-carbonic anhydrase inhibitor hybrids for multitargeted antiglaucoma therapy. J Med Chem. 2018;61:5380–5394.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7:168–181.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem. 2016;31:345–360.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Pat. 2000;10:575–600.
- Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov. 2017;12:61–88.
- Lusthaus JA, Goldberg I. Brimonidine and brinzolamide for treating glaucoma and ocular hypertension; a safety evaluation. Expert Opin Drug Saf. 2017;16:1071–1078.
- Lu LJ, Tsai JC, Liu J. Novel pharmacologic candidates for treatment of primary open-angle glaucoma. Yale J Biol Med. 2017;90:111–118.
- Perrino E, Uliva C, Lanzi C, et al. New prostaglandin derivative for glaucoma treatment. Bioorg Med Chem Lett. 2009;19:1639–1642.
- Spadoni G, Bedini A, Furiassi L, et al. Identification of bivalent ligands with melatonin receptor agonist and fatty acid amide hydrolase (FAAH) inhibitory activity that exhibit ocular hypotensive effect in the rabbit. J Med Chem. 2018;61:7902–7916.
- Fabrizi F, Mincione F, Somma T, et al. A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem. 2012;27:138–147.
- Supuran CT. Carbonic anhydrase inhibitor – NO donor hybrids and their pharmacologic applications. In: Bonavida B, Morbidelli L, editors. Therapeutic applications of nitric oxide in cancer and inflammatory-related disorders. New York: Elsevier; 2019:229–242.
- Nocentini A, Supuran CT. Adrenergic agonists and antagonists as antiglaucoma agents: a literature and patent review (2013-2019). Expert Opin Ther Pat. 2019;29(10)805–815. Article in this special issue. DOI:10.1080/13543776.2019.1665023
- Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat. 2018;28:709–712.
- Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat. 2018;28:713–721.
- Supuran CT, Altamimi ASA, Carta F. Carbonic anhydrase inhibition and the management of glaucoma: a literature and patent review 2013-2019. Expert Opin Ther Pat. 2019:29(10); 781–792. Article in this special issue. DOI:10.1080/13543776.2019.1679117.
- Nocentini A, Supuran CT, Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov. 2019 Aug 22;14(11):11751–1197. DOI:10.1080/17460441.2019.1651289.
- Berrino E, Supuran CT. Rho-kinase inhibitors in the management of glaucoma. Expert Opin Ther Pat;2019;29(10):817–827. Article in this special issue. DOI:10.1080/13543776.2019.1670812.
- Angeli A, Supuran CT. Prostaglandin receptor agonists as antiglaucoma agents (a patent review 2013 – 2018). Expert Opin Ther Pat. 2019;29(10):793–803. Article in this special issue. DOI:10.1080/13543776.2019.1661992
- Bua S, Supuran CT. Diagnostic markers for glaucoma: a patent and literature review (2013-2018). Expert Opin Ther Pat. 2019;29(10)829–839. Article in this special issue. DOI:10.1080/13543776.2019.1667336
- Supuran CT. Agents for the prevention and treatment of age-related macular degeneration and macular edema: a literature and patent review. Expert Opin Ther Pat. 2019;29(10):761–767. Article in this special issue. DOI:10.1080/13543776.2019.1671353
- Capasso C, Winum JY. Novel method of treating macular degeneration: A patent evaluation (WO2018/107005). Expert Opin Ther Pat. 2019;29(10):749–752. Article in this special issue. DOI:10.1080/13543776.2019.1661991
- Bonardi A, Supuran CT, Treatment of glaucoma and ocular hypertension using rho kinase inhibitors: patent evaluation of US2018244666 and US2018256595. Expert Opin Ther Pat. 2019 Aug 22;29(10)753–759. Article in this special issue. DOI:10.1080/13543776.2019.1658743.