Protozoans are widespread organisms in most habitats worldwide, being a paraphyletic group with an intricate evolutionary history [Citation1,Citation2]. There is a multitude of phyla, genera, and protozoan species, many of which ecologically and industrially relevant, but some of them are also parasites infecting humans and wild as well as domestic animals. The caused diseases after such infections range from mild to moderate (e.g. infections with Toxoplasma gondii or Entamoeba histolytica), may be rather serious conditions (in case of Cryptosporidium parvum, Giardia lamblia, Trichomonas vaginalis, Babesia spp. infections) or very serious and widespread ones, such as malaria (disease caused by at least five different Plasmodium species), leishmaniasis (a multitude of Leishmania species provoke disease), Chagas disease (produced by Trypanosoma cruzi), or African sleeping disease (caused by subspecies of T. brucei, i.e. T. b. gambiense and T.b.rhodesiense) [Citation3,Citation4], etc. Several fatal protozoal diseases are also produced by amoebae, such as Naegleria fowleri [Citation5] Acanthamoeba spp [Citation6]. or Balamuthia mandrillaris [Citation6] which are fortunately rare. Albeit the 12 protozoan genera that produce human disease are now well studied (), the drug armamentarium to treat them is rather scarce. Indeed, few available drugs for effectively treating these conditions are available, they show relevant toxicity, low therapeutic index, and even worse, extensive resistance has been developed to most of them [Citation7]. This situation has several causes, which will be briefly presented here.
Table 1. Pathogenic protozoans and the diseases provoked they provoked as well as drugs used for their management.
Most parasitic protozoans have complicated life cycles, with many different stages and also more than one host, with the vertebrate (human) being generally just one component in their cycle [Citation3,Citation4,Citation7]. For example, Plasmodia, the organisms provoking malaria, have at least six different stages/phases during their life cycle, with the various forms of the pathogen present in different organs and tissues, but also with many different genes that are expressed in the different phases, and with a substantial capability to evade the host immune defenses [Citation8]. Other pathogenic protozoans have insects as secondary hosts (e.g. T. cruzi, T. brucei, and Leishmania spp.), or even other mammals, such as Toxoplasma gondii (usually cats and other Felidae) [Citation4,Citation5,Citation9]. For Cryptosporidium spp., Giardia lamblia, Entamoeba spp. or Trichomonas vaginalis infections, it seems that there is not an intermediate host [Citation3]. Thus, such complicated life cycles constitute the first serious challenge to identify druggable antiprotozoal targets and in some cases also to grow various forms of the pathogen in laboratory for testing drug candidates [Citation3,Citation9–14].
The second factor involves the perception that most people have about protozoan diseases. Although considered tropical diseases, which affect a relatively low number of patients from developing countries, nowadays protozoan diseases are widespread all over the world, with only malaria provoking a huge number of infections and many casualties yearly [Citation7,Citation8,Citation14]. Due to climate change, many protozoans and their intermediate hosts are nowadays found in parts of Europe, North America, and Australia leading to the possibility of serious outbreaks also in these parts of the world.
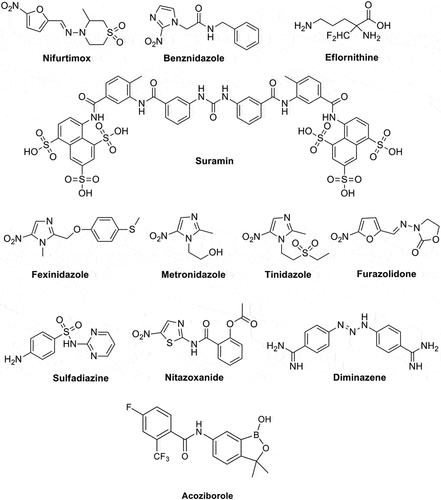
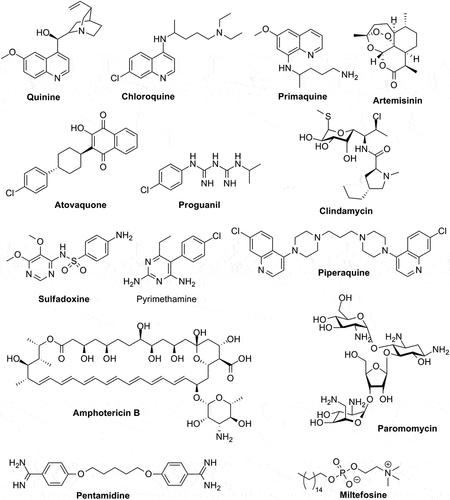
As seen from and , in 2023 only 25 drugs are in clinical use for the treatment of these infections. With a few exceptions, all these drugs were discovered in the ‘30-‘70s (except artemisinin and its derivatives, found in the ‘80s) and are characterized by high toxicity, low therapeutic index, and many side effects [Citation3,Citation12–16]. Furthermore, just a limited number of chemotypes are present in the armamentarium of the anti-protozoal drugs, with the nitro-azoles being predominant, followed by the dihydrofolate reductase and dihydropteroate synthase inhibitors [Citation7] (). Although the recent approval of the two new agents, Fexinidazole and Nitazoxanide, is remarkable and demonstrates that relevant achievements can be obtained, both of them belong to the same class of nitroazoles [Citation3] – see , in clinical use for many decades and with all drug resistance problems typical for the entire class [Citation7]. Acoziborole (SCYX-7158) () is one of the few compounds in Phase III clinical trials for the treatment of T. brucei infection, belonging to an entirely new class, as it is a benzoxaborole derivative, the first to be employed as an antiprotozoal drug [Citation17,Citation18]. One should anyhow stress that benzoxaboroles possess a range of well-known pharmacological activities [Citation17] in addition to the anti-protozoal one observed for acoziborole, among which anti-bacterial, anti-fungal, antiviral as well as carbonic anhydrase inhibitory action, which raises questions regarding the potential side effects of the new drug [Citation19–21]. Several new generation azoles, such as ravuconazole and its prodrug (fos-ravuconazole) seem to be promising anti-T. cruzi agents [Citation22], but there is limited information regarding their clinical trials. It is rather disheartening to see that even for malaria, the worst of the documented protozoan diseases, most of the clinical trials that are registered in EU deal with various vaccine candidates or combination therapies of existing drugs, but do not consider novel chemical entities. However, there are promising and encouraging results about the vaccines, as I will discuss later in the paper.
Figure 1. Chemical structure of the 25 clinically used drugs for the management of protozoan infections.
This special issue of Expert Opinion on Therapeutic Patents presents a topical collection of review articles dealing with most of the protozoan pathogenic organisms mentioned in the introduction. Vermelho’s group discusses Chagas disease treatment options in a paper where both scientific and patent literature is documented [Citation23]. Drugs and nanoformulations for the management of Leishmania infection are dealt with in a review article from Nico’s group [Citation24], whereas the management of malaria with drugs [Citation25] and vaccines [Citation26] is extensively documented in two different review articles. Indeed, immunization with recombinant proteins and multiantigen antimalaria vaccines have shown promising results in several clinical trials, which are discussed in detail in the review from Rovero’s group [Citation26]. The management of babesia, amoeba, and other zoonotic diseases provoked by protozoans are discussed by Capasso and Supuran [Citation27], whereas drugs for the treatment of Trichomonas, giardia, toxoplasma, and other ‘minor’ protozoan infections: are dealt with in a paper by Guglielmi [Citation28]. Carradori presents a detailed review on emerging strategies to design new anti-Trypanosoma brucei agents [Citation29].
As briefly discussed here, although relevant scientific advances were done in understanding the life cycle of pathogenic protozoa, translational studies from the lab to the clinic are few and there are no major new classes of antimalarial drugs being developed (the exception being the benzoxaborole derivative acoziborole mentioned above). Specifically, with the exception of two nitroazoles (see above), which were approved in the last 5 years, and acoziborole (), no other new drugs have emerged to treat protozoan-based diseases. However, these three good examples constitute an important new step toward the drug design of antiprotozoan drugs. This special issue thus has also the role to bring to the attention of the scientific community and pharmaceutical industries that such neglected tropical diseases should be properly considered also due to their potential to develop pandemic events. With the disruptions and terrible medical/social situations experienced due to the SARS-CoV-2 pandemic for the last three years [Citation30,Citation31], scientists from academia and industry have become aware of these challenges and it is envisageable that high-quality drug design studies and new antiprotozoal drugs will emerge.
Declaration of interests
CT Supuran is Editor-in-Chief of the Expert Opinion on Therapeutic Patents. He was not involved in the assessment, peer review, or decision-making process of this paper. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lax G, Lee WJ, Eglit Y, et al. Ploeotids represent much of the phylogenetic diversity of euglenids. Protist. 2019;170(2):233–257.
- Cavalier-Smith T, Chao EE, Snell EA, et al. Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts (animals, fungi, choanozoans) and amoebozoa. Mol Phylogenet Evol. 2014;81:71–85.
- Vermelho AB, Supuran CT. Antiprotozoal drug development and delivery. Cham Switzerland: Springer Nature; 2022. p. 1–330.
- Vermelho AB, Rodrigues GC, Supuran CT. Why hasn’t there been more progress in new Chagas disease drug discovery? Expert Opin Drug Discov. 2020;15(2):145–158.
- Debnath A. Drug discovery for primary amebic meningoencephalitis: from screen to identification of leads. Expert Rev Anti Infect Ther. 2021;19(9):1099–1106.
- Kofman A, Guarner J, Humphries RM. Free living amoebic infections: review. J Clin Microbiol. 2022;60(1):e0022821.
- Vermelho AB, Mori M, Donald WA, et al. Challenges and promises for obtaining new antiprotozoal drugs: what’s going wrong? In: Vermelho A Supuran C, editors. Antiprotozoal drug development and delivery. Cham Switzerland: Springer Nature; 2022. p. 321–330.
- Consalvi S, Tammaro C, Appetecchia F, et al. Malaria transmission blocking compounds: a patent review. Expert Opin Ther Pat. 2022;32(6):649–666.
- Krungkrai J, Prapunwatana P, Wichitkul C, et al. Molecular biology and biochemistry of malarial parasite pyrimidine biosynthetic pathway. Southeast Asian J Trop Med Public Health. 2003;34(2):32–43.
- Müller J, Hemphill A. In vitro culture systems for the study of apicomplexan parasites in farm animals. Int J Parasitol. 2013;43(2):115–124.
- Wang B, Castellanos-Gonzalez A, AC W Jr. Novel drug targets for treatment of cryptosporidiosis. Expert Opin Ther Targets. 2020;24(9):915–922.
- Pessanha de Carvalho L, Kreidenweiss A, Held J. Drug repurposing: a review of old and new antibiotics for the treatment of malaria: identifying antibiotics with a fast onset of antiplasmodial action. Molecules. 2021;26(8):2304.
- Vallejo M, Reyes PP, Martinez Garcia M, et al. Trypanocidal drugs for late-stage, symptomatic Chagas disease (Trypanosoma cruzi infection). Cochrane Database Syst Rev. 2020 Dec 11;12(12):CD004102.
- de Araújo RV, Santos SS, Sanches LM, et al. Malaria and tuberculosis as diseases of neglected populations: state of the art in chemotherapy and advances in the search for new drugs. Mem Inst Oswaldo Cruz. 2020;115:e200229.
- Roatt BM, de Oliveira Cardoso JM, De Brito RCF, et al. Recent advances and new strategies on leishmaniasis treatment. Appl Microbiol Biotechnol. 2020;104(21):8965–8977.
- Mansoldo FRP, Carta F, Angeli A, et al. Chagas disease: perspectives on the past and present and challenges in drug discovery. Molecules. 2020;25(22):5483.
- Wall RJ, Rico E, Lukac I, et al. Clinical and veterinary trypanocidal benzoxaboroles target CPSF3. Proc Natl Acad Sci U S A. 2018;115(38):9616–9621.
- Nocentini A, Supuran CT, Winum JY. Benzoxaborole compounds for therapeutic uses: a patent review (2010- 2018). Expert Opin Ther Patents. 2018 Jun;28(6):493–504.
- Bonardi A, Nocentini A, Cadoni R, et al. Benzoxaboroles: new potent inhibitors of the carbonic anhydrases of the pathogenic bacterium vibrio cholerae. ACS Med Chem Lett. 2020;11(11):2277–2284.
- Nocentini A, Cadoni R, Dumy P, et al. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J Enzyme Inhib Med Chem. 2018;33(1):286–289.
- Alterio V, Cadoni R, Esposito D, et al. Benzoxaborole as a new chemotype for carbonic anhydrase inhibition. Chem Commun (Camb). 2016;52(80):11983–11986.
- Mazzeti AL, Capelari-Oliveira P, Bahia MT, et al. Review on experimental treatment strategies against trypanosoma cruzi. J Exp Pharmacol. 2021 Mar 31;13:409–432. DOI:10.2147/JEP.S267378.
- Pestana Caroli A, Mansoldo FRP, Cardoso VS, et al. Are patents important indicators of innovation for chagas disease treatment? Expert Opin Ther Pat. 2023;33:1–17. this issue. DOI:10.1080/13543776.2023.2176219.
- Verdan M, Taveira I, Lima F, et al. Drugs and nanoformulations for the management of Leishmania infection: a patent and literature review (2015-2022). Expert Opin Ther Pat. 2023;33: this issue.
- Lopes EA, Santos MMM, Mori M. Anti-malaria drugs: what’s new in the patents? Expert Opin Ther Pat. 2023;33:in press (this issue).
- Quagliata M, Papini AM, Rovero P. Malaria vaccines. Expert Opin Ther Pat. 2023;33:1–10. in press (this issue). DOI:10.1080/13543776.2023.2190884.
- Capasso C, Supuran CT. The management of babesia, amoeba and other zoonotic diseases provoked by protozoa. Expert Opin Ther Pat. 2023;33:in press (this issue).
- Francesca A, Granese A, Guglielmi P. Novel therapeutic opportunities for Toxoplasma gondii, Trichomonas vaginalis and Giardia intestinalis infections. Expert Opin Ther Pat. 2023;33:in press (this issue).
- Melfi F, Carradori S, Campestre C, et al. Emerging compounds and therapeutic strategies to treat infections from trypanosoma brucei: an overhaul of the last 5-years patents. Expert Opin Ther Pat. 2023;33:1–17. in press (this issue). DOI:10.1080/13543776.2023.2193328.
- Mori M, Capasso C, Carta F, et al. A deadly spillover: sARS-CoV-2 outbreak. Expert Opin Ther Pat. 2020;30(7):481–485.
- Supuran CT. Coronaviruses. Expert Opin Ther Pat. 2021;31(4):291–294.