1. Rationale for the development of bispecific agents
Psoriasis is a chronic inflammatory skin disorder affecting 2.5% of the population worldwide.
Its pathogenic mechanism involves tissue cells, in particular keratinocytes, and a wide array of immune cells. In the psoriatic microenvironment, cytokines play a critical role in the initiation and maintenance of the psoriatic phenotype. The introduction of anti-cytokine antibodies, fusion proteins and small molecules (e.g. apremilast), profoundly changed the therapeutic paradigm as they demonstrated high efficacy in the majority of patients, though not all patients respond to therapy and loss of treatment responsiveness usually occurs over time.[Citation1,Citation2] Classically, TNF-α has been considered one of the key-cytokines in psoriasis, albeit the essential role of the IL-23/IL-17A axis has recently emerged, leading to the identification of new therapeutic targets and, thus, to the development of novel drugs that resulted more effective than TNF-α agents.[Citation3–Citation6] Head-to-head clinical trials testing IL-17A-blocking agents vs. anti-TNF-α inhibitors or p40IL-12/IL-23 neutralizing agent showed a faster response and a higher effectiveness in terms of psoriasis area severity index (PASI175), and particularly, PASI90, and PASI100, using anti-IL-17A agents.[Citation6,Citation7] The remarkable clinical efficacy achieved with IL-17A blockers confirmed the centrality of IL-17A signaling to psoriasis and a more relevant pathogenic role compared to other cytokines. Notwithstanding the superior effectiveness, IL-17A blockers did not obtain clinical response in all patients nor all responder patients achieved a complete resolution of the clinical manifestations.[Citation6,Citation7] Thereby, a novel therapeutic strategy is still needed.
Seminal studies suggested the concurrent blockade of two cytokines as future winning strategy in the treatment of psoriasis because of (i) the more efficient suppression of critical pathogenic steps or pathways; and (ii) the potential reduced risk of developing alternative circuits driving disease inflammation. Specifically, TNF-α and IL-17A may represent two targets to be simultaneously neutralized as they are considered the most relevant mediators in the psoriatic pathogenic process.[Citation3] They act in synergism on keratinocytes inducing the expression of psoriasis-signature genes involved in the key-pathogenic, inflammatory, and reverberating circuits. Notably, the therapeutic response to either a TNF-α or an IL-17A blocker correlated with the reduction of IL-17A/TNF-α synergistic gene expression.[Citation3,Citation8] This intimate link between IL-17A and TNF-α occurs at different levels. TNF-α activates myeloid dendritic cells in producing IL-23, and the blockade by a TNF-α inhibitor, namely etanercept, suppressed the IL-23-driven Th17 response.[Citation9] Strikingly, etanercept efficacy is strictly linked to the IL-17A signaling suppression and not to TNF-α.[Citation10] Early responses to etanercept included both the reduced expression of IL-17RC, an IL-17 receptor subunit, and an attenuation of the IL-17 receptor signaling activity.[Citation11] Nevertheless, the earliest changes induced by etanercept in lesional psoriatic skin did not include the decreased expression of T cell-derived products, namely IL-17A, IL-22 and IFN-γ, indicating that adaptive immune response is subsequently affected by TNF-α blockade.[Citation11] Along these lines, another study described an early suppression of the innate immune response after 10-day treatment with adalimumab, another TNF-α inhibitor.[Citation12] Because TNF-α inhibition affects the innate immune system well before blunting the adaptive immune response, that is instead suppressed by the IL-17A neutralization, the use of bispecific agents blocking one ‘early’ mediator belonging to the innate immunity, TNF-α, and one essential product mainly secreted by the adaptive immune compartment, IL-17A, may result in superior effectiveness compared to single-cytokine targeting agents.
2. Bispecific agents targeting psoriasis-signature cytokines
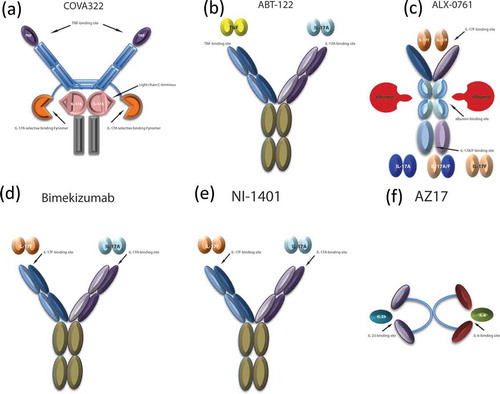
Up to date, a few bispecific agents have been designed and tested for the treatment of psoriasis ( and ). The most advanced in the development program is COVA322, a bispecific TNF-α/IL-17A inhibitor that is currently being tested in phase Ib/IIa study in psoriasis patients (NCT02243787).[Citation13] COVA 322 is a fynomer antibody, resulting from the fusion of high-affinity IL-17A binding fynomer to the light chain of the fully human anti-TNF-α antibody, adalimumab (). Fynomers are small 7-kDa globular proteins derived from the SH3 domain of the human Fyn kinase that can be engineered to bind with antibody-like affinity and specificity to virtually any target.[Citation13]
Table 1. Bispecific agents currently tested for psoriasis.
Figure 1. Bispecific therapeutic agents in development for the treatment of psoriasis. (a) COVA322, constituted by a high-affinity IL-17A selective binding Fynomer fused to the C-terminal light chain of the already-marketed fully human anti-TNF-α antibody, adalimumab, maintaining TNF-α binding actvity. (b) ABT-122, a dual variable domain immunoglobulin targeting both TNF-α and IL-17. (c) ALX-0761, a trivalent anti-IL-17A/F nanobody, consisting of an N-terminal IL-17F specific moiety, a C terminal moiety that binds both IL-17A and IL-17F, and a central portion binding albumin. (d) Bimekizumab, a humanized monoclonal antibody inhibiting both IL-17A and IL-17F. (e) NI-1401, a fully human monoclonal antibody neutralizing both IL-17A and IL-17F. (f) AZ17, consisting of two single-chain Fragment variables (scFvs), each one binding IL6 or IL23, and linked by a polyethylene glycol (PEG) moiety.
COVA322 improves acute inflammation with a satisfactory safety profile in preclinical models, demonstrating pharmacokinetics similar to adalimumab, without in vivo degradation of the fynomer portion.[Citation13] COVA322 was well tolerated in single and repeat doses administered to cynomolgus monkeys with no cardiovascular, respiratory and central nervous system adverse effects being observed.[Citation13]
A first-in-man, single dose escalation, tolerability, safety, pharmacokinetic and efficacy phase Ib/IIa is currently underway. Patients with stable chronic moderate-to-severe plaque psoriasis will receive ascending single-doses of COVA322 or placebo as a constant-rate i.v. infusion, followed by 12 weeks of evaluation.
Another bispecific antibody (BsAb) neutralizing IL-17A/TNF-α, ABT-122, is not being investigated yet for psoriasis treatment, as it is exclusively tested in several phase II trials for psoriatic arthritis (NCT02429895 and NCT02349451) and rheumatoid arthritis (NCT02141997, NCT01853033 and NCT02433340).
Beside the synergism with TNF-α, IL-17A effects could also be potentiated by IL-17F, which shows similar biological activity and, possibly, even a non-redundant role in vivo. IL-17F and IL-17A show about 50% sequence homology and signal through the same receptor, though, IL-17A is approximately 10–30 times more potent than IL-17F in triggering downstream gene expression.
ALX-0761 (40 kD) is a trivalent, bispecific nanobody that blocks both IL-17A and IL-17F and that binds to human serum albumin for plasma half-life extension (). This novel therapeutic entity may provide a more effective way to block inflammatory responses as demonstrated by the improvement of arthritis and X-ray score in a preclinical model of rheumatoid arthritis.[Citation14] Currently, a phase Ib study with ALX-0761 in patients with psoriasis is ongoing.
Bimekizumab, (UCB4940), is another humanized monoclonal antibody inhibiting both IL-17A and IL-17F (). A phase I study assessing safety, pharmacokinetics, and pharmacodynamics in patients with psoriasis (NCT02529956) or with psoriatic arthritis (NCT02141763) have been completed. Likewise, NI-1401, a fully human monoclonal antibody neutralizing both IL-17A and IL-17F, completed phase Ia and Ib trials testing its safety, pharmacokinetics, and immunogenicity in healthy volunteers (NCT01540760 and NCT01480310) but no results have been released yet.
Another bispecific agent therapeutically relevant for the treatment of psoriasis, AZ17, neutralizes both IL-6 and IL-23, which are involved in the Th17 differentiation process. Whilst IL-6 acts early in the initiation phases of Th17 cell differentiation, inducing IL-23 receptor expression, IL-23 is thought to promote Th17 cell lineage stabilization, expansion, and maintenance, stimulating Th17 cytokine production. AZ17 was successfully tested in two relevant preclinical in vivo mouse models, showing greater efficacy in improving psoriasiform inflammation and epidermal thickness, compared to individual anti-IL-6 or anti-IL-23 antibodies.[Citation15] Of note, this BsAb demonstrated selectivity in neutralizing IL-23, but not IL-12.[Citation15]
3. Conclusion
This new therapeutic approach constituted by bispecific agents derives from a better understanding of the pathogenic mechanisms underlying the psoriatic plaque formation. Currently, key cytokines such as IL-17, IL-23, IL-6, and TNF, have been identified as targets, but other pathogenically relevant cytokines such as IL-19, IL21, and IL-22, could also be take into account for developing novel bispecific agents. Scarce clinical preliminary data do not allow a profound discussion about benefit/risk ratio related to this class of agents. However, their future use in psoriasis could expand the therapeutic armamentarium providing a further option to effectively control psoriasis.
4. Expert opinion
BsAbs bind to two different antigens, or two different epitopes on the same antigen. They were first described over 30 years ago, and many different bispecific molecules have been produced since then.[Citation16]
One of the potential advantages of BsAbs over standard antibodies relates to their avidity. Although yet to prove, the ‘avidity hypothesis’ holds that BsAbs may more likely bind to cells that bear both targets than to cells that express only one, while monospecifics antibodies bind indiscriminately. Thereby, BsAbs may be more potent and safer.
However, their use in the clinic has been relatively limited so far, mostly due to disappointing initial clinical studies. Loss of parental antibody affinity, severe adverse events, immunogenicity, poor stability and low production yields has been the main reasons for this failure.[Citation17] In fact, to date, only two agents have been approved for clinical use, catumaxomab (anti-EpCAM/anti-CD3) and blinatumomab (anti-CD19/anti-CD3), both for cancer treatment.[Citation16]
Excessive immune suppression leading to unacceptable risk of infections and other adverse effects may be one of the major caveats associated with dual cytokine inhibition. In fact, the results of clinical trials in rheumatoid arthritis with combinations of biologics (anakinra and etanercept or abatacept and etanercept) led to increased infections with no significant clinical benefits.[Citation18,Citation19] Another peculiar safety aspect that needs to be investigated is the immunogenicity owned by bispecific agents.
To optimize the administration of two biological agents, low co-administered doses of anti-TNF and anti-IL-17 agents in a collagen-induced arthritis mouse model resulted in significant anti-inflammatory effect, in contrast to monotherapies, showing that partial inhibition of both cytokines may be sufficient to provide clinical efficacy.[Citation20] In fact, this dual TNF/IL-17A inhibition may possibly dissociate clinical efficacy from immune suppression.
Because the management of immune-mediated disorders, such as rheumatoid arthritis, psoriatic arthritis, and psoriasis, is still characterized by unmet medical needs, alternative strategies are necessary to further improve patients’ lives and, overall, dual cytokine inhibition may represent a very promising and innovative approach for the treatment of psoriasis and other immune-mediated disorders. However, to maintain the favorable safety profile of the current biologic agents and to obtain high efficacy will be challenging for bispecific agents, in particular if one considers the novel therapeutic paradigm that will include IL-17 blockers. Indeed, with anti-TNF agents, the improvement of at least 75% of baseline PASI score could be considered a valuable therapeutic outcome to be pursued. Recently, the achievement of PASI75 arose dissatisfaction as patient’s needs of a clear or almost clear skin emerged.[Citation21] The striking superiority of IL-17 blockers in achieving PASI90 or PASI100 response, compared to previous therapies, could fulfill, at least for a consistent part of treated patients, the expectation of a clear or almost clear skin, maintaining a satisfactory safety profile. Thus, the therapeutic goals used in recent clinical trials testing anti-IL-17 agents lifted up to PASI90 or PASI100 response.[Citation21] In this new therapeutic scenario, bispecific will be called to demonstrate a very high clinical profile. Promisingly, the preliminary clinical outcomes on bimekizumab presented at the 2016 American Academy of Dermatology, held in Washington, have brought high expectations. A first-in-human, placebo-controlled, single-dose-escalating study randomizing 13 subjects to placebo and 26 to escalating doses of bimekizumab: 8, 40, 160, 480, and 640 mg.[Citation22] All subjects received one dose at the baseline, and then were followed for 20 weeks. Clinical response was detected after 160, 480, and 460 mg injection, by Week 2, reaching the maximal improvement between Week 4 and 6, and that response was maintained through 16–20 weeks.[Citation22] In patients treated with higher bimekizumab doses, PASI90 response was more frequently observed: in the top two dose groups, PASI 90 was achieved by 83% of patients from Week 6–12, and in 90% at Week 12. Overall, 78 adverse events occurred in all 39 participants have been reported and classified as mild or moderate, except one. The serious adverse event, experienced by one 40 mg bimekizumab-treated patient, was represented by vomiting that required hospitalization, though the adverse event was not considered treatment-related. To evaluate bimekizumab safety and efficacy in psoriasis, as well as for other bispecific agents, large-scale population and head-to-head studies are necessary. For other immune-mediated disorders, namely rheumatoid arthritis and psoriatic arthritis, the clinical development of this class of agents is in a more advanced stage, with ongoing head-to-head trials (i.e. adalimumab vs. ABT-122 in rheumatoid arthritis and psoriatic arthritis), or ongoing ‘add-on’ trials with other biologics (i.e. bimekizumab added to certolizumab pegol in rheumatoid arthritis).
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Levin EC, Gupta R, Brown G, et al. Biologic fatigue in psoriasis. J Dermatolog Treat. 2014;25:78–82.
- Chiricozzi A, Caposiena D, Garofalo V, et al. A new therapeutic for the treatment of moderate to severe plaque psoriasis: apremilast. Expert Rev Clin Immunol. 2016 Mar;12:237–249.
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677–687.
- Lowes MA, Russell CB, Martin DA, et al. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013;34(4):174–181.
- Krueger JG, Fretzin S, Suárez-Fariñas M, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130:145–154.
- Griffiths CEM, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409.
- Chiricozzi A, Nograles KE, Johnson-Huang LM, et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One. 2014;9:e90284.
- Zaba LC, Cardinale I, Gilleaudeau P, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194.
- Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022–1395.
- Johnston A, Guzman AM, Swindell WR, et al. Early tissue responses in psoriasis to the antitumour necrosis factor-α biologic etanercept suggest reduced interleukin-17 receptor expression and signalling. Br J Dermatol. 2014;171:97–107.
- Hendriks AG, van der Velden HM, Wolberink EA, et al. The effect of adalimumab on key drivers in the pathogenesis of psoriasis. Br J Dermatol. 2014;170:571–580.
- Silacci M, Lembke W, Woods R, et al. Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases. MAbs. 2016;8:141–149.
- Vanheusden K, Detalle L, Hemeryck A, et al. Pre-clinical proof-of-concept of ALX-0761, a nanobody neutralising both IL-17A and IL-17F in a cynomolgus monkey collagen induced arthritis model. Poster n. 1287, presented at the Annual Meeting of the American College of Rheumatology (ACR); 2013 Oct 26–30; San Diego, CA.
- Stenderup K, Rosada C, Shanebeck K, et al. AZ17: a new bispecific drug targeting IL-6 and IL-23 with potential clinical use-improves psoriasis in a human xenograft transplantation model. Protein Eng Des Sel. 2015;28:467–480.
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316.
- Mabry R, Gilbertson DG, Frank A, et al. A dual-targeting PDGFRbeta/VEGF-A molecule assembled from stable antibody fragments demonstrates anti-angiogenic activity in vitro and in vivo. MAbs. 2010;2(1):20–34.
- Weinblatt M, Schiff M, Goldman A, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis. 2006;66:228–234.
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412–1419.
- Fischer JA, Hueber AJ, Wilson S, et al. Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 2015;67:51–62.
- Puig L. PASI90 response: the new standard in the therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645–648.
- Glatt S, Helmer E, Strimenopoulou F, et al. First-in-human IL-17A and IL-17F blockade with bimekizumab in patients with mild-to-moderate psoriasis: results of a randomized, placebo-controlled, single-dose-escalating study. 74th Annual Meeting American Academy of Dermatology; Washington, DC, 2016 Mar 4–8. Abstract F053.
