ABSTRACT
Introduction: Discovery of oncogenic mutations in the KIT and PDGFRA tyrosine kinase receptor was a crucial step for the development of tyrosine kinase inhibitors (TKIs). Since then, GIST became a model for the development of molecular-targeted therapy, which led to dramatically improved median overall survival of advanced GIST. Still, further progress is needed after third-line or for TKI resistant mutations.
Areas covered: In this review, after a brief introduction on imatinib, sunitinib, and regorafenib, an overview of TKIs that was evaluated beyond these drugs is provided, with a main focus on the novel approved TKIs.
Expert opinion: Combination therapies have thus far not fulfilled their promise in GIST, nor did immunotherapy. Increased understanding of GIST and advances in the development of molecular-targeted drugs led to the introduction of ripretinib and avapritinib. Furthermore, NTRK inhibitors became available for ultrarare NTRK fusions. Solutions for NF1 and BRAF mutated and SDH-deficient GIST are still to be awaited. This all underlines the need for adequate molecular profiling of high-risk GISTs before treatment is started. Possibly by using circulating tumor DNA in the future, targeting resistance mutations with specific drugs along the course of the disease would be easier, avoiding multiple tumor biopsies.
1. Introduction
Although gastrointestinal stromal tumors (GISTs) are a rare type of cancer with an incidence of around 15 patients per million per year [Citation1], they are the most prevalent mesenchymal neoplasm. Reported incidence rates of GIST are variable across different geographical regions, although most population-based studies share similar epidemiological features of GIST around the globe [Citation2]. At diagnosis, patients have a median age of mid-sixties with a slight predominance in males [Citation1]. While GIST can arise along the entire gastrointestinal tract, most primary are found in the stomach (56%) and in the small intestine (32%). The minority of GISTs is located in the colon and rectum (6.0%), esophagus (0.7%), and other sites (5.5%) [Citation2]. The recognition of overexpression of the KIT protein, a receptor tyrosine kinase, also known as CD-117 was crucial for accurately diagnosing GIST more than two decades ago. Simultaneously, the discovery of a gain of function mutation resulting in uncontrolled activation of KIT was a practice-changing breakthrough [Citation3]. These mutations in KIT or platelet-derived growth factor receptor (PDGFRA) genes lead via activation of sustained growth, proliferation, and inhibition of apoptosis to – development of GIST [Citation3,Citation4]. Approximately 80% of GISTs arise from oncogenic KIT mutations while PDGFRA mutations are in 10–15% responsible for GIST. In the remaining 5–10% of GIST, the formally so-called wild-type GIST, other stigmata such as SDH-deficiency, NF1 mutation, and occasional NTRK and BRAF mutations are identified.
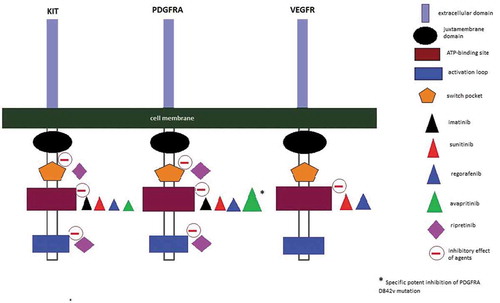
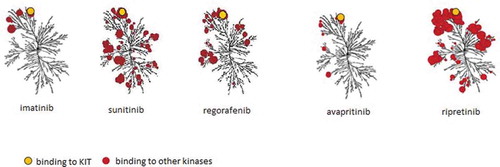
The discovery of KIT and PDGFRA receptor tyrosine kinase inhibitors (TKIs) allowed GIST to be one of the first types of cancer successfully treated with molecular-targeted therapy. Despite this, most advanced GIST will eventually exhibit resistance to a TKI, making the switch to a subsequent line of therapy necessary. In this review, we will first briefly describe the currently approved first-, second-, and third-line TKIs. We will focus on an update of progress beyond these approved TKIs. An overview of the human kinome that is targeted by currently registered drugs for advanced GIST is given in and a schematic mechanism of action is shown in .
Figure 1. Kinome illustrations of imatinib, sunitinib, regorafenib, avapritinib, and ripretinib. Red circle represent various kinases. Avapritinib specifically inhibits PDGFRA D842V. Illustration reproduced courtesy of Cell Signaling Technology, Inc. (www.cellsignal.com)
2. Approved first-, second-, and third-line TKI’s: imatinib, sunitinib, and regorafenib
Since GIST is insensitive to conventional chemotherapy [Citation5], the introduction of imatinib, a selective TKI of BCR-ABL, KIT, and PDGFR (), revolutionary changed the prognosis of patients with advanced and metastatic GIST. A single case report in 2000 [Citation6] led to rapid initiation of phase 1 and 2 trials [Citation7–9] and eventually to confirmation of efficacy and safety in two parallel phase 3 trials [Citation10,Citation11], studying 400 versus 800 mg once daily. No difference in response rates between the two doses was observed in both trials. Based on these results, an imatinib dose of 400 mg daily is the standard first-line therapy. An exception applies to the patients with a KIT exon 9 mutation, where an imatinib dose of 800 mg daily is more effective as demonstrated by the MetaGIST combined analysis [Citation12]. Long-term results in patients receiving imatinib, with a median follow-up of over 10 years, demonstrate a median progression-free survival (PFS) of 1.7–2.0 years, with an estimated PFS at 10 years of 9.2%–9.5% [Citation13].
When the failure of imatinib occurs, due to the development of secondary mutations [Citation14,Citation15], sunitinib is the drug of choice. Similar to imatinib, it targets KIT and PDGFR, however, sunitinib is effective in imatinib-resistant GIST probably through to different and broader binding characteristics and affinities (). Sunitinib is more effective in KIT exon 13 and exon 14 mutations and less active in KIT exon 17 and exon 18 mutations [Citation16]. Patients treated with sunitinib after progression on imatinib, have a median PFS of nearly 6 months [Citation17]. Two standard dosing schedules are common; 50 mg daily in 6-week cycles with 4 weeks on and 2 weeks off-treatment and the continuous daily dose of 37.5 mg [Citation18].
The approved third-line therapy for advanced GIST is regorafenib. This TKI has the ability to block a broad range of kinases (). Treatment with regorafenib provides benefit for patients with resistance to both imatinib and sunitinib with a median PFS of 4.8 months versus 0.9 months in the placebo group [Citation19].
3. Results of studies with TKIs beyond standard therapy for GIST
3.1. Nilotinib
Before regorafenib was approved as third-line therapy, the activity of nilotinib was tested to serve as a solution for patients with advanced GIST resistant to both imatinib and sunitinib. A phase 3 study was undertaken based on a retrospective study of patients treated with nilotinib within a compassionate use program and a phase 2 trial that demonstrated encouraging results, suggesting activity of nilotinib in imatinib and sunitinib resistant GIST [Citation20,Citation21].
In the phase 3 trial [Citation22], 165 patients were treated with nilotinib as third-line therapy and 85 patients in the control arm were treated with: (1) best supportive care alone, (2) best supportive care plus imatinib or (3) best supportive care plus sunitinib. Although PFS assessed by the unblinded local investigator was longer in the nilotinib group compared to control (median 4.0 months vs 2.3 months, p = 0.0007), the primary endpoint defined as PFS assessed by blinded central radiology review was not significantly different between the 2 arms (median 3.7 months in both groups). Post-hoc analysis of patients that truly received third-line therapy (n = 197, thus excluding the so-called prior imatinib or sunitinib intolerant patients in the control group) showed a median overall survival advantage of 4 months in patients treated with nilotinib. However, the primary endpoint for this study was not met and exploratory analyses did not support registration and therefore further development of nilotinib as third-line treatment for GIST was ceased.
3.2. Sorafenib
In vitro studies demonstrated the activity of sorafenib against secondary mutations in imatinib-resistant cell lines by inhibition of tumor angiogenesis and inhibition of several kinases such as KIT, BRAF, and FLT-3 [Citation23,Citation24]. A retrospective analysis of 124 patients with sorafenib as third- or fourth-line treatment for advanced GIST showed a median PFS of 6.4 months [Citation25]. In two phase 2 trials, safety evaluation showed moderate tolerability with managable adverse effects. One phase 2 trial including 31 patients, with sorafenib mainly given as third-line therapy, demonstrated a median PFS of 4.9 months and a disease control rate of 36% [Citation26], while in the other phase 2 trial performed with 38 patients, an even higher disease control rate of 68% was observed [Citation27] with a median PFS of 5.2 months. No phase 3 trials were performed in GIST, possibly due to the registration of a similar drug of the same company in GIST (regorafenib).
3.3. Pazopanib
Pazopanib, which is approved for metastatic nonadipocytic soft-tissue sarcoma is also investigated as a third-line treatment for advanced GIST. In a single-arm, open-label, phase 2 study [Citation28] including 25 GIST patients with resistance to imatinib and sunitinib, 36% of the participants received pazopanib as third-line treatment and 40% as fourth-line treatment. Due to the low number of patients without progression after 6 months (3 of 15 first included patients), enrollment of new patients in the trial was stopped according to a predefined study design. In the total cohort, the progression free rate after 24 weeks was 17% with a PFS of 1.9 months. The investigators concluded that the activity of pazopanib in advanced GIST is minimal.
In contrast, PAZOGIST, a French multicenter, randomized phase 2 trial [Citation29] showed more positive results. Eighty-one patients with failure of at least 2 TKIs, were treated with pazopanib plus best supportive care (n = 40) or best supportive care only (n = 41). The 4-month PFS rate, assessed by local investigators was significantly higher with pazopanib compared to the best supportive care only group, 45.2% vs 17.6, respectively. However, this significant difference was lost when the 4-month PFS rate was assessed by central reviewers. The median PFS was only 1.1 months different with 3.4 months in the pazopanib plus supportive care group and 2.3 months in the supportive care only group. Based on the results of these phase 2 trials, pazopanib seems to be less active compared to regorafenib as third-line agent for advanced GIST.
3.4. Cabozantinib
In addition to KIT and VEGFR2, cabozantinib is active against MET and AXL. These receptors are important in processes of tumor growth, angiogenesis, and metastasis [Citation13]. When the activity of KIT is inhibited by imatinib in GIST, compensatory MET activation and upregulation of AXL follows, resulting in resistance to imatinib [Citation14,Citation15]. In patient-derived mouse GIST xenografts, cabozantinib exhibits anti-tumor activity by inhibition of tumor growth proliferation and angiogenesis in imatinib-resistant models [Citation16]. In the EORTC 1317 phase 2 CaboGIST study [Citation30], 50 patients with metastatic GIST, progressive on imatinib and sunitinib were included. The study decision rule to justify further investigation with cabozantinib was defined as, if at least 21 of the first 41 evaluated patients were progression-free at week 12. This condition was met, as 24 (58.5%) of the first 41 patients had no signs of progression at week 12. Of 50 patients, 30 (60%) were progression-free at 12 weeks after starting cabozantinib. The disease control rate (sum of partial response and stable disease) was 82%. A median PFS of 5.5 months was observed. Notably, 4 (8%) of 50 patients were still using cabozantinib at the clinical cutoff point. Safety analysis showed similar adverse events as sunitinib and regorafenib, such as hypertension, hand-foot syndrome, and diarrhea. The major reason (84.8%) for discontinuation of cabozantinib was a progressive disease and in 8.7% of the cases cabozantinib was ceased due to toxicity. The effectiveness, in terms of PFS rate at 12 weeks and median PFS is comparable with regorafenib. The same applies for adverse events rate and severity. The exact position of cabozantinib in the treatment of GIST can only be established if a phase 3 trial would be performed.
3.5. Buparlisib
The hypothesis that buparlisib has potential activity in GIST is based on the activation of alternative signaling pathways such as phosphoinositide 3-kinase (PI3K) in imatinib resistant GIST [Citation31,Citation32]. Buparlisib, an inhibitor of the PI3K pathway, had demonstrated anti-growth effects in patient-derived GIST xenograft models [Citation33]. In a multicenter, phase 1b study [Citation34], a combination of imatinib with buparlisib was given in patients with resistance to both imatinib and sunitinib. In the dose-escalation phase, 35 patients received the maximum-tolerated dose of 80 mg buparlisib along with 400 mg imatinib. The best overall response observed was in 54.3% of the patients a stable disease, while none of the patients achieved a partial response or complete response. The median PFS was 3.5 months. The toxicity profile showed, besides the common side effects of imatinib (nausea, diarrhea), neuropsychiatric adverse events, probably due to blood-barrier penetration of buparlisib. Overall the combination of imatinib and buparlisib has limited activity.
3.6. Dasatinib
Similar to imatinib, dasatinib is a potent inhibitor of BCR-ABL and KIT; additionally, it inhibits the nonreceptor kinases (SRC family) and ephrin receptor kinases. Dasatinib has shown activity in imatinib-resistant cells [Citation35,Citation36] and its efficacy has been observed in chronic myelogenous leukemia, as first-line and second-line treatment [Citation37,Citation38]. The efficacy and safety of dasatinib in GIST as first-line treatment has been explored in a single-arm phase 2 trial [Citation39]. Seventy-four percent of the 42 treated patients had a metabolic response (complete plus partial), assessed by 18 F-fluorodeoxyglucose-positron emission tomography combined with CT (FDG-PET/CT). At a median follow up of 67.2 months, all patients were off trial having a median PFS of 13.6 months. Grade 3 toxicity was observed in 38% of the patients with gastrointestinal and pulmonary (dyspnea or pleural effusion) as the most common reported adverse events. Although dasatinib has activity as first-line therapy, imatinib provides more benefit and has a more favorable toxicity profile.
Another single-arm phase 2 trial studied the activity of dasatinib as a third-line treatment in 58 GIST patients with failure on both imatinib and sunitinib. The preliminary results illustrate a disease control rate of 62% and a median PFS of 3.0 months 40. While dasatinib is active as a third-line treatment, compared to regorafenib it seems to be less effective.
3.7. Heat shock protein 90 (HSP90) inhibitor
HSP90 is a molecular chaperone, assisting many proteins, including KIT and PDGFRA, in normal cellular functions by folding and stabilizing them [Citation40]. Activation of KIT and PDGFRA depends on their stabilization by HSP90 [Citation41,Citation42]. In GIST xenograft models, inhibition of HPS90 resulted in anti-tumor activity [Citation43]. In an open-label phase 2 trial [Citation40,Citation44] patients with advanced GIST refractory to imatinib, sunitinib, and regorafenib received the oral HPS90 inhibitor TAS-116. A median PFS of 4.4 months, which is valuable as fourth-line therapy, was achieved. None of the patients had a complete or partial response; the disease control rate and progression-free rate at 12 weeks were 85% and 73.4%, respectively. The most common adverse events were gastrointestinal disorders, while 20% of the patients experienced ocular toxicity (visual impairment, night blindness) which may impair quality of life. However, in this trial the ocular adverse events and all other grade 3 or higher adverse events were resolved after dose modification. This encouraging result is promising for HSP90 inhibition as a potential novel therapy in patients with advanced GIST. Currently, a phase 3 trial in Japan is ongoing to evaluate TAS-116 in GIST (JapicCTI no. 184094).
3.8. Fibroblast growth factor pathway inhibitors
Various physiological processes during embryogenic and postnatal devolvement, among others differentiation and angiogenesis, are regulated through the fibroblast growth factor (FGF) signaling pathway [Citation45]. The dysregulation of the FGF pathway (e.g. as a result of mutations) is involved in oncogenic development in different types of cancer [Citation46]. In GIST, particularly in wild type and SDH-deficient molecular subgroups, abnormalities in FGF receptor (FGFR) kinases is implicated to contribute to the pathogenesis of GIST [Citation47,Citation48]. With this rationale, targeting the FGFR could be effective in GIST. TKIs such as pazopanib, dovitinib, and regorafenib with multitarget activity are evaluated in the treatment of GIST. They inhibit one or more FGFR kinases in addition to their broader activity against multiple other kinases.
Since the specific role of the FGF pathway is suspected in the development of GIST, selective inhibition of FGFR in GIST might be promising. The only reported study with a selective inhibitor in GIST is a combination therapy of BGJ398 and imatinib [Citation49]. In this phase 1 trial, 16 patients with advanced GIST, with failure on imatinib were included. The study was closed early, due to the toxicity of the combination therapy. The efficacy results showed limited clinical benefit, as stable disease (best clinical response) was observed in 7 (44%) patients with a median PFS of 12.1 weeks. However, the studied population was mostly pretreated with three or more TKIs and none of the patients had an alternation in the FGFR gene. It would be interesting to study the effects of a selective FGF inhibitor in SHD-deficient or wild-type GIST.
3.9. Neurotropic receptor tyrosine kinase (NTRK) inhibitors
The NTRK genes (NTRK1, NTRK2, NTRK3) encode the tropomyosin receptor kinase (TRK) proteins. NTRK gene fusions leading to the sustained activity of TRK, occur in various cancer types [Citation50,Citation51]. In genomic profiling of 24 GIST patients with no mutations in the KIT/PDGFRA/RAS pathway, two patients with an ETV6-NTRK3 gene fusion were identified [Citation47]. A phase 1 study 52, evaluating the efficacy of larotrectinib, a selective NTRK inhibitor, included 55 patients with various tumors exhibiting NTRK fusions. Three of them were GIST patients. A partial response was observed in all 3 GIST patients. Although an NTRK fusion is rare in GIST, results with NTRK inhibitors are promising and therefore a screening strategy to identify patients with NTRK fusions will be needed.
3.10. Immunotherapy
There is limited knowledge on the role of the immune system in the pathogenesis and progression of GIST. However, the possible prognostic value of immune infiltrates in GIST has been reported [Citation52] and preclinical studies in transgenic mice show the enhanced antitumor activity of imatinib when immunotherapy is given [Citation53]. Two clinical trials with immune therapy in GIST patients have been published. In a phase 2 study, pembrolizumab (programmed death-1 receptor inhibitor) in combination with cyclophosphamide was studied in patients with various sarcoma subtypes. Ten patients with GIST refractory to at least imatinib and sunitinib showed a median PFS of 1.3 months expressing the limited clinical benefit of combination therapy.
Another phase 1 study included 20 GIST patients with failure on at least two TKIs. In this trial, ipilimumab (cytotoxic T-lymphocyte antigen-4 inhibitor) was combined with dasatinib, resulting in a median PFS of 2.8 months and no objective responses. The contribution of ipilimumab in this study is likely to be limited as dasatinib itself has activity in GIST showing a PFS of 3.0 months in another trial, as third-line therapy [Citation40]. Based on published results so far, there is limited evidence yet for use of immunotherapy in GIST.
3.11. Ongoing clinical trials
Various agents and therapy strategies are studied in ongoing clinical trials. An overview of ongoing, recruiting clinical trials with novel therapy strategies involving GIST is given in .
Table 1. Overview of ongoing recruiting clinical trials for metastatic or locally advanced GIST with agents in development according to clinicaltrials.gov, date assessed: 6 October 2020
4. Novel registered agents in advanced/metastatic GIST
4.1. Ripretinib
Ripretinib is a so-called switch-control TKI. It is recognized that both KIT and PDGFRA contain an inhibitory switch in the juxtamembrane domain and an activating loop switch in the kinase domain [Citation2,Citation54]. These switches regulate kinase activity by binding to the kinase switch pocket, which is located near the kinase domain 1. In contrast to most primary GIST mutations, which are loss-of-function mutations in the inhibitory switch, virtually all secondary mutations are gain-of-function mutations located in the activating loop switch or the related switch pocket. These mutations lead to sustained on-state of the switch pocket resulting in uncontrolled activation of the kinase [Citation55].
Ripretinib turns the kinase into an off-state, leading to inhibition of downstream signaling. It binds to the switch pocket and to the activation loop preventing its entry to the switch pocket. This dual mechanism of action results in locking KIT and PDGFRA in its inactive state [Citation56]. Accordingly, broad inhibition of multiple secondary (and some primary) mutations can be accomplished. The strong antineoplastic effect of ripretinib was illustrated in in vitro studies by inhibition of mutated KIT receptors (exons 9, 11, 13, 14, 17, and 18) and PDGFRA receptors (exons 12, 14, and 18) [Citation57,Citation58].
The first evaluation of ripretinib in GIST patients was a phase 1 study [Citation59] in which a recommended phase 2 dose of 150 mg once a day was established. Ripretinib was well tolerated and no maximum-tolerated dose was reached. The efficacy result was promising with a median PFS of 5.5 months in patients received ripretinib as fourth line. Patients treated with ripretinib as second line had a median PFS of 10.7 months.
In patients with advanced GIST, the activity of ripretinib as a fourth-line treatment was recently established in the INVICTUS trial [Citation60]. In this phase 3, double-blind, placebo-controlled trial, 129 patients with advanced GIST with failure to at least imatinib, sunitinib, and regorafenib, were randomized to receive ripretinib (n = 85) or placebo (n = 44). Although the objective response rate (9.4%) of ripretinib is similar to other TKIs, it demonstrated a significant improvement in PFS, as patients in ripretinib group had a median PFS of 6.3 months compared to 1.0 month in the placebo group (p < 0.001). At 6 months, the PFS rate was 51% for ripretinib and 3.2% for placebo. Analysis of subgroups (such as age, gender, number of previous treatments) all showed a significant longer PFS compared to placebo. Besides the improvement in PFS, a superior overall survival was observed in patients receiving ripretinib compared to placebo: 15.1 months vs 6.6 months, respectively. Gastrointestinal adverse events and hypertension were common, but alopecia was more often observed than in other TKIs, as it was reported in half of the patients receiving ripretinib. However, in general, adverse events were of low grade and manageable, leading to a low rate of dose reductions (8.2%) and interruptions (21.2%).
After recently obtained approval by FDA, ripretinib has provided the clinicians a potent agent in the treatment of patients with advanced GIST resistant to approved first-, second-, and third-line therapy. In the EU, the regulatory assessment by EMA has started and an expanded access program is ongoing.
In the Intrigue study (ClinicalTrials.gov, ID No NCT03673501), a global open-label randomized trial, ripretinib is compared to sunitinib as second-line therapy with PFS (assessed by blinded independent central review) as the primary outcome.
4.2. Avapritinib
In contrast to imatinib, sunitinib, and regorafenib, avapritinib is a selective type I inhibitor of KIT and PDGFRA, binding to the activation loop in its active conformation [Citation61]. Distinctively from all the other TKIs, avapritinib is a strong inhibitor of the imatinib-insensitive PDGFRA D842V mutated kinase activity [Citation62]. The D842V mutation is the most common subtype of PDGFRA mutation as it constitutes 50% of the total PDGFRA mutations [Citation63]. Moreover, KIT exon 11/17 mutations in patient-derived xenograft models with insensitivity to imatinib were successfully inhibited by avapritinib [Citation61].
In a phase 1 trial [Citation64], 56 patients with a PDGFRA D842V mutation received avapritinib at a starting dose of 400 mg daily, which eventually because of toxicity, was reduced to 300 mg daily. The efficacy results are impressive, with the primary endpoint overall response rate being met in 87.5% of the patients. In these patients, complete response was observed in 8.9%, and 78.6% of the patients achieved a partial response. Patients benefited from avapritinib during a long period of time with a median duration of response of 27.6 months. While median PFS was not reached, the 12-month PFS rate was 81%. These clinical results led to FDA and EMA approval of avapritinib for treatment of patients with unresectable or metastatic GIST having a PDGFRA D842V mutation.
It is important to give consideration to the adverse effects of avapritinib. Although most adverse effects (e.g. edema, nausea, vomiting, diarrhea) are similar to other TKIs and feasible to manage, special attention should be given to cognitive side effects that were observed in 48% of the patients. In patients with cognitive adverse effects (mostly grade 1 or 2), memory impairment (29%), confused mental state (7%), encephalopathy (1%), and other cognitive disorder (11%) were reported. The long-term cognitive side effect is not known yet, but how it might impair a patient’s quality of life remains to be studied in more depth.
Apart from the treatment of D842V mutation, the activity of avapritinib was explored as a fourth-line treatment of advanced GIST. In an international, open-label, randomized phase 3 trial (VOYAGER), 240 patients treated with avapritinib were compared to 236 patients receiving regorafenib. The overall response rate was 17% for patients treated with avapritinib and 7% for the regorafenib group. But, most importantly, no improvement of PFS, the primary outcome, was observed. Treatment with avapritinib resulted in a median PFS of 4.2 months while patients on regorafenib had a median PFS of 5.6 months [Citation65].
Thus, despite the failure of avapritinib to improve clinical outcomes as a fourth-line treatment in a broad GIST population, it has firmly demonstrated its potency in the treatment of advanced GIST exhibiting the imatinib-insensitive PDGFRA D842V mutation, becoming the first registered TKI ever for this type of mutation.
5. Conclusion
Based on result of the clinical trials, imatinib, sunitinib, and regorafenib have established positions as first-, second-, and third-line therapy in the treatment of unresectable or metastatic GIST. Various TKIs, such as sorafenib, nilotinib, dasatinib, and pazopanib, that are registered for non-GIST indications have been investigated to serve as an alternative for later line treatments. Some of them are being used off-label, but none of them showed increased activity directly compared to the registered TKIs. More recently activity of two different TKIs with high affinity to specific GIST mutations have been approved and were added to the armamentarium for the treatment of GIST. Ripretinib, active against multiple resistance mutations, was successful in fourth-line therapy, achieving a median PFS of 6.3 months compared to 1.0 months in the patients treated with placebo and is now being explored in the second line. Patients with a PDGFRA D842V mutation, which is insensitive to currently available TKIs in GIST, have been successfully treated with avapritinib in a phase 1 trial. In the VOYAGER trial, avapritinib failed to improve PFS as a fourth-line treatment.
6. Expert opinion
When accessing the results of clinical trials performed with GIST patients, it is critical to realize that in general, the clinical outcomes of phase 2 trials are more positive than phase 3 trials. In other words, promising results of a phase 2 trial with a certain drug do not guarantee the same effectiveness in a phase 3 trial performed with the same drug. This discrepancy is caused, amongst others, by the selection of patients with non- or slowly progressing GIST in phase 2 trials when PFS is the primary endpoint. The indolent behavior of metastatic GIST is in that case more likely related to the nature of the disease and not necessarily to the drug treatment. Therefore, randomized studies are extremely important to account for this bias. These trials are still based on RECIST-criteria for their primary endpoint, while it has been known for a long time that this method is not ideal for GIST. However, other criteria, including CHOI have their drawbacks as well. Regulatory agencies should be open for discussion with GIST clinicians to explore better endpoints.
The position of imatinib as first-line treatment of advanced GIST is irrefutable, as its efficacy is extensively evidenced along with its favorable toxicity profile. Imatinib provides an overall median PFS of 20 months, while nearly 10% of the patients have a PFS of 10 years 13. Unlike in CML, in responders, being patients with prolonged stable disease, partial response, or complete remission, imatinib is continued, even if no evidence of disease is present after a long period of treatment. Interruption of treatment after prolonged (>10 years) response could be an interesting strategy to explore further. However, early termination of imatinib seems not advisable as it was demonstrated in the BRF14-trial that, regardless of the timing of interruption (after 1-,3-, or 5 years), GIST progressed after an interruption in most patients, even in patients who had complete remission before discontinuation of imatinib [Citation66,Citation67].
Besides the imatinib insensitivity of PDGFRA D842V mutated GIST, treatment of NF1 mutated and SDH-deficient GIST with imatinib remains a challenge. Despite the success of imatinib in patients with other mutations, most patients develop secondary resistance. A potential solution might be an alternating TKI regimen, as tried in renal cancer. The recently reportedly ALT-GIST trial (ClinicalTrials.gov, ID No NCT00793871) [Citation68], studied an alternating regimen of imatinib and regorafenib compared to imatinib as first-line treatment. With the use of drug-free intervals in this combination therapy, it was investigated whether allowing tumor stem cells to reenter the cell cycle will lead to more sensitivity to TKIs, prolonging the interval to the occurrence of secondary resistance. However, the efficacy results of the ALT-GIST trial were negative.
Patients with treatment failure on imatinib will gain approximately 6 months of PFS when switching to second-line therapy with sunitinib, although more side effects are experienced compared to imatinib. Depending on the result of the Intrigue trial (ClinicalTrials.gov, ID No NCT03673501), in which sunitinib and ripretinib are compared, a shift in second-line therapy is possible.
Regorafenib is now the established third-line therapy, since its superiority in terms of clinical outcomes is proven. The median PFS of patients using regorafenib is almost 4 months longer than the control arm. Sunitinib and regorafenib have illustrated their complementary activity against secondary mutations [Citation69], and therefore an alternating regimen of these TKIs was studied in a phase 1 trial [Citation70] as fourth-line therapy in 14 patients with advanced GIST. Alternation therapy of sunitinib and regorafenib was well tolerated. Four patients (28.6%) had stable disease at the best response, and the median PFS was 1.9 months. Although the clinical benefit seems to be marginal, combination therapy of TKIs with complementary activity might be a strategy to overcome secondary mutations. Cabozantinib seems to have the most promising phase 2 results beyond standard therapy; however, a phase 3 trial should then confirm the results of the published phase 2 trial.
Ripretinib, a so-called switch-control TKI, has the unique ability to bind both the inhibitory switch and activating switch of the kinase, locking it in an inactivated state. More importantly, this mechanism of action targets all the secondary mutations, which arise in the activating switch. As a result, inhibition of a very broad spectrum of secondary mutations is achieved. The results of a phase 3 trial confirm the efficacy of ripretinib, as patients who received it as fourth-line treatment, had a median PFS of over 6 months compared to 1.0 months in the placebo arm. This beneficial result led to the approval of ripretinib by the FDA, providing clinicians the first and the only, potent, fourth-line TKI in the treatment of advanced GIST.
Despite the approval of four lines of treatment, and exploration of multiple agents in clinical trials, no TKI was yet found to be a successful treatment for the PDGFRA D842V mutated GIST. Avapritinib, a type 1 inhibitor, distinguishes itself from other TKIs, by its capability of strong inhibition of the D842V mutation. While this mutation is virtually insensitive for all the approved TKIs, treatment with avapritinib leads to an overall response of 87.5%. This achievement made avapritinib the only FDA-approved drug for the treatment of GIST exhibiting a PDGFRA D842V mutation. However, the cognitive side effects should be taken into account depending on the results of long-term follow-up of these side effects. Due to the rarity of the PDGFRA D842V mutation in advanced/metastatic GIST patients, no phase 3 setting for registration is feasible, similar to the registration of neurotrophic receptor tyrosine kinase (NTRK) inhibitors in rare NTRK fusion-positive cancer, including GIST. The FDA should be congratulated for approving drugs with such high activity in ultrarare diseases (less than 1 per 100.000) based on single-arm trials. And this may be the way forward for the future to targeted treatment of other ultrarare subtypes of GIST such as BRAF mutated GIST. This also shows that mutational testing is mandatory in any high-risk or metastatic GIST.
Article highlights
GIST is one of the first type of cancers that served as a successful model for molecular-targeted therapy.
Despite the success of TKIs, most advanced GISTs eventually exhibit resistance to TKI due to secondary mutations or rare primary resistance.
Multiple other TKIs, immune and combination therapies have failed to be successful beyond the standard treatment of GIST.
Increased knowledge of molecular pathogenesis led recently to the development of highly potent agents.
Ripretinib is a novel TKI targeting secondary mutations with activity as fourth-line therapy in advanced GIST which recently led to its approval by the FDA.
Avapritinib is the first FDA and EMA approved TKI with high potency in the treatment of GIST exhibiting PDGFRA D842V mutation.
Molecular profiling of high-risk GIST at diagnosis and identifying secondary mutations in the course of the disease is crucial for the application of adequate-targeted therapy.
This box summarizes the key points contained in the article.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Verschoor AJ, Bovee J, Overbeek LIH, et al. The incidence, mutational status, risk classification and referral pattern of gastro-intestinal stromal tumours in the Netherlands: a nationwide pathology registry (PALGA) study. Virchows Arch. 2018;472(2):221–229.
- Soreide K, Sandvik OM, Soreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46.
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580.
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710.
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58.
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14):1052–1056.
- van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358(9291):1421–1423.
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480.
- Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC soft tissue and bone sarcoma group phase II study. Eur J Cancer. 2003;39(14):2006–2011.
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–1134.
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–632.
- Gastrointestinal Stromal Tumor Meta-Analysis G. Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28(7):1247–1253.
- Casali PG, Zalcberg J, Le Cesne A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: long-term analysis of the european organisation for research and treatment of cancer, Italian Sarcoma Group, and Australasian gastrointestinal trials group intergroup phase III randomized trial on imatinib at two dose levels. J Clin Oncol. 2017;35(15):1713–1720.
- Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11(11):4182–4190.
- Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216(1):64–74.
- Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15(24):7510–7518.
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338.
- George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959–1968.
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302.
- Montemurro M, Schoffski P, Reichardt P, et al. Nilotinib in the treatment of advanced gastrointestinal stromal tumours resistant to both imatinib and sunitinib. Eur J Cancer. 2009;45(13):2293–2297.
- Sawaki A, Nishida T, Doi T, et al. Phase 2 study of nilotinib as third-line therapy for patients with gastrointestinal stromal tumor. Cancer. 2011;117(20):4633–4641.
- Reichardt P, Blay JY, Gelderblom H, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23(7):1680–1687.
- Heinrich MC, Marino-Enriquez A, Presnell A, et al. Sorafenib inhibits many kinase mutations associated with drug-resistant gastrointestinal stromal tumors. Mol Cancer Ther. 2012;11(8):1770–1780.
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109.
- Montemurro M, Gelderblom H, Bitz U, et al. Sorafenib as third- or fourth-line treatment of advanced gastrointestinal stromal tumour and pretreatment including both imatinib and sunitinib, and nilotinib: a retrospective analysis. Eur J Cancer. 2013;49(5):1027–1031.
- Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30(6):2377–2383.
- Kindler HL, Campbell NP, Wroblewski K, et al. Sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): final results of a University of Chicago Phase II Consortium trial. J clin oncol. 2011;29(15). DOI:10.1200/jco.2011.29.15_suppl.10009
- Ganjoo KN, Villalobos VM, Kamaya A, et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol. 2014;25(1):236–240.
- Mir O, Cropet C, Toulmonde M, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol. 2016;17(5):632–641.
- Schoffski P, Mir O, Kasper B, et al. Activity and safety of the multi-target tyrosine kinase inhibitor cabozantinib in patients with metastatic gastrointestinal stromal tumour after treatment with imatinib and sunitinib: European Organisation for Research and Treatment of Cancer phase II trial 1317 ‘CaboGIST’. Eur J Cancer. 2020;134:62–74.
- Duensing A, Heinrich MC, Fletcher CD, et al. Biology of gastrointestinal stromal tumors: KIT mutations and beyond. Cancer Invest. 2004;22(1):106–116.
- Wozniak A, Gebreyohannes YK, Debiec-Rychter M, et al. New targets and therapies for gastrointestinal stromal tumors. Expert Rev Anticancer Ther. 2017;17(12):1117–1129.
- Van Looy T, Wozniak A, Floris G, et al. Phosphoinositide 3-kinase inhibitors combined with imatinib in patient-derived xenograft models of gastrointestinal stromal tumors: rationale and efficacy. Clin Cancer Res. 2014;20(23):6071–6082.
- Gelderblom H, Jones RL, George S, et al. Imatinib in combination with phosphoinositol kinase inhibitor buparlisib in patients with gastrointestinal stromal tumour who failed prior therapy with imatinib and sunitinib: a Phase 1b, multicentre study. Br J Cancer. 2020;122(8):1158–1165.
- O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500–4505.
- Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399–401.
- Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123(4):494–500.
- Shah NP, Guilhot F, Cortes JE, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014;123(15):2317–2324.
- Montemurro M, Cioffi A, Domont J, et al. Long-term outcome of dasatinib first-line treatment in gastrointestinal stromal tumor: a multicenter, 2-stage phase 2 trial (Swiss Group for Clinical Cancer Research 56/07). Cancer. 2018;124(7):1449–1454.
- Li J, Zhou Y, Zhang X, et al. Safety and efficacy of dasatinib in patients with advanced gastrointestinal stromal tumors refractory to imatinib and sunitinib: a single arm, multicenters, phase 2 trial. J clin oncol. 2019;37(4_suppl):138.
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772.
- Bauer S, Yu LK, Demetri GD, et al. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66(18):9153–9161.
- Smyth T, Van Looy T, Curry JE, et al. The HSP90 inhibitor, AT13387, is effective against imatinib-sensitive and -resistant gastrointestinal stromal tumor models. Mol Cancer Ther. 2012;11(8):1799–1808.
- Doi T, Kurokawa Y, Sawaki A, et al. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: a phase II, single-arm trial. Eur J Cancer. 2019;121:29–39.
- Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149(2):121–130.
- Tanner Y, Grose RP. Dysregulated FGF signalling in neoplastic disorders. Semin Cell Dev Biol. 2016;53:126–135.
- Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14(1):339.
- Urbini M, Indio V, Tarantino G, et al. Gain of FGF4 is a frequent event in KIT/PDGFRA/SDH/RAS-P WT GIST. Genes Chromosomes Cancer. 2019;58(9):636–642.
- Kelly CM, Shoushtari AN, Qin LX, et al. A phase Ib study of BGJ398, a pan-FGFR kinase inhibitor in combination with imatinib in patients with advanced gastrointestinal stromal tumor. Invest New Drugs. 2019;37(2):282–290.
- Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19(11):1469–1472.
- Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2(5):367–376.
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378(8):731–739
- Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–U1099.
- Ksienski D. Imatinib mesylate: past successes and future challenges in the treatment of gastrointestinal stromal tumors. Clin Med Insights Oncol. 2011;5:365–379.
- Gajiwala KS, Wu JC, Christensen J, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009;106(5):1542–1547.
- Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell. 2019;35(5):738–751 e739.
- Schneeweiss M, Peter B, Bibi S, et al. The KIT and PDGFRA switch-control inhibitor DCC-2618 blocks growth and survival of multiple neoplastic cell types in advanced mastocytosis. Haematologica. 2018;103(5):799–809.
- Schneeweiss MA, Peter B, Blatt K, et al. The multi-kinase inhibitor DCC-2618 inhibits proliferation and survival of neoplastic mast cells and other cell types involved in systemic mastocytosis. Blood. 2016;128(22):1965.
- Janku F, Abdul Razak AR, Chi P, et al. Switch control inhibition of KIT and PDGFRA in patients with advanced gastrointestinal stromal tumor: a phase I study of ripretinib. J Clin Oncol. 2020;38(28):3294–3303.
- Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923–934.
- Evans EK, Gardino AK, Kim JL, et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med. 2017;9:414.
- Gebreyohannes YK, Wozniak A, Zhai ME, et al. Robust activity of avapritinib, potent and highly selective inhibitor of mutated KIT, in patient-derived xenograft models of gastrointestinal stromal tumors. Clin Cancer Res. 2019;25(2):609–618.
- Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865–878.
- Heinrich MC, Jones RL, von Mehren M, et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 2020;21(7):935–946.
- Corporation BM. Blueprint medicines announces top-line results from phase 3 VOYAGER trial of avapritinib versus regorafenib in patients with advanced gastrointestinal stromal tumor. 2020.
- Patrikidou A, Chabaud S, Ray-Coquard I, et al. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol. 2013;24(4):1087–1093.
- Ray-Coquard I, Bui N, Adenis A. Risk of relapse with imatinib (IM) discontinuation at 5 years in advanced GIST patients: results of the prospective BFR14 randomized phase III study comparing interruption versus continuation of IM at 5 years of treatment: a French Sarcoma Group Study. J clin oncol. 2010;28:10032.
- Yop D, Zalcberg JR, Blay JY, et al. ALT-GIST: randomised phase II trial of imatinib alternating with regorafenib vs imatinib alone for the first line treatment of metastatic gastrointestinal stromal tumor (GIST). ASCO. 2019;Chicago:2019.
- Serrano C, Marino-Enriquez A, Tao DL, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2019;120(6):612–620.
- Serrano C, Leal A, Kuang Y, et al. Phase I study of rapid alternation of sunitinib and regorafenib for the treatment of tyrosine kinase inhibitor refractory gastrointestinal stromal tumors. Clin Cancer Res. 2019;25(24):7287–7293.