ABSTRACT
Introduction: Although international clinical practice guidelines recognize a continued role for menopausal hormone therapy (HT), particularly for symptomatic women <60 years of age or within 10 years of menopause, safety and tolerability concerns have discouraged HT use due to potential links with a perceived increased risk of hormone-dependent cancers, and an established risk of stroke and venous thromboembolism. There is therefore a need for safe, effective non-hormonal therapy for relief of menopausal vasomotor symptoms (VMS).
Areas covered: This narrative review summarizes the dataset accrued for fezolinetant, a neurokinin-3 receptor (NK3R) antagonist in clinical development for menopause-associated VMS.
Expert opinion: Altered signaling in neuroendocrine circuits at menopause leads to VMS wherein NK3R activity plays a key role to modulate the thermoregulatory center in a manner conducive to triggering the ‘hot flash’ response. Thus, a new generation of NK3R antagonists has entered clinical development to specifically target the mechanistic basis of VMS. Fezolinetant is the most advanced NK3R antagonist in terms of stage of clinical development. Results to date have demonstrated rapid and substantial reduction in VMS frequency and severity and associated improvements in health-related quality of life. NK3R antagonists offer a non-hormonal alternative to HT for the treatment of menopause-related VMS.
1. Introduction
Ovarian function progressively declines with reproductive aging, to the extent that the ovaries fail to support circulating estrogen levels during menopause. Up to 80% of postmenopausal women experience vasomotor symptoms (VMS) during the menopausal transition [Citation1]. VMS, which may persist for a median of 7.4 years [Citation2], are characterized by hot flashes (also known as hot flushes) and/or night sweats, and are caused by a loss of thermoregulatory control due to withdrawal of estrogen feedback on the hypothalamus [Citation3]. The discomfort caused by hot flashes and night sweats is the main menopause-related reason that women seek medical attention [Citation4]. Moderate-to-severe VMS is also linked to significant problems related to sleep, quality of life (QoL), and depression [Citation5–9]. There are significant associated healthcare resource costs and societal costs caused by the impact of VMS on work productivity [Citation5,Citation10,Citation11].
The standard of care for VMS associated with menopause is hormone therapy (HT) with combined estrogen and progesterone, or estrogen alone [Citation12]. Although international clinical practice guidelines recognize a continued role for HT, particularly for symptomatic women <60 years of age or within 10 years of menopause [Citation13–16], safety and tolerability concerns have discouraged HT use due to a potential increased risk of hormone-dependent cancers (such as breast cancer) [Citation17], and a measured increased risk of stroke and venous thromboembolism [Citation13,Citation18]. HT should therefore only be prescribed with regular monitoring and assessment [Citation15,Citation19–21]. A significant number of peri- and postmenopausal women in the US and Europe choose not to use HT [Citation22,Citation23].
Other treatments including selective serotonin reuptake inhibitors (SSRIs), gabapentin, and clonidine, as well as cognitive behavioral therapy and herbal remedies, have limited efficacy or may be associated with adverse effects such as sedation and nausea [Citation24]. As such, there is a considerable need for safe, effective non-hormonal therapy for relief of VMS associated with menopause. Neurokinin-3 receptor (NK3R) antagonists offer a new therapeutic approach and various candidates have been advanced into clinical development for the treatment of VMS. Fezolinetant is the candidate at the most advanced stage of clinical development and the dataset accrued in its discovery and development is the primary focus of this review.
2. Development of fezolinetant (ESN364), an NK3R antagonist
2.1. NK3 receptor pharmacology and first-generation NK3R antagonists
The three main mammalian neurokinins are substance P (SP), Neurokinin A (NKA), and Neurokinin B (NKB). These neuropeptides have been characterized as the endogenous ligands for the neurokinin receptors NK1 (encoded by the gene tacr1), NK2 (tacr2), and NK3 (tacr3), respectively [Citation25]. NK1 and NK2 receptors are broadly expressed across the central and peripheral nervous systems whereupon NK1 antagonists have been developed for treatment of emesis [Citation26], and also evaluated for addiction [Citation27], asthma, and irritable bowel [Citation28], whereas NK2 antagonists have been evaluated principally for the treatment of gastrointestinal (GI) and inflammatory disorders [Citation29].
In contrast, the NK3 receptor (NK3R) is predominantly expressed in the central nervous system, with some peripheral expression detected in the gut, bladder, and reproductive tract [Citation30–32]. NK3R antagonists were initially developed for the treatment of cognitive disorders, such as schizophrenia, where the initial development candidates, osanetant, talnetant, and pavinetant (the latter also known as AZD2624/AZD4901/MLE4901), were abandoned in early clinical trials due to lack of efficacy [Citation33,Citation34]. These clinical failures are speculated to be due, at least in part, to poor brain bioavailability [Citation33,Citation34], thereby emphasizing the importance of optimizing this feature in next-generation compounds, as described in the following section for fezolinetant.
2.2. Discovery of fezolinetant
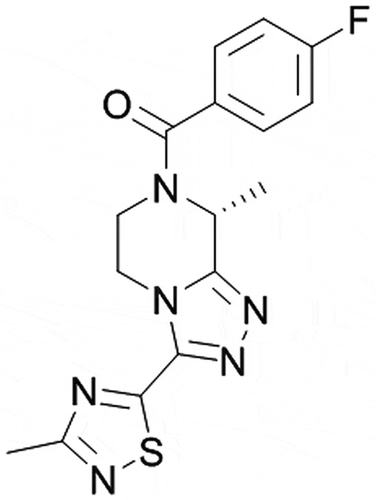
The discovery linking human genomics to phenotype to decipher the role of NK3R in the hypothalamic-pituitary-gonadal (HPG) axis [Citation35] inspired the research culminating in the discovery of fezolinetant (also known as ‘ESN364ʹ, ), the first NK3R antagonist developed for the bespoke purpose of treating Women’s Health disorders. Prior publications have described features of the chemistry and pharmacological profiling that supported development of fezolinetant [Citation36–38]. Initial pharmacology was benchmarked against the first-generation NK3R antagonists, osanetant and talnetant, where there were no published observations of effects on the HPG axis despite extensive preclinical and clinical testing. The discrepant pharmacology on the HPG axis of fezolinetant relative to these first-generation antagonists may be explained by the free drug hypothesis, a key advantage of fezolinetant. The free drug hypothesis states that only the ‘free’ drug concentration at the receptor is responsible for efficacy [Citation39]. As shown in , the superior in vivo efficacy of fezolinetant is a function of its receptor occupancy at NK3R in brain, in turn, stemming from its favorable PK profile as demonstrated by its low, unbound clearance, high brain penetration, and high unbound (‘free’) fraction in brain. It therefore effectively modulates the HPG axis in vivo in rat at a minimum effective oral dose of 3 mg/kg, whereas efficacy of osanetant and talnetant was undetectable at 100 mg/kg (see the Panel B figure accompanying ). The lead optimization effort driving improvements in potency, pharmacokinetic and physicochemical parameters concluding in the delivery of fezolinetant is recognized by experts in the field [Citation40].
Table 1. Comparison of fezolinetant to first-generation NK3R antagonists osanetant and talnetant in rat. (A) Minimum effective dose correlates to brain receptor occupancy (e.g. unbound (‘free’) concentration of drug relative to receptor potency), (B) in vivo efficacy of NK3R antagonists measured by dose-dependent inhibition of plasma LH in castrate rat
Fezolinetant has higher potency at the human NK3R (Ki = 21.8 nM, ) compared with that of the rat [Citation37]. Also, fezolinetant is >450-fold selective for human NK3R versus the NK1 and NK2 receptors, which is a distinctive feature in relation to certain other NK3 antagonists (for example, osanetant), as well as the stated nonselective NK receptor antagonist, elinzanetant (NT-814, originally known as GSK1144814, also now known as BAY3427080) [Citation41]. Furthermore, fezolinetant proved >450-fold selective against related HPG axis receptors (κ-opioid receptor [KOR], GPR54 [or KiSS1R], the GnRH receptor, the gonadotropin inhibitory hormone receptor GnIH-R [or NPFF1]); this experiment was performed as previously described to provide confidence that the mechanism of action underlying its observed drug efficacy on the HPG axis is ascribed to selective antagonism of NK3R [Citation36]. Fezolinetant also demonstrated broad target selectivity for NK3R following standard testing against panels of G protein-coupled receptors (GPCRs), kinases, and ion channels [Citation37].
Table 2. Receptor binding potency and selectivity at the human isoforms of the neurokinin receptor subtypes for fezolinetant, osanetant, and talnetant
2.3. The neuroendocrine role of the NK3 receptor and relevance to VMS
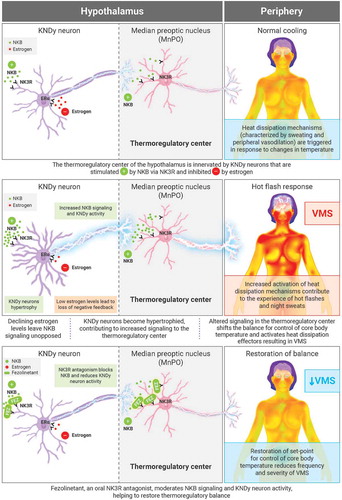
NK3R drug development in the field of Women’s Health disorders was prompted by the seminal report describing the first indication of loss-of-function mutations in NKB (TAC3) or NK3R (tacr3) and the consequent phenotype of familial hypogonadotropic hypogonadism [Citation35]. Further research demonstrated that NKB activation of NK3R is a positive regulator of tonic gonadotropin-releasing hormone (GnRH) release [Citation42]. A working model reconciling the roles of NKB and other neuroendocrine peptides in the modulation of the hypothalamic-pituitary gonadal (HPG) axis is the subject of various, recent review articles [Citation43,Citation44]. Briefly, NK3R is expressed on Kisspeptin/Neurokinin B/Dynorphin (‘KNDy’) neurons serving as the GnRH pulse generator [Citation43], popularly recognized for regulating sex hormone levels over the phases of the menstrual cycle. However, KNDy neurons also integrate neuroendocrine control of various other functions including thermoregulation, circadian rhythms, and sleep [Citation45,Citation46].
VMS are caused by a loss of thermoregulatory control coincident with the altered KNDy signaling triggered by menopause. Thus, KNDy neurons are stimulated by NKB and inhibited by estrogen as part of the homeostatic mechanisms supporting reproductive endocrinology. At menopause, the decline in estrogen levels disrupts this balance and the consequential, preponderant activity of KNDy neurons changes the activity in the brain regions that these neurons innervate, such as the thermoregulatory control center in the median preoptic nucleus (MnPO) [Citation3].
This mechanistic explanation for the basis of VMS is derived from various observations in both preclinical and ex vivo studies. Ovariectomy is known to increase tail skin temperature in rats, and this phenomenon is reversed by estradiol treatment [Citation47,Citation48], consistent with the clinical sequelae of menopausal hot flashes [Citation49]. Rance and coworkers recognized hypertrophy in NKB-expressing neurons in the hypothalamic infundibular nucleus of postmenopausal women [Citation50,Citation51], and the corresponding neural network in ovariectomized rats [Citation52]. They further demonstrated that these NKB-expressing KNDy neurons were critical to estrogen modulation of body temperature and the observation of cutaneous vasodilation in ovariectomized rats [Citation46]. More recent work has demonstrated that KNDy neurons project to additional NK3R-expressing neurons in the median preoptic nucleus (MnPO) [Citation53], the site for thermoregulatory control. These findings have culminated in the recognition of this NK3R-sensitive neural circuit as key to the flushing response which arises from a shift in the balance point for core body temperature [Citation46,Citation54], thereby activating heat dissipation effectors [Citation55]. This mechanistic explanation, based on preclinical research, appears to translate well to the clinical setting based on various reports implicating NKB/NK3R signaling in VMS [Citation45,Citation56,Citation57]. Activation of heat dissipation effectors manifests in the clinical symptoms of sweating and cutaneous vasodilation, experienced as hot flashes ().
Figure 1. Mechanism of action of NK3R antagonists in VMS ERα: estrogen receptor alpha; FEZ: fezolinetant (NK3R antagonist); KNDy: Kisspeptin/Neurokinin B/Dynorphin; NKB: neurokinin B; NK3R: neurokinin-3 receptor; VMS: vasomotor symptoms
Fezolinetant was initially advanced into relevant preclinical models as a preamble to its entry into clinical development for the treatment of VMS. As previously described for the rat, ovariectomy in the ewe causes changes in core body temperature considered analogous to menopausal hot flashes [Citation58]. The first demonstration of efficacy for fezolinetant in a VMS model was performed in ovariectomized ewes, where drug treatment significantly prevented the elevation of core body temperature in comparison to that of vehicle-treated controls [Citation38]. This antipyrogenic effect in ovariectomized ewes was coincident with a significant prolongation of the luteinizing hormone (LH) pulse interval and a selective decrease in plasma LH, but not FSH, consistent with the proposed mechanism of action to block NKB/NK3R signaling to regulate KNDy neuron signaling [Citation38]. These findings are complemented by mechanistic studies conducted in ovariectomized rats to demonstrate the direct actions of fezolinetant on the thermoregulatory center of the brain [Citation59]. Thus, a significant increase in c-fos expression, an indirect marker of neuronal activity, was observed in MnPO neurons in the brain of ovariectomized rats that coincidentally exhibited hot flash-like symptoms, as shown by increased skin temperature. After 1 week of fezolinetant administration, activation of c-fos expression in MnPO neurons was significantly inhibited, concomitant with a significant diminution of skin temperature in treated rats relative to controls.
These preclinical studies, together with the clinical observation that fezolinetant appeared to modulate the HPG axis in a manner consistent with a mechanism of action on KNDy neurons [Citation60], formed the basis of our decision to test the clinical hypothesis that NK3R antagonism may restore normal sensitivity of the temperature control center for the treatment of VMS.
2.4. Clinical development of fezolinetant
2.4.1. Pharmacokinetics and pharmacodynamics
A first-in-human study in healthy premenopausal women and healthy men evaluated the safety, PK, and pharmacodynamics (PD) of fezolinetant after single-dose (3 to 180 mg) and multiple-dose administration (20, 60, or 180 mg/day for 21 days in women; 20, 60, or 180 mg/day for 10 days in men) [Citation60]. Fezolinetant was well tolerated during single-dose escalation and multiple-dose escalation. There were no clinically significant changes across dose groups in any laboratory parameters, vital signs, or electrocardiographic measurements.
Fezolinetant was rapidly absorbed, with Cmax achieved at a median time of 1.5–2 h after dosing; and showed linear PK, with mean plasma half-life ranging from 3.35 to 4.19 h across the dose groups [Citation60]. In men, fezolinetant elicited significant and rapid dose-dependent decreases in plasma LH and testosterone with recovery to baseline levels at 16–24 h post-dosing [Citation60]. In premenopausal women, fezolinetant treatment initiated at days 3–5 of the menstrual cycle delayed the rise of estradiol over the follicular phase, thereby delaying the presentation of a dominant follicle, endometrial thickening, the LH surge, and causing prolongation of the overall menstrual cycle (P < 0.05, in all cases), as anticipated based on the proposed drug action to modulate KNDy neuron activity. Estradiol levels did not decline below those characteristic of the early follicular phase and therefore plasma concentrations were maintained above levels associated with bone mineral density loss [Citation61]. No inhibition of FSH levels occurred at any time throughout the menstrual cycle. Fezolinetant effects reversed rapidly after discontinuation [Citation60].
Fezolinetant was next evaluated in patients with VMS associated with menopause in phase II studies. These included a 12-week double-blind proof-of-concept phase IIa study conducted in eight Belgian clinics from September 2015 to October 2016 (EudraCT 2015-002578-20), in which healthy postmenopausal women 40–65 years of age with moderate/severe VMS (≥49 episodes/week) were randomized 1:1 to fezolinetant 90 mg BID or placebo [Citation62]. In addition, a 12-week, randomized, placebo-controlled, double-blind dose-ranging phase IIb study (VESTA; [ClinicalTrials.gov NCT03192176]) was conducted at 51 sites in the US from July 2017 to September 2018 in postmenopausal women 40–65 years of age with moderate/severe VMS (≥50 episodes/week) who were randomized to fezolinetant 15, 30, 60, or 90 mg BID or 30, 60, or 120 mg QD or placebo [Citation63]. Population PK evaluations in these two studies indicated that fezolinetant maintained a similar PK profile in VMS patients as previously described in premenopausal women [Citation60].
Pharmacodynamic biomarkers were evaluated in these menopausal subjects to compare with results of the previously described phase I study results in premenopausal women [Citation60]. In the phase IIa study, at peak drug levels (3 h postdose), fezolinetant decreased plasma LH by 49.8% relative to baseline vs 16.4% with placebo [Citation62]. Fezolinetant showed little impact on plasma levels of estradiol, FSH, and sex hormone-binding globulin (SHBG). In the phase IIb study, fezolinetant was associated with dose-dependent LH reductions at 3 h postdose vs relatively stable levels in the placebo group [Citation63]. All groups, including placebo, showed small decreases from baseline in FSH levels, with no treatment- or dose-related effects. No clear trends or differences from placebo in estradiol or SHBG levels were observed. Lowering of LH levels coincident with peak levels of drug exposure is consistent with the proposed mechanism of action of KNDy neuron inhibition.
2.4.2. Safety in phase II studies
Fezolinetant was well tolerated in the phase IIa study [Citation62]. Treatment-emergent adverse events (TEAEs) were reported by 29 (67.4%) subjects receiving fezolinetant and 35 (79.5%) subjects receiving placebo; all TEAEs were mild or moderate in severity. In total, 13 (30.2%) TEAEs in the fezolinetant group were considered to be treatment related vs 11 (25.0%) in the placebo group. The most common fezolinetant-related adverse events (AEs) were gastrointestinal (GI)-related in six subjects (14.0%); there were no GI AEs in the placebo group. GI-related AEs may be attributable to a pharmacological response to the compound as NK3R is also expressed in the GI tract [Citation64,Citation65]. Two subjects (4.7%) in the fezolinetant group discontinued due to TEAEs considered possibly related to treatment (one subject reported worsening of preexisting fibromyalgia, depression, dry mouth, headache, palpitations, diarrhea, and vomiting; one subject reported headache and vertigo). No placebo subjects discontinued due to a TEAE [Citation62].
Increased alanine aminotransferase (ALT) values were more frequent in the fezolinetant group (five subjects, 11.9%) vs. the placebo group (one subject, 2.3%). In all cases, the increases in ALT were mild and transient and did not exceed 3x the upper limit of normal (ULN). None of these abnormalities were considered clinically significant, with the exception of one occurrence of raised ALT and aspartate aminotransferase (AST) in the placebo group. No consistent changes in vital signs, ECG, or bone density markers were observed during the study.
Fezolinetant was also well tolerated in the phase IIb study [Citation63]. Overall TEAE rates were similar across treatment groups, and were largely mild/moderate. The most common TEAEs were nausea, diarrhea, fatigue, urinary tract infection, upper respiratory tract infections, sinusitis, headache, and cough (). No serious treatment-related TEAEs occurred. A sum total of nine subjects from fezolinetant treatment groups experienced transient ALT or AST elevations >3x ULN detected at routine, prescheduled testing, typically after between 4 and 8 weeks of treatment. There were no cases of bilirubin >2x ULN. ALT/AST levels returned to baseline following discontinuation of treatment and trended toward baseline values in those participants who remained on treatment. None of these elevations were associated with evidence of liver functional impairment or liver-associated symptoms.
Table 3. Treatment-emergent adverse events in the phase IIb study
There were no clinically meaningful changes in vital signs, nor differences from placebo in ECG results with fezolinetant. Changes in bone turnover markers were not different across treatment groups. Fezolinetant was not associated with any changes in baseline estradiol levels and, relatedly, no increase in the thickness of the endometrial lining nor any sign of endometrial hyperplasia (the latter being monitored as a risk factor for endometrial cancer) [Citation63]. The absence of any changes to estradiol levels, and no increases in FSH levels, in response to fezolinetant, is a common finding also reported for other NK3 antagonists [Citation66], and a reassuring outcome for women with a contraindication to conventional HT due to risks of estrogen-sensitive cancers [Citation17,Citation18], or thromboembolism [Citation67,Citation68].
2.4.3. Efficacy in treatment of VMS in phase II studies
Fezolinetant is the first NK3R antagonist evaluated for the treatment of VMS in a phase IIa randomized trial conducted according to FDA guidelines [Citation62]. The primary outcome was change from baseline to week 12 in daily total VMS score (averaged over the course of a week). Total VMS score is a composite of VMS severity and frequency data. It is calculated by multiplying the number of mild, moderate, or severe VMS episodes (scored as 1, 2, or 3, respectively) during the period, summing the values and dividing by the number of days in the period. A higher score indicates worse symptoms [Citation69]. Secondary outcomes included changes from baseline over time in weekly VMS frequency and severity scores, and QoL assessments including the Hot Flash Related Daily Interference Scale (HFRDIS), the Greene Climacteric Scale (GCS) to measure changes in climacteric symptoms, the Leeds Sleep Evaluation Questionnaire (LSEQ) to assess sleep quality, and the Sheehan Disability Scale (SDS) to assess daily functional impairment.
Of 122 subjects screened, 87 were randomized to receive fezolinetant (n = 43; 93% completed the study) or placebo (n = 44; 91% completed the study). On the primary endpoint, fezolinetant resulted in significantly greater reduction in daily total VMS score from baseline to week 12 (–26.5; 95% CI –30.8, –22.2) vs placebo (–12.2; 95% CI – 16.5, –7.8; least squares mean difference (LSMD) – 12.3; 95% CI – 16.9, –7.8; P < 0.001). Mean daily total VMS score was also reduced with fezolinetant at weeks 4 and 8 ().
Figure 2. Effect of fezolinetant on VMS over time. (A) Daily total VMS score during week 4 and week 12. (B) Frequency of moderate/severe hot flashes during the duration of the study. (C) Moderate/severe VMS score during the duration of the study. Data are presented as mean ± 95% CI (mITT population, observed data). BID: twice daily; CI: confidence interval; FUP: follow-up; HF: hot flashes; mITT: modified intention to treat; VMS: vasomotor symptoms. Adapted from Depypere H, Timmerman D, Donders G, et al., Treatment of Menopausal Vasomotor Symptoms with Fezolinetant, a Neurokinin 3 Receptor Antagonist: A Phase 2a Trial, The Journal of Clinical Endocrinology & Metabolism 2019; 104 (12): 5893–5905 doi:10.1210/jc.2019-00677. Reprinted (and adapted) by permission of Oxford University Press on behalf of the Endocrine Society
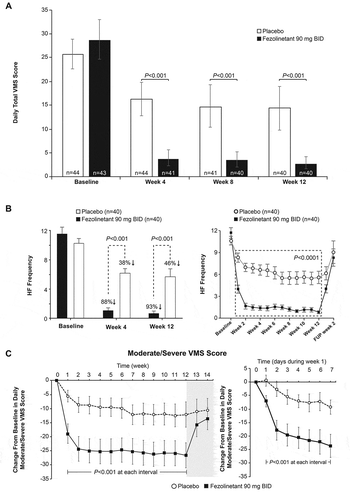
Fezolinetant significantly reduced hot flash (HF) frequency vs placebo. The frequency of weekly moderate and severe VMS decreased by 93% over 12 weeks in the fezolinetant group, from a mean of 80.7 weekly episodes at baseline to 5.7 at week 12. In the placebo group, the reduction in frequency was 46%, from a mean of 72.0 weekly episodes at baseline to 39.0 at week 12 (). Severity and frequency of moderate/severe VMS were reduced from the first day of treatment.
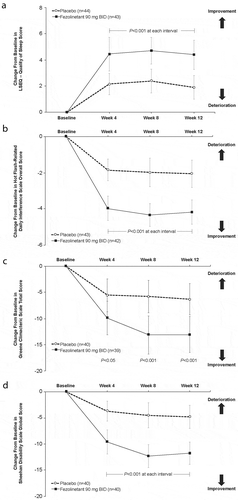
Fezolinetant treatment resulted in improvement from baseline in sleep quality, overall daily interference, climacteric symptoms, and function at weeks 4, 8, and 12. These measures are components of the patient-reported outcomes (PROs) collected as part of the HFRDIS, GCS, LSEQ, and SDS, where overall outcomes for each of these scales showed significant improvement ().
Figure 3. Effect of fezolinetant on quality of life measures. (A) LSEQ (does not include a total mean score that incorporates all four dimensions assessed). (B) HFRDIS. (C) GCS. (D) SDS. Data are presented as mean ± 95% CI (mITT population). BID; twice daily; CI: confidence interval; GCS: Greene Climacteric Scale; LSEQ: Leeds Sleep Evaluation Questionnaire; mITT: modified intention to treat; SDS: Sheehan Disability Scale. From Depypere H, Timmerman D, Donders G, et al., Treatment of Menopausal Vasomotor Symptoms with Fezolinetant, a Neurokinin 3 Receptor Antagonist: A Phase 2a Trial, The Journal of Clinical Endocrinology & Metabolism 2019; 104 (12): 5893–5905 doi:10.1210/jc.2019-00677. Reprinted by permission of Oxford University Press on behalf of the Endocrine Society
In the phase IIb study, primary outcomes were reduction in moderate/severe VMS frequency and severity at weeks 4 and 12: (severity calculation: [number of moderate VMS x2] + [number of severe VMS x3]/[total daily moderate/severe VMS]) [Citation63]. A key secondary outcome was the responder rate, defined as ≥50% reduction in moderate/severe VMS frequency [Citation70].
Of 352 treated participants, 287 completed the study: fezolinetant twice daily 15 mg (n = 40), 30 mg (n = 38), 60 mg (n = 33), 90 mg (n = 32); fezolinetant once daily 30 mg (n = 34), 60 mg (n = 36), 120 mg (n = 37); placebo (n = 37). All fezolinetant regimens significantly reduced the frequency of moderate/severe VMS at weeks 4 and 12 (). Fezolinetant reduced moderate/severe VMS in the range of 62–81% at week 4 (depending on dose) compared with about a 39% reduction with placebo; at week 12, moderate/severe VMS were reduced by 74–87% with fezolinetant vs 55% with placebo.
Table 4. Primary efficacy outcomes: frequency of moderate/severe VMS per 24 h, full analysis set
PROs were investigated in a secondary analysis of this phase IIb study [Citation70]. Changes from baseline in Menopause-Specific Quality of Life (MENQoL) questionnaire, HFRDIS, and GCS were compared with published minimally important differences (MIDs). For all doses, mean changes from baseline in MENQoL VMS scores exceeded the MID (1.2) [Citation71] at weeks 4 (placebo: −1.8; fezolinetant: −1.9 to −3.6) and 12 (placebo: −2.3; fezolinetant: −2.9 to −4.4). Mean changes in HFRDIS at weeks 4 (placebo: −2.2; fezolinetant: −2.5 to −3.8) and 12 (placebo: −2.9; fezolinetant: −3.3 to −4.3) exceeded the MID (1.76) [Citation70]. GCS-VMS domain scores improved for most fezolinetant doses vs placebo (week 4, placebo: −1.7; fezolinetant: −2.1 to −3.3; week 12, placebo: −2.1; fezolinetant: −2.7 to −3.6).
2.4.4. Phase III studies
Fezolinetant is under further investigation for treatment of moderate-to-severe VMS in ongoing phase III trials as part of the BRIGHT SKY program: Skylight 1 (NCT04003155), Skylight 2 (NCT04003142), Skylight 4 (NCT04003389), Moonlight 1 (NCT04234204), and Moonlight 3 (NCT04451226) [Citation72]. Skylights 1 and 2 are 12-week randomized, placebo-controlled, double-blind studies of fezolinetant 30 mg or 45 mg once daily, with each study enrolling approximately 450 women, followed by an extension period to 52 weeks in total to assess efficacy and safety. Co-primary endpoints are change from baseline to weeks 4 and 12 in VMS severity and frequency. Secondary outcome measures include mean change in the Patient-reported Outcomes Measurement Information System Sleep Disturbance (PROMIS) and overall safety, including endometrial safety. Skylight 4 is a 52-week double-blind safety and tolerability study of fezolinetant vs placebo in approximately 1,150 women seeking treatment for VMS associated with menopause. Secondary endpoints include an assessment of endometrial safety and bone health. Moonlight 1 is a 12-week randomized, placebo-controlled, double-blind study with a 12-week non-controlled extension period of fezolinetant in approximately 300 menopausal women in Asia (China, South Korea, Taiwan). The primary outcome is change from baseline to weeks 4 and 12 in VMS severity and frequency. Moonlight 3 is a 52-week single-arm safety and tolerability study of fezolinetant vs placebo in approximately 150 women seeking treatment for VMS associated with menopause.
3. Conclusion
Fezolinetant is the most advanced NK3R antagonist in its stage of clinical development for VMS, with the most extensive, accrued database of clinical safety and efficacy data in women. Results to date have demonstrated rapid and substantial reduction in VMS frequency and severity, which has translated into improvements in health-related quality of life. Fezolinetant has the attributes of high NK3R target selectivity and a favorable PK profile based on the efficiency of ‘free’ drug to attain target receptor engagement such that moderate, daily doses achieve drug efficacy with clear safety margins in trials of up to 12-weeks’ duration. Fezolinetant has advanced into multiple phase III clinical trials to measure efficacy and long-term safety, with study closure and data reporting anticipated in 2021.
4. Expert opinion
VMS is the symptom for which the majority of peri- or postmenopausal women seek treatment. Despite HT being the most effective available treatment for VMS, only 3–10% of affected women in the US and Europe use HT, in part due to the safety concerns of stroke, breast cancer, coronary heart disease, and other illnesses as identified by the Women’s Health Initiative (WHI) [Citation67], and corroborated in recent clinical reports [Citation17,Citation68,Citation73,Citation74]. Although current guidelines recommend HT as first-line treatment, particularly for symptomatic women <60 years of age or within 10 years of menopause [Citation13–16], many women cannot take HT due to safety and tolerability concerns driven by medical history or concomitant medical conditions, or make an informed decision to not take it.
Alternative non-hormonal treatments include the SSRI paroxetine at a dose of 7.5 mg/day. This is currently the only approved non-hormonal agent for VMS, but has modest efficacy and some tolerability concerns, including significant nausea and dizziness and, in rare cases, suicidal thought [Citation24,Citation75]. Clonidine, gabapentin, and other SSRIs and serotonin norepinephrine reuptake inhibitors are used off-label in many cases to manage VMS, but also have only modest efficacy [Citation24]. In addition, herbal medicines such as black cohosh, ginseng, gingko biloba, St. John’s wort, and dong quai have been studied for VMS; however, there is conflicting evidence regarding their efficacy [Citation76,Citation77]. Thus, there is a need for a suitable non-hormonal treatment option for many postmenopausal women.
Recent breakthroughs indicate that NKB/NK3R signaling is a key point for pharmacological intervention in the treatment of menopause-associated VMS. Specifically, NK3R antagonists offer a targeted approach to directly restore thermoregulatory balance by modulating the brain circuits triggered by the diminution of estrogen levels at menopause. Therefore, NK3R antagonists are currently being evaluated as a novel non-hormonal therapeutic approach to alleviate VMS, an advancement from the discontinuation in schizophrenia trials of the first-generation compounds osanetant, talnetant, and pavinetant (). Fezolinetant is the NK3R antagonist at the most advanced stage of clinical development for VMS and is currently in multiple, international phase III trials. Four other NK antagonists (elinzanetant, pavinetant, SJX-653, and MLE-301) have also been advanced into clinical development for VMS. In the years ahead, NK3R antagonists could potentially provide an alternative non-hormonal treatment for women with menopause-associated VMS unsuitable for HT or who are unwilling to take HT.
Figure 4. Structures of NK3R antagonists advanced into clinical development – (A) osanetant, (B) talnetant, pavinetant and SJX-653, (C) elinzanetant, (D) fezolinetant
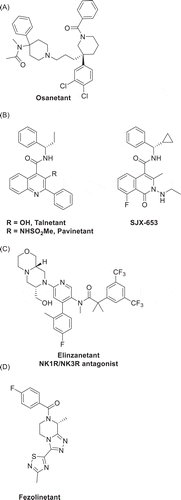
Elinzanetant is a nonselective NK1R/NK3R pseudo-irreversible antagonist with greater potency at the NK1 receptor () [Citation41]. The compound was initially developed by GlaxoSmithKline for the treatment of addiction disorders [Citation78], prior to being spun-out into KaNDy Therapeutics and most recently acquired by Bayer [Citation79]. KaNDy Therapeutics demonstrated that elinzanetant is effective in the treatment of VMS in a phase II trial [Citation80]. The potent action of elinzanetant on NK1 receptors is posited to bring some benefit with regard to peripheral flushing and sleep in VMS [Citation80], whereas the alternate effects of NK1 antagonists on centrally mediated emesis and addiction circuits [Citation26,Citation27], gastrointestinal pharmacology [Citation28], and the hypothalamic-pituitary-adrenal axis [Citation81] is of unknown consequence in menopausal women. Additional clinical testing will further characterize the NK1R/NK3R pharmacology of elinzanetant.
Pavinetant (MLE4901, originally AZD2624) is an NK3R antagonist that also displayed reduction of VMS in clinical trials [Citation66,Citation82]. However, development of pavinetant was discontinued after assessment of clinical risks and benefits, driven by concerns about the hepatic safety profile, an AE proposed to be related to the chemical structure of pavinetant rather than a general class effect for NK3R antagonists [Citation83]. SJX-653 is an NK3R antagonist with a chemical structure similar to that of pavinetant (). SJX-653 was evaluated in a single ascending dose phase I clinical study in men, which included characterizing its effect on pharmacodynamic markers known to be associated with NK3R engagement [Citation84]. A SJX-653 phase II trial (NCT04278872) to evaluate its efficacy in moderate-to-severe VMS associated with the menopause was prematurely terminated on the basis that the anticipated target profile was not met. MLE-301 is an NK3R antagonist with an unspecified chemical structure from US Patent No. 8.507.535 that has recently been discontinued following a phase I clinical trial [Citation85,Citation86].
Fezolinetant has a distinct chemical structure from the competing antagonists in clinical development (). As previously summarized, the clinical results to date have demonstrated rapid and substantial reduction in VMS frequency and severity, which has translated into improvements in quality-of-life outcomes. Fezolinetant has the attributes of high NK3R target selectivity and a favorable PK profile and it has demonstrated efficacy with clear safety margins in the completed trials of up to 12-weeks’ duration. Fezolinetant has advanced into multiple phase III clinical trials with study closure and data reporting anticipated in 2021.
The long-term safety of NK3R antagonists is to be established in phase III trials. Hepatic safety is highlighted as an aspect to be appropriately monitored, particularly following the discontinuation of pavinetant [Citation83]. The relative advantages of long-term treatment with NK3R antagonists versus HT in menopausal women also remain to be determined. NK3R antagonists are not anticipated to elicit the peripheral side effects of hormones; for example, there are no signs of endometrial hyperplasia, to-date, in response to fezolinetant [Citation63], although this needs to be confirmed in long-term studies. On the other hand, there are potential benefits of HT on certain symptoms that appear in some women contemporaneously with menopause, such as vulvovaginal atrophy and loss of bone density, where NK3R antagonists are not anticipated to offer direct benefit. HT has direct effects on various tissues whereas NK3R antagonists act on the neural circuit underlying the hot flash response and, therefore, are targeted specifically for the treatment of VMS. To this end, an expanded role for NK3R antagonists may be in the treatment of VMS triggered by hormone deprivation therapy in women treated for estrogen-sensitive cancers (e.g. breast cancer) [Citation87], as well as survivors of such cancers where HT treatment would be contraindicated [Citation88], although appropriate clinical trials are required to establish NK3R antagonist efficacy and safety in these specific, underserved populations.
Article highlights
Up to 80% of postmenopausal women experience vasomotor symptoms (VMS) during the menopausal transition period.
Although current guidelines recommend hormone therapy (HT) as first-line treatment, many women cannot take HT due to safety and tolerability concerns driven by medical history or concomitant medical conditions.
There is a need for safe, effective non-hormonal therapy for relief of VMS associated with menopause.
Neurokinin-3 receptor (NK3R) antagonists offer a targeted approach to directly restore thermoregulatory balance by modulating the brain circuits triggered by the diminution of estrogen levels at menopause.
Fezolinetant is the NK3R antagonist at the most advanced stage of clinical development for VMS and is currently in multiple international phase III trials.
Clinical evidence indicates that fezolinetant elicits a rapid and substantial reduction in VMS frequency and severity, and associated improvements in health-related quality of life.
Declaration of interest
Christopher Lademacher and Emad Siddiqui are employees of Astellas Pharma. Graeme Fraser was formerly an employee and shareholder of OGEDA and is currently a consultant to Astellas. Herman Depypere has no disclosures to report. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Box 1. Drug summary box
Box 1. Drug summary box
Acknowledgments
The authors would like to thank the fezolinetant study investigators, all patients and their legal representatives who took part in the studies relating to this manuscript. The authors also acknowledge the contribution of Seiji Kaku to research and development of fezolinetant. Medical writing support for this manuscript was provided by Sue Cooper of Elevate Scientific Solutions and funded by Astellas Pharma.
Data availability statement
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Additional information
Funding
References
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006 Jul;96(7):1226–1235.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015 Apr;175(4):531–539.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013 Aug;34(3):211–227.
- Williams RE, Kalilani L, DiBenedetti DB, et al. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007 Dec 20;58(4):348–358.
- Whiteley J, DiBonaventura M, Wagner JS, et al. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Womens Health (Larchmt). 2013 Nov;22(11):983–990.
- Williams RE, Levine KB, Kalilani L, et al. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009 Feb 20;62(2):153–159.
- Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: who is most bothered by vasomotor symptoms? Menopause. 2008 Sep-Oct;15(5):841–847.
- Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the study of women’s health across the Nation. Obstet Gynecol Clin North Am. 2011 Sep;38(3):489–501.
- Worsley R, Bell RJ, Gartoulla P, et al. Moderate-severe vasomotor symptoms are associated with moderate-severe depressive symptoms. J Womens Health (Larchmt). 2017 Jul;26(7):712–718.
- Whiteley J, Wagner JS, Bushmakin A, et al. Impact of the severity of vasomotor symptoms on health status, resource use, and productivity. Menopause. 2013 May;20(5):518–524.
- Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015 Mar;22(3):260–266.
- The NAMS Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017 Jul;24(7):728–753.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015 Nov;100(11):3975–4011.
- de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Maturitas. 2016 Sep;91:153–155.
- Committee on Practice Bulletins - Gynecology. ACOG practice bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014 Jan;123(1):202–216.
- Okano H, Higuchi T, Kurabayashi T, et al. Japan Society of Obstetrics and Gynecology and Japan Society for Menopause and Women’s Health 2017 guidelines for hormone replacement therapy. J Obstet Gynaecol Res. 2018 Aug;44(8):1355–1368.
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019 Sep 28;394(10204):1159–1168.
- Furness S, Roberts H, Marjoribanks J, et al. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst Rev. 2009 Apr 15;(2):CD000402.
- Pfizer Canada Inc. Complete prescribing information PREMARIN® Vaginal Cream 2018 [cited 2021 March 15]. Available from: https://www.pfizer.ca/sites/default/files/201806/Premarin_VC_PM_E_204671_07Jun2018.pdf
- Evorel® Sequi patient leaflet 2019 [cited 2021 March 15]. Available from: https://www.medicines.org.uk/emc/files/pil.10930.pdf
- Lumsden MA. The NICE Guideline - Menopause: diagnosis and management. Climacteric. 2016 Oct;19(5):426–429.
- Bair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008 Jan-Feb;15(1):32–43.
- Cumming GP, Currie H, Morris E, et al. The need to do better – Are we still letting our patients down and at what cost? Post Reprod Health. 2015 Jun;21(2):56–62.
- The North American Menopause Society. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015 Nov;22(11):1155–1172. quiz1173–1174.
- Steinhoff MS, von Mentzer B, Geppetti P, et al. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014 Jan;94(1):265–301.
- Di Maio M, Baratelli C, Bironzo P, et al. Efficacy of neurokinin-1 receptor antagonists in the prevention of chemotherapy-induced nausea and vomiting in patients receiving carboplatin-based chemotherapy: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2018 Apr;124:21–28.
- Schank JR, Heilig M. Substance P and the neurokinin-1 receptor: the new CRF. Int Rev Neurobiol. 2017;136:151–175.
- Duffy RA. Potential therapeutic targets for neurokinin-1 receptor antagonists. Expert Opin Emerg Drugs. 2004 May;9(1):9–21.
- Lecci A, Capriati A, Maggi CA. Tachykinin NK2 receptor antagonists for the treatment of irritable bowel syndrome. Br J Pharmacol. 2004 Apr;141(8):1249–1263.
- Mayer EA, Carlos Marvizon J. Neurokinin 3 receptors in the gut: a new target for the treatment of visceral pain? Gastroenterology. 1999 May;116(5):1250–1252.
- Templeman L, Sellers DJ, Chapple CR, et al. Investigation of neurokinin-2 and -3 receptors in the human and pig bladder. BJU Int. 2003 Nov;92(7):787–792.
- Crane LH, Williams MJ, Nimmo AJ, et al. Estrogen-dependent regulation of neurokinin 3 receptor-mediated uterine contractility in the rat. Biol Reprod. 2002 Nov;67(5):1480–1487.
- Litman RE, Smith MA, Desai DG, et al. The selective neurokinin 3 antagonist AZD2624 does not improve symptoms or cognition in schizophrenia: a proof-of-principle study. J Clin Psychopharmacol. 2014 Apr;34(2):199–204.
- Dawson LA, Porter RA. Progress in the development of neurokinin 3 modulators for the treatment of schizophrenia: molecule development and clinical progress. Future Med Chem. 2013 Sep;5(13):1525–1546.
- Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009 Mar;41(3):354–358.
- Hoveyda HR, Fraser GL, Roy MO, et al. Discovery and optimization of novel antagonists to the human neurokinin-3 receptor for the treatment of sex-hormone disorders (part I). J Med Chem. 2015 Apr 9;58(7):3060–3082.
- Hoveyda HR, Fraser GL, Dutheuil G, et al. Optimization of novel antagonists to the neurokinin-3 receptor for the treatment of sex-hormone disorders (part II). ACS Med Chem Lett. 2015 Jul 9;6(7):736–740.
- Fraser GL, Hoveyda HR, Clarke IJ, et al. The NK3 receptor antagonist ESN364 interrupts pulsatile LH secretion and moderates levels of ovarian hormones throughout the menstrual cycle. Endocrinology. 2015 Nov;156(11):4214–4225.
- Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010 Dec;9(12):929–939.
- Young RJ, Leeson PD. Mapping the efficiency and physicochemical trajectories of successful optimizations. J Med Chem. 2018 Aug 9;61(15):6421–6467.
- Ridler K, Gunn RN, Searle GE, et al. Characterising the plasma-target occupancy relationship of the neurokinin antagonist GSK1144814 with PET. J Psychopharmacol. 2014 Mar;28(3):244–253.
- Rance NE, Krajewski SJ, Smith MA, et al. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010 Dec 10;1364:116–128.
- Herbison AE. A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front Neuroendocrinol. 2020 Apr;57:100837.
- Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol. 2012 Apr;33(2):160–168.
- Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015 Feb;16(5):8466.
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012 Nov 27;109(48):19846–19851.
- Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010 Mar;151(3):1187–1193.
- Okada M, Hayashi N, Kometani M, et al. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn J Pharmacol. 1997 Jan;73(1):93–96.
- Kobayashi T, Tamura M, Hayashi M, et al. Elevation of tail skin temperature in ovariectomized rats in relation to menopausal hot flushes. Am J Physiol Regul Integr Comp Physiol. 2000 Apr;278(4):R863–R869.
- Rance NE, Young WS 3rd. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991 May;128(5):2239–2247.
- Rometo AM, Krajewski SJ, Voytko ML, et al. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007 Jul;92(7):2744–2750.
- Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994 Oct;60(4):337–345.
- Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, et al. Neurokinin 3 receptor-expressing neurons in the median preoptic nucleus modulate heat-dissipation effectors in the female rat. Endocrinology. 2015 Jul;156(7):2552–2562.
- Padilla SL, Johnson CW, Barker FD, et al. A neural circuit underlying the generation of hot flushes. Cell Rep. 2018 Jul 10;24(2):271–277.
- Krajewski-Hall SJ, Miranda Dos Santos F, McMullen NT, et al. Glutamatergic neurokinin 3 receptor neurons in the median preoptic nucleus modulate heat-defense pathways in female mice. Endocrinology. 2019 Apr 1;160(4):803–816.
- Rance NE, McMullen NT, Smialek JE, et al. Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990 Jul;71(1):79–85.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the women’s health initiative study. Menopause. 2017 Mar;24(3):252–261.
- MacLeay JM, Lehmer E, Enns RM, et al. Central and peripheral temperature changes in sheep following ovariectomy. Maturitas. 2003 Nov 20;46(3):231–238.
- Tahara A, Takamatsu H, Ohtake A, et al. Effects of neurokinin 3 receptor antagonist fezolinetant on hot flash-like symptoms in ovariectomized rats. Eur J Pharmacol 2021. In press https://doi.org/10.1016/j.ejphar.2021.174207.
- Fraser GL, Ramael S, Hoveyda HR, et al. The NK3 receptor antagonist ESN364 suppresses sex hormones in men and women. J Clin Endocrinol Metab. 2016 Feb;101(2):417–426.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992 Feb;166(2):740–745.
- Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019 Dec 1;104(12):5893–5905.
- Fraser GL, Lederman S, Waldbaum A, et al. A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause. Menopause. 2020 Apr;27(4):382–392.
- Jaafari N, Hua G, Adelaide J, et al. Expression of the tachykinin receptor mRNAs in healthy human colon. Eur J Pharmacol. 2008 Dec 3;599(1–3):121–125.
- Houghton LA, Cremonini F, Camilleri M, et al. Effect of the NK(3) receptor antagonist, talnetant, on rectal sensory function and compliance in healthy humans. Neurogastroenterol Motil. 2007 Sep;19(9):732–743.
- Prague JK, Abbara A, Comninos AN, et al. Neurokinin 3 receptor antagonists do not increase FSH or estradiol secretion in menopausal women. J Endocr Soc. 2020 Feb 1;4(2):bvz009.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–333.
- Vickers MR, MacLennan AH, Lawton B, et al. Main morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ. 2007 Aug 4;335(7613):239.
- Sloan JA, Loprinzi CL, Novotny PJ, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001 Dec 1;19(23):4280–4290.
- Santoro N, Waldbaum A, Lederman S, et al. Effect of the neurokinin 3 receptor antagonist fezolinetant on patient-reported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 2020 Dec;27(12):1350–1356.
- Bushmakin AG, Abraham L, Pinkerton JV, et al. Evaluation of the measurement model and clinically important differences for menopause-specific quality of life associated with bazedoxifene/conjugated estrogens. Menopause. 2014 Aug;21(8):815–822.
- Astellas Pharma Inc. Astellas initiates phase 3 clinical trials for fezolinetant in postmenopausal women with vasomotor symptoms. Press Release. August 6, 2019.
- Crawford SL, Crandall CJ, Derby CA, et al. Menopausal hormone therapy trends before versus after 2002: impact of the women’s health initiative study results. Menopause. 2018 Dec 21;26(6):588–597.
- Ameye L, Antoine C, Paesmans M, et al. Menopausal hormone therapy use in 17 European countries during the last decade. Maturitas. 2014 Nov;79(3):287–291.
- Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013 Oct;20(10):1027–1035.
- Borrelli F, Ernst E. Alternative and complementary therapies for the menopause. Maturitas. 2010 Aug;66(4):333–343.
- Mintziori G, Lambrinoudaki I, Goulis DG, et al. EMAS position statement: non-hormonal management of menopausal vasomotor symptoms. Maturitas. 2015 Jul;81(3):410–413.
- te Beek ET, Hay JL, Bullman JN, et al. Pharmacokinetics and central nervous system effects of the novel dual NK1/NK3 receptor antagonist GSK1144814 in alcohol-intoxicated volunteers. Br J Clin Pharmacol. 2012;75(5):1328–1339.
- Cross R. Bayer to acquire KaNDy Therapeutics. Chemi Eng News 2020 98(31).
- Trower M, Anderson RA, Ballantyne E, et al. Effects of NT-814, a dual neurokinin 1 and 3 receptor antagonist, on vasomotor symptoms in postmenopausal women: a placebo-controlled, randomized trial. Menopause. 2020 Feb 17;27(5):498–505.
- Wils J, Duparc C, Cailleux AF, et al. The neuropeptide substance P regulates aldosterone secretion in human adrenals. Nat Commun. 2020 May 29;11(1):2673.
- Prague JK, Roberts RE, Comninos AN, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017 May 6;389(10081):1809–1820.
- Modi M, Dhillo WS. Neurokinin B and neurokinin-3 receptor signaling: promising developments in the management of menopausal hot flushes. Semin Reprod Med. 2019 May;37(3):125–130.
- Anderson RA, Cormier J, Thieroff-Ekerdt R, et al. Pharmacodynamic activity of the novel neurokinin-3 receptor antagonist SJX-653 in healthy men. J Clin Endocrinol Metab. 2020 Dec 1; 105(12):e4857-e4865.
- License Agreement between Hoffmann-La Roche and Millendo Therapeutics. [cited 2021 March 15]. Available from: https://www.sec.gov/Archives/edgar/data/1544227/000155837019007932/mlnd-20190630ex104d45207.htm
- Business Wire Press Release. Millendo Therapeutics Provides Pipeline and Business Update. [cited 2021 March 15]. Available from: https://www.businesswire.com/news/home/20210105005561/en/Millendo-Therapeutics-Provides-Pipeline-and-Business-Update.
- Leon-Ferre RA, Majithia N, Loprinzi CL. Management of hot flashes in women with breast cancer receiving ovarian function suppression. Cancer Treat Rev. 2017 Jan;52:82–90.
- Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017 Oct 1;102(10):3647–3661.