ABSTRACT
Introduction
Antibody–drug conjugates (ADCs) represent a revolutionary approach in the systemic treatment for both solid and hematologic tumors. Constituted by an antibody, a cytotoxic payload, and a linker, ADCs aim to selectively deliver cytotoxic agents to tumors while sparing normal tissues. Various ADCs have been tested and approved for multiple solid tumors so far, but if there is one that had a major impact on clinical practice, this is Trastuzumab-deruxtecan (T-DXd). Notably, T-DXd was approved for HER2-positive and HER2-low metastatic breast cancer (MBC), HER2-positive gastric cancer (GC), HER2-mutant non-small cell lung cancer (NSCLC) and HER2 3+ solid tumors. Moreover, it received Breakthrough Therapy Designation for HER2-positive colorectal cancer (CRC).
Areas covered
We review preclinical and clinical data of T-DXd, focusing on early-phase ongoing trials exploring combination therapies to enhance the activity of T-DXd in HER2-expressing solid tumors.
Expert opinion
The clinical use of T-DXd still raises questions about selection of patients, treatment duration, prioritization over other approved ADCs, and management of resistance. Concerns regarding the toxicity of T-DXd remain, particularly with combinations involving potentially toxic drugs. Advancements in biomarker identification and combination therapies offer promising avenues to enhance efficacy and overcome resistance to T-DXd, ultimately improving outcomes for patients with cancer.
1. Introduction
Antibody–drug conjugates (ADCs) are revolutionizing systemic cancer care of both solid and liquid tumors [Citation1]. ADCs are composed of three main parts: the antibody, that is directed usually against an antigen expressed on cancer cells, a cytotoxic payload and a linker [Citation2]. The properties of the ADC are determined by all its three components, each of them conveying important features [Citation1].
The basic idea under the development of ADCs is the attempt to deliver the cytotoxic agent selectively to the tumor, sparing normal tissues [Citation1]. This makes it possible to reach higher concentrations of chemotherapeutic agent without increasing the toxicity.
The first ADC was approved for acute myeloid leukemia in 2000 (Gemtuzumab-ozogamicin) [Citation3]. Trastuzumab-emtansine (TDM1) gained Food and Drug Administration (FDA) approval in 2013 for the treatment of pretreated human epidermal growth factor receptor 2 (HER2) positive metastatic breast cancer (MBC), on the basis of the results of EMILIA trial [Citation4]. HER2 positivity was defined as immunohistochemistry (IHC) 3+ staining or IHC 2+ and amplification detected by in-situ hybridization (ISH) [Citation5]. Other ADCs were successively tested and approved in various solid tumors [Citation6–14].
Trastuzumab-deruxtecan (T-DXd), an HER2-targeting ADC, was subsequently developed and entered clinical practice, being approved for HER2-positive and HER2-low MBC, as well as for other HER2-addicted solid tumors, specifically HER2-positive gastric cancer (GC) and HER2-mutant non-small cell lung cancer (NSCLC) [Citation6,Citation9,Citation13,Citation14]. Recently, FDA approved T-DXd also for HER2 expressing (IHC 3+) solid tumors, and granted Breakthrough Therapy Designation for HER2-overexpressing colorectal cancer (CRC) [Citation15–17]. See for the timeline of T-DXd development and approval.
Figure 1. Timeline of development and approval of Trastuzumab-deruxtecan for solid tumors. In bold FDA approvals. Abbreviations: D: DESTINY (e.g. DBREAST01: DESTINY-Breast01).
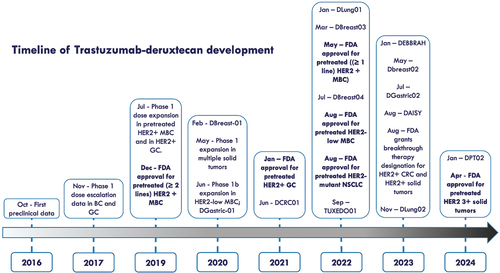
Interestingly, clinical data of T-DXd suggests that this ADC is active even in the presence of low expression of HER2. In facts, DESTINY-Breast04 trial showed a survival benefit in patients receiving T-DXd over physician choice chemotherapy in patients with HER2 1+ or HER2 2+ without amplification detected by ISH, thus overcoming the historical dualistic classification of MBC in HER2-positive or HER2-negative [Citation13]. Disquisitions are still ongoing to determine if the HER2-low MBC represents a different biological entity [Citation18]. The awaited results of the phase III DESTINY-Breast06 trials, which include a cohort of patients with HER2 0 MBC, may provide decisive evidence in this regard, despite the already available data of the phase II DAISY trial [Citation19].
Alongside the positive results in terms of drug efficacy, T-DXd presents a toxicity profile that has been raising concerns during its development, especially for the emergence of potentially fatal lung toxicity (interstitial lung disease – ILD) [Citation20]. Lung toxicity occurs in a significant proportion of patients: a pooled analysis showed that 16.8% of patients with advanced cancer experienced ILD of any grade [Citation21]. The risk factors associated with ILD risk were Japanese ancestry, BC compared to other tumor types, and higher drug dose [Citation21]. Subsequent studies demonstrated a generally lower incidence of ILD (around 10%), as well as a reduction of fatal cases, thanks to the awareness of ILD potential severity and to the optimization of its management [Citation20]. The mechanisms leading to ILD development are not yet fully understood, but it has been demonstrated to occur also with different ADCs, targeting HER2 or other antigens, even if with generally lower incidence [Citation20]. Some drugs other than ADCs were also shown to cause ILD, such as chemotherapeutic agents, mTOR inhibitors, tyrosine kinase inhibitors (TKI), immune checkpoint inhibitors (ICI), and CDK4/6 inhibitors [Citation22–30]. Despite being under special observation, ILD is not the only toxicity to burden treatment with T-DXd. Hematological toxicity, as well as nausea, vomiting, diarrhea and alopecia have been reported as frequent adverse events (AEs), and should be taken into consideration when designing combination trials [Citation31].
In this manuscript, we aim to review the data of early-phase drug development of T-DXd and we report the combination therapies actually under early-phase development to enhance the activity of T-DXd in solid tumors.
2. Trastuzumab-deruxtecan characteristics and preclinical data
T-DXd is composed by Trastuzumab, an IgG1 humanized monoclonal antibody targeting HER2 [Citation32], an enzymatically cleavable peptide linker (maleimide glycyn-glycyn-phenylalanyn-glycyn – GGFG) and a cytotoxic payload, deruxtecan (DXd) [Citation33]. DXd is a topoisomerase-I inhibitor derivative of exatecan mesylate [Citation33]. Each antibody is conjugated with eight DXd molecules, thus having a drug–antibody ratio (DAR) of 8, more than double of TDM1 (3.5) [Citation33,Citation34].
In vitro preclinical studies of T-DXd demonstrated remarkable cell growth inhibition in HER2-expressing cell lines [Citation33]. This activity relied on the conjugation of the DXd with the anti-HER2 antibody, as the control drugs (anti-HER antibody alone and other IgG-DXd ADC) showed weaker cell growth inhibition [Citation33].
In vivo experiments showed that T-DXd activity was dependent on the dose and on HER2-binding, as no activity was detected for the control ADC carrying DXd [Citation33].
Preclinical data further suggested that T-DXd retains trastuzumab mechanisms of action in terms of induction of antibody-dependent cell-mediated cytotoxicity (ADCC) and downregulation of phosphorylated Akt, which upregulates p27 and impairs cell proliferation [Citation33]. Aside of these mechanisms of action, T-DXd demonstrated also to induce DNA damage and apoptosis thanks to the Topoisomerase I inhibition by DXd, thus combining the pharmacologic properties of both the antibody and the payload [Citation33].
The activity of T-DXd was experimented also in mice xenograft models expressing HER2 at various levels, demonstrating activity in all the models with expression of HER2 (intense, moderate and weak), but not in the HER2 negative model [Citation33]. The control ADC (TDM1, DAR 3.5) displayed activity only in the HER2-intense model, suggesting that the higher DAR allows T-DXd to convey a sufficient amount of payload to the tumor even in presence of low expression of the antigen [Citation33]. T-DXd demonstrated higher activity than TDM1 in HER2-positive PDX models, while in HER2 1+, FISH negative only T-DXd was shown to be active [Citation33].
Regarding toxicity profile, tests on monkeys demonstrated bone marrow, intestinal and lung toxicities as the major concerns [Citation33].
One of the features attributed to ADCs is the so-called bystander effect, meaning that the cytotoxic payload exerts activity also on cells not expressing the antigen, but near to the ones that express it [Citation35]. The proposed mechanism is the release of the payload in the extracellular space and its uptake by the surrounding cells [Citation35]. The bystander effect may thus explain the efficacy of some ADCs even in the presence of HER2 heterogenous expression in some cancers, as high expression of the antigen by all the cells is not required. A preclinical study addressing the bystander effect properties of T-DXd showed the drug was capable of killing both HER2-positive and HER2-negative co-cultured cells, as well as when both were inoculated in mice [Citation36]. Interestingly, it was also shown that the bystander effect was not elicited when the different cells were inoculated in distant sites, thus highlighting the importance of spatial proximity in this process [Citation36]. On the contrary, TDM1 did not display bystander effect, and the reason was attributed to the high membrane permeability of DXd compared to Emtansine and to the higher DAR of T-DXd [Citation36].
Alongside with the HER2 expression, there are mechanistic evidences regarding the rationale of administering T-DXd also on the basis of the mutational status of HER2. In fact, the presence of a HER2 activating mutation may indicate an addiction to its pathway, which can be targeted thanks to the inhibition exerted by the antibody. Moreover, preclinical evidence suggested that HER2-mutant cancer cells may have increased ubiquitination and internalization of HER2, possibly leading to an increase of T-DXd internalization and then enhanced activity [Citation37].
3. Phase I clinical trials of T-DXd monotherapy
The results of the dose escalation part of the first phase I clinical trial of T-DXd were published in 2017 [Citation38]. The trial enrolled 24 patients (17 BC and 7 GC) in Japan. Among the 23 patients evaluable for response, 21 (91%) had disease control, while 10 (43%) achieved a partial response (PR) to the treatment, including 2 HER2-low patients [Citation38]. High objective response rates (ORR) and disease control rates (DCR) were observed in HER2+ BC patients who had previously received TDM1 (58% ORR, 100% DCR) and both HER2+ BC and GEC who had previously received trastuzumab (BC: 53% ORR, 94% DCR; GEC: 50% ORR, 100% DCR) [Citation38]. The responses were dose-dependent, with most of them observed at 5.4 mg/kg or above dose level [Citation38]. The maximum tolerated dose (MTD) was not reached in any of the cohorts, and the most common AEs where hematological and gastrointestinal, 9 patients (37%) needed a dose reduction (all from 5.4 mg/kg or higher dose levels) and 3 discontinued the treatment due to AEs, 2 of them treatment-related (1 pneumonitis, 1 decrease platelet count) [Citation38]. The established doses for expansion were 5.4 mg/kg and 6.4 mg/kg, and the results of the part 2 were published separately for BC and GC cohorts. Two hundred seventy-four patients were enrolled between August 2015 and August 2018, among which 118 HER2+ ABC pretreated with TDM1 and 44 HER2+ pretreated GC [Citation39,Citation40]. In the BC cohort, 57 (50%) the 115 patients who received at least one dose of T-DXd had a grade 3 or higher AE, 21 (18%) had dose reductions and 13 (11%) discontinued the treatment due to treatment-related AEs (9 ILD or pneumonitis, 2 grade 5) [Citation39]. Six patients had ILD (5 grade 1 or 2, 1 grade 3) and 8 pneumonitis (6 grade 1 or 2, 2 grade 5) [Citation39].
Sixty-six (59.5%) of the 111 patients evaluable achieved a response and 104 (93.7%) had clinical benefit [Citation39]. Median progression-free survival (mPFS) was 22.1 months, while median overall survival (mOS) was not reached [Citation39].
Regarding the 44 HER2+ GC patients, 28 (64%) had at least one grade 3 or higher AE (21 (48%) drug-related), 11 (25%) a serious AE (4 (9%) drug-related) [Citation40]. Six patients discontinued the treatment because of AE, 5 drug related (3 ILD, 1 decreased appetite, 1 platelet count decreased) [Citation40]. The ORR was 43.2% (19/44) and the CBR was 79.5% (35/44) [Citation40]. mPFS and mOS were 5.6 and 12.8 months, respectively [Citation40].
Sixty non-BC/non-GC (20 CRC, 18 NSCLC, 21 other tumor types) were enrolled in the HER2-expressing (≥1+) and/or HER2-mutant solid tumors expansion cohort of the same trial [Citation41]. The most common AEs were confirmed to be hematological and gastrointestinal, 30.5% (18 out of the 59 patients who received at least one dose of T-DXd) had serious AE, 8.5% (5/59) discontinued the treatment due to an AE (among which 2 ILD and 1 pneumonitis) [Citation41]. One NSCLC patient died for drug-related ILD [Citation41]. In the entire cohort, the ORR was 28.3% (17/60), mPFS 7.2 months, mOS 23.4 months [Citation41]. CRC patients had worse PFS if compared to other cancer types, and HER2-mutant NSCLC had higher tumor shrinkage than NSCLC without HER2 mutation, irrespectively of HER2 expression [Citation41]. ORR among all NSCLC patients was 55.6%, while 77.2% (8/11) along with 11.3 months of mPFS in HER2-mutant NSCLC [Citation41]. Patients with HER2-expressing CRC seemed to derive less benefit from T-DXd: only 1 out of 20 patients (5%) had a PR and the mPFS was 4 months [Citation41].
T-DXd showed promising activity also in the HER2-low BC cohort, which included 54 patients: ORR was 37%, mPFS 11.1 months and mOS 29.4% [Citation42]. Seven patients experienced pneumonitis and 3 ILD; among these 10 patients, 3 had grade 5 lung toxicity. All of them were in the 6.4 mg/kg cohort [Citation42].
4. Phase II and III clinical trials of T-DXd monotherapy
The encouraging results of T-DXd in the phase I trial warranted the design of multiple phase II and III trials, some of which disease-specific while other agnostic.
4.1. Breast cancer
4.1.1. Phase II
DESTINY-Breast 01 is a phase II single-arm trial that evaluated the efficacy T-DXd at the dose of 5.4 mg/kg in 184 patients with HER2-positive MBC pretreated with TDM1, and the median number of previous treatments for advanced disease was 6 [Citation43]. The primary endpoint was confirmed to be ORR, which resulted to be 62% [Citation44]. The updated survival results were recently published: mOS was 29.1 months (95% CI 24.6–36.1 months), while mPFS and median duration of response (mDoR) were 19.4 months (95% CI 14.1–25.0 months) and 18.2 months (95% CI 15.0 months-not evaluable), respectively [Citation44]. Grade 3 or higher AEs occurred in 99 patients (53.8%), while drug-related ILD occurred in 29 (15.8%) patients, of which 5 deceased (2.7%) [Citation44].
These results granted T-DXd the FDA approval for HER2-positive MBC pretreated with at least two lines of systemic therapy for advanced disease [Citation45].
DEBBRAH trial is an ongoing 5 cohorts phase II clinical trial enrolling patients with HER2+ or HER2-low MBC with stable, untreated, or progressing brain metastases (BMs), and/or leptomeningeal carcinomatosis [Citation46]. The results published so far of the cohorts 1, 2 and 3 (non-progressing BMs after local therapy (n = 8), asymptomatic untreated BMs (n = 4), or progressing BMs after local therapy (n = 9)) showed an intracranial ORR of 46.2% in patients with active BMs [Citation46].
TUXEDO-01 was a single-arm phase II clinical trial aiming to assess T-DXd performance in patients affected by HER2-positive MBC with active brain metastases [Citation47]. Among the 15 patients enrolled, 11 (73%) showed intracranial response to the treatment, including 2 CR [Citation47]. Despite the small sample size, these trials support the use of T-DXd in patients with active BMs.
The phase II DAISY trial tested T-DXd in patients with HER2-overexpressing (n = 72, cohort 1), HER2-low (n = 74, cohort 2) and HER2 non-expressing (n = 40, cohort 3) MBC [Citation19]. Among the 177 enrolled patients, ORR was 70.6% (95% CI 58.3–81) in cohort 1, 37.5% (95% CI 26.4–49.7) in cohort 2 and 29.7% (95% CI 15.9–47) in cohort 3 [Citation19]. Median PFS was also associated with HER2 expression: 11 versus 4 months in patients with HER2 IHC 3+ or IHC 2+/ISH+ and HER2 IHC 0, respectively (p < 0.0001) [Citation19]. A machine-learning unsupervised clustering analysis showed that a specific cluster characterized by a low HER2 staining and a moderate cell density with high fibroblasts and immune cell representation was associated with lack of response, indicating the importance of the HER2 expression patterns as a determinant of response [Citation19]. Interestingly, ERBB2 expression levels did not correlate with response among patients with HER2 0 MBC, despite the activity seen also in cohort 3, suggesting that other mechanisms are involved [Citation19].
4.1.2. Phase III
DESTINY-Breast02 was a randomized 2:1, multicenter, open-label phase III trial comparing T-DXd (5.4 mg/kg) versus treatment of physician choice in patients with HER2-positive MBC that had previously received TDM1 [Citation6]. The median number of previous treatments for advanced disease was 2. Among the 608 patients enrolled, mPFS was 17.8 months (95% CI 14.3–20.8) in the T-DXd group versus 6.9 months (95% CI 5.5–8.4) in the treatment of physician’s choice group (HR 0.36, 95% CI 0.28–0.45; p < 0.0001) [Citation6]. Grade 3 or higher AEs occurred in 213 (53%) patients receiving T-DXd versus 86 (44%) receiving treatment of physician’s choice; drug-related ILD occurred in 42 (10%; including two grade 5 death events) versus one (<1%) [Citation6].
In the DESTINY-Breast03 randomized, open-label phase III trial T-DXd (5.4 mg/kg) was compared to TDM1 in 524 patients affected by HER2-positive MBC previously treated with trastuzumab and a taxane [Citation48]. The median number of previous therapies for advanced disease was 2. mPFS was 28.8 months (95% CI 22.4–37.9) with T-DXd and 6.8 months (95% CI 5.6-,·2) with TDM1 (HR 0.33, 95% CI 0.26–0.43; p < 0·0001) [Citation48]. mOS was not reached (95% CI 40.5 months-not estimable), in the T-DXd group and was not reached (34.0 months-not estimable), in the TDM1 group (HR 0.64; 95% CI 0.47–0.87; p = 0.0037) [Citation48]. Drug-related ILD or pneumonitis occurred in 39 (15%) patients treated with T-DXd and 8 (3%) patients treated with TDM1, with no grade 4 or 5 events [Citation48].
The open-label phase III trial DESTINY-Breast04 enrolled patients with HER2-low (HER2 2+ without ISH amplification or HER2 1+) pretreated with 1 or 2 lines of chemotherapy for advanced disease to be randomized 2:1 to receive T-DXd (5.4 mg/kg) or chemotherapy of physician choice [Citation13]. The median number of previous treatments for advanced disease received was 3. In the hormone receptor-positive cohort (494/557 total patients), the mPFS was 10.1 months in the T-DXd group and 5.4 months in the physician’s choice group (HR, 0.51; p < 0.001), and mOS was 23.9 months and 17.5 months, respectively (HR, 0.64; p = 0.003). Among all patients, the mPFS was 9.9 months in the T-DXd group and 5.1 months in the physician’s choice group (HR, 0.50; p < 0.001), and mOS was 23.4 months and 16.8 months, respectively (HR, 0.64; p = 0.001) [Citation13]. Drug-related ILD or pneumonitis occurred in 12.1% of the patients who received T-DXd, among which 0.8% were fatal [Citation13]. The results of DESTINY-Breast04 trial led to the approval of T-DXd by FDA for pretreated HER2-low MBC in 2022 [Citation49].
4.2. Gastric cancer
DESTINY-Gastric01 is a randomized phase II trial comparing the efficacy of T-DXd (6.4 mg/kg) with chemotherapy in 187 HER2-positive GC or GEJ adenocarcinoma progressing after at least 2 lines of therapy for advanced disease, including trastuzumab [Citation9]. The median number of previous treatments for advanced disease was 2. The ORR was 51% in T-DXd group, as compared to 14% in chemotherapy group [Citation9]. mOS was longer with T-DXd (12.5 vs. 8.4 months; HR 0.59; 95% CI, 0.39 to 0.88; p = 0.01) [Citation9]. A total of 12 patients had T-DXd – related ILD or pneumonitis (3 = grade ≥ 3) [Citation9].
DESTINY-Gastric01 also included two exploratory cohorts for HER2-low advanced GC (cohort1 HER2 2+ and ISH-negative, cohort 2 HER2 1+) [Citation50]. The ORR was 26.1% (95% CI, 9.1 to 51.2) in cohort 1 (5/19 patients) and 9.5% (95% CI, 1.2 to 30.4) in cohort 2 (2/21 patients) [Citation50]. mOS was 7.8 months (95% CI, 4.7 to non-evaluable) in cohort 1 and 8.5 months (95% CI, 4.3 to 10.9) in cohort 2; mPFS was 4.4 months (95% CI, 2.7 to 7.1) and 2.8 months (95% CI, 1.5 to 4.3), respectively [Citation50]. Drug-related ILD or pneumonitis occurred in one patient in each cohort (grade 1 or 2) [Citation50].
The phase II single-arm DESTINY-Gastric02 enrolled 79 patients with HER2-positive GC or GEJC progressing to a previous line of treatment for advanced disease containing trastuzumab, and the median number of previous treatments was 1 [Citation51]. ORR was 42% (95% CI 30.8–53.4, 33 of 79 patients), including 4 (5%) complete responses [Citation51]. Two patients died due to ILD or pneumonitis [Citation51].
The DESTINY-Gastric03 study is an ongoing Phase Ib/II trial in patients with pre-treated HER2-positive gastric and gastroesophageal junction carcinomas, which involves the use of T-DXd in combination with certain chemotherapy drugs [Citation52]. Currently, only data regarding the T-DXd cohort in combination with fluoropyrimidines have been published [Citation52]. In part 1, 15 pts received T-DXd +5-fluorouracil (5-FU) and 10 pts T-DXd + capecitabine [Citation52]. The most common grade ≥3 AEs were hematological: anemia 33% and 30%, decreased neutrophil count 33% and 40% with T-DXd +5-FU or Capecitabine, respectively [Citation52]. Patients started with T-DXd 5.4 mg/kg + 5-FU 800 mg/m2 and T-DXd 5.4 mg/kg + Capecitabine 1000 mg/m2 twice daily [Citation52]. Two dose-limiting toxicities occurred (grade 3 stomatitis) with T-DXd 6.4 mg/kg + 5-FU 800 mg/m2 [Citation52]. Recommended phase II doses therefore were T-DXd 6.4 mg/kg + 5-FU 600 mg/m2 and T-DXd 6.4 mg/kg + Capecitabine 1000 mg/m2 BID. Five out the seven patients receiving T-DXd + Capecitabine responded to the treatment, as well as three out of the seven patients receiving T-DXd +5-FU [Citation52].
The activity of T-DXd in pretreated HER2-positive GC cancers was also tested in Chinese patients in the DESTINY-Gastric06 trial, which showed an ORR of 28.8% (21/73 patients), a mPFS of 5.7 (95% CI 4.0, 6.8), and a mOS 10.2 (95% CI 7.5, 14.3) [Citation53]. The drug-related cases of ILD or pneumonitis were 3 [Citation53].
4.3. Non-small cell lung cancer
The DESTINY-Lung01 open label, phase II clinical trial enrolled HER2-mutant NSCLC refractory to standard treatments (median number of previous treatments for advanced disease = 2), all treated with T-DXd 6.4 mg/kg [Citation14]. The primary endpoint, ORR, resulted to be 55% (95% CI, 44 to 65). The mDoR was 9.3 months (95% CI, 5.7 to 14.7). mPFS was 8.2 months (95% CI, 6.0 to 11.9), while mOS 17.8 months (95% CI, 13.8 to 22.1) [Citation14]. ILD occurred in 26% of patients (2 fatal events) [Citation14]. Interestingly, responses occurred across different HER2 mutation subtypes, and in patients with no detectable HER2 expression or HER2 amplification [Citation14].
In the DESTINY-Lung2 trial, 152 HER2-mutant pretreated NSCLC (median number of previous treatments for advanced disease = 2) were randomized to receive 2:1 to receive T-DXd at 5.4 and 6.4 mg/kg dose levels, respectively [Citation54]. ORR was 49.0% (95% CI, 39.0 to 59.1) and 56.0% (95% CI, 41.3 to 70.0) [Citation54]. mDoR was 16.8 months (95% CI, 6.4 to not estimable) and not estimable (95% CI, 8.3 to not estimable) with 5.4 and 6.4 mg/kg, respectively [Citation54]. Thirteen of 102 (12.9%) and 14 of 50 (28.0%) patients had drug-related ILD (2.0% grade ≥3 in each arm) with 5.4 and 6.4 mg/kg, respectively [Citation54]. On the basis of these results, FDA granted accelerated approval to T-DXd for pretreated HER2-mutant NSCLC in 2022 [Citation55].
4.4. Colorectal cancer
DESTINY-CRC01 was an open-label phase II trial that enrolled HER2-expressing, RAS and BRAF V600 non-mutant CRC to be treated with T-DXd (6.4 mg/kg) [Citation16]. The median number of previous treatments (early or advanced setting) was 4. Seventy-eight patients were enrolled into three different cohorts according to HER2 expression levels: cohort A (53): HER2 3+ or HER2 2+ and ISH-positive; cohort B (7): HER2 2+ and ISH-negative; cohort C (18): HER2 1+ [Citation16]. ORR was reported in 24 (45.3%, 95% CI 31.6–59.6) patients in cohort A, while no responses were observed in cohorts B and C [Citation16]. Five patients (6%) had ILD or pneumonitis (2 fatal) [Citation16].
Another phase II trial (DESTINY-CRC02) enrolled 122 pretreated HER2+ CRC patients to receive T-DXd (82 5.4 mg/kg, 40 6.4 mg/kg) [Citation56]. The median number of previous treatments was 3 and 4 in the 5.4 mg/kg and 6.4 mg/kg cohorts, respectively. ORR was 37.8% (95% CI, 27.3–49.2%) in the 5.4 mg/kg arm and 27.5% (95% CI, 14.6–43.9%) in the 6.4 mg/kg arm [Citation56]. One fatal ILD AE occurred in the 6.4 mg/kg arm [Citation56]. On the whole, these results granted T-DXd a breakthrough therapy designation by FDA for pretreated HER2-positive CRC.
4.5. Osteosarcoma
A Phase 2 study tested T-DXd (5.4 mg/kg) in 9 adolescents and young adults with HER2-positive advanced osteosarcoma [Citation57]. Only a single patient had more than 24 weeks of stable disease (SD) of the 9 planned in the first stage, so the study did not meet criteria to progress to stage 2 [Citation57]. The estimated ORR was 11.1% [Citation57].
4.6. Agnostic trials
T-DXd was tested in two phase II non-randomized basket trials enrolling patients with HER2 addicted solid tumors, namely DESTINY-Pantumor01 and DESTINY-Pantumor02. The results of DESTINY-Pantumor02 were presented at ESMO Congress 2023 and then published in January 2024 [Citation15]. In this open-label non-randomized phase II trial, 267 patients affected by pretreated HER2-expressing (3+ or 2+) advanced solid tumors other than BC, GC, CRC and NSCLC received T-DXd (5.4 mg/kg) [Citation15]. The patients were enrolled in seven cohorts according to their tumor type: endometrial, cervical, ovarian, bladder, biliary tract, pancreatic, and other. The median number of previous treatments for advanced disease was 2 for all the tumor types except ovarian cancer (3). The global ORR was 37.1% (95% CI, 31.3 to 43.2), while the mDoR was 11.3 months (95% CI, 9.6 to 17.8), the mPFS 6.9 months (95% CI, 5.6 to 8.0), and the mOS 13.4 months (95% CI, 11.9 to 15.5) [Citation15]. Among the 75 patients with central HER2 IHC 3+ expression, the ORR was 61.3% (95% CI, 49.4 to 72.4), the mDoR was 22.1 months (95% CI, 9.6 to not reached), the mPFS 11.9 months (95% CI, 8.2 to 13.0), and the mOS was 21.1 months (95% CI, 15.3 to 29.6) [Citation15]. Thus, T-DXd demonstrated to have a remarkable clinical activity in various HER2-expressing tumor types including some HER2 2+ tumors, even if this finding is not completely generalizable [Citation15]. Indeed, T-DXd seemed to have a lower activity in pancreatic cancer patients (ORR 4%, 1/25), as well as in the HER2 2+ biliary tract cancers (0%, 0/14) [Citation15]. Drug-related ILD occurred in 10.5% of patients, with 3 fatal AEs.
On the contrary, the complete publication of the DESTINY-Pantumor01, an open-label, non-randomized phase II trial enrolling patients with HER2-mutant solid tumors (except HER2+ MBC and GC and HER2-mutant NSCLC) is still awaited, but the preliminary results were presented at ESMO 2023 [Citation58]. Among the 102 patients that received T-DXd (5.4 mg/kg), the ORR was 29.4% (95% CI 20.8, 39.3), mDoR was not reached, mPFS was 5.4 mo (95% CI 2.7, 7.1) [Citation58]. HER2 expression levels did not correlate with response. Drug-related ILD or pneumonitis occurred in 11 pts (10.8%, 2 fatal cases) [Citation58]. Despite the overall low number of patients not allowing to draw definitive conclusions, it is remarkable that the response rate was numerically different in patients harboring mutations in tyrosine kinase (36.5%) and extracellular (29.4%) domains if compared to transmembrane/juxtamembrane domain (5.9%) [Citation58].
contains the ongoing clinical trials employing T-DXd as monotherapy both in the early and advanced setting. Clinical trials data was downloaded by Clinicaltrials.gov on 17 March 2024 searching for ‘trastuzumab deruxtecan.’
Table 1. Ongoing clinical trials employing T-DXd as monotherapy in the early and advanced setting. NCT: National clinical trial.
5. Early-phase clinical trials with T-DXd combinations
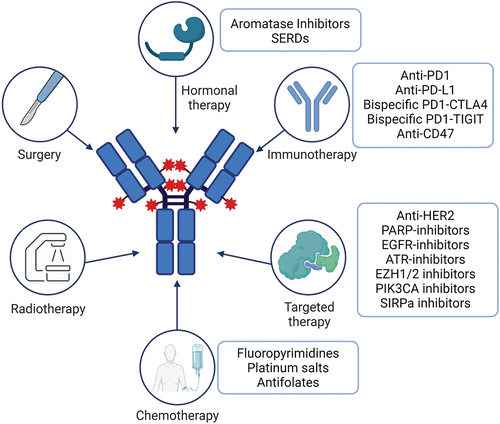
Combination regimens including ADCs are gaining a great deal of interest in order to improve the efficacy shown by the single agents and to overcome resistance to previous treatments. T-DXd is being tested in multiple phase I-II trials in combination with agents of different classes and with various targets, such as immunotherapy, PARP-inhibitors, hormonal therapy, other anti-HER2 drugs (mainly tyrosine kinase inhibitors). The main goal is to identify synergistic mechanisms of action without excessively increasing the toxicity. shows the combinations of T-DXd under development.
Figure 2. Combination therapies under study in clinical trials. Abbreviations: SERDs: Selective Estrogen Receptor Degraders. Created with BioRender.com.
As instance, preclinical evidences suggest that Cyclin E1 (CCNE1) is often co-amplified with ERBB2 and may confer resistance to T-DXd [Citation59]. The WEE1 inhibitor Adavosertib was shown to enhance T-DXd activity in HER2-low, CCNE1 gastroesophageal cancer patient-derived xenografts (PDX) and prolonged event-free survival (EFS) in models overexpressing HER2 [Citation59]. These preclinical results still require clinical validation.
Nevertheless, the available clinical data about T-DXd combination therapies are scarce so far, and encompass mainly hormonal therapies and immunotherapy, in addition to the combination with fluoropyrimidines previously reported as per DESTINY-Gastric03 trial. See for a schematization of the ongoing combination trials with T-DXd.
Table 2. Ongoing trials of T-DXd in combination with other drugs. NCT: National clinical trial.
A preliminary analysis of the phase Ib trial DS8201-A-U105 employing T-DXd in combination with nivolumab for the treatment of HER2-expressing MBC showed that AEs grade ≥3 occurred in 50.0% of patients; 7 (14.6%) HER2+ patients had drug-related ILD (6 grade 2; 1 grade 5) [Citation60].
The ongoing phase Ib DESTINY-Breast08 trial explores T-DXd safety and activity in combination with multiple therapies in HER2-low MBC. Preliminary results of T-DXd with anastrozole or fulvestrant were presented at 2023 San Antonio Breast Cancer Symposium. In the T-DXd plus anastrozole arm (n = 21) ORR was 71.4% (95% CI, 47.8%–88.7%) vs 40.0% (95% CI, 19.1%–64.0%) in the T-DXd plus fulvestrant arm (n = 20). The mPFS was 13.4 months (95% CI, 8.5–19.4) in the anastrozole arm vs not estimable (95% CI, 5.6-not estimable) in the fulvestrant arm [Citation61]. The combination of T-DXd with hormonal therapy was also studied in the neoadjuvant setting in the phase II TALENT trial [Citation62]. This trial enrolled HER2-low HR+ early BC that were randomized to receive T-DXd with or without anastrozole [Citation63]. One out of the 17 patients in T-DXd alone arm had pathological complete response (pCR). The ORR was 75% (12/16, 1 CR, 11 PR) in the monotherapy arm and 63.2% (12/19, 2 CR, 10 PR) in the combination arm [Citation63]. ILD occurred in one patient and the safety profile was similar in the two arms [Citation63].
BEGONIA trial is an open-label platform trial testing combination of Durvalumab with other anticancer agents as frontline treatment for triple-negative breast cancer (TNBC) [Citation64,Citation65]. The cohort 6 enrolled patients with HR-negative, HER2-low MBC to be treated with T-DXd and Durvalumab. As per the updated results of this cohort, the ORR was 57% (26/46, 95% CI, 41–71, with 1 complete response). The response was not correlated with PD-L1 expression. mPFS was 12.6 months (95% CI, 8–not reached). The safety profile was consistent with the one of single agents: the most common AEs were nausea (41, 73%), fatigue (26, 46%), and vomiting (17, 30%). Drug-related ILD or pneumonitis occurred for five patients (9%, 1 fatal concomitant with COVID pneumonia) [Citation64].
T-DXd in combination with Durvalumab is also tested in the platform phase II trial HUDSON, enrolling so far 43 patients affected by HER2-altered NSCLC (23 overexpressing HER2 and 20 with HER2 mutation) progressed to anti-PD(L)1 treatment [Citation66]. Confirmed ORR was 26.1% (80% CI, 14.3–41.3) in HER2 overexpressing patients and 35% (80% CI, 20.7–51.8) in HER2 mutant patients. mPFS was 2.8 months (80% CI, 2.2–5.5) in HER2 overexpressing and 5.7 months (80% CI, 5.5–6.5) in HER2 mutant patients [Citation66]. mOS was 9.5 mo (80% CI, 6.6–12.4) in the HER2 overexpressing and 10.6 months (80% CI, 8.9-NC) in the HER2 mutant groups [Citation66]. Pneumonitis, pulmonary embolism and anemia were the most common grade ≥3 AEs [Citation66]. All grade drug-related ILD occurred in 9.3% of patients [Citation66].
6. Expert opinion
If ADCs are currently considered a game-changer in the treatment of solid tumors, much of the credit is attributed to T-DXd. In fact, the remarkable results that this compound obtained across multiple tumor types, different HER2 alteration types (mutations and amplifications) and expression levels boosted the development of new ADCs, some of them already entering clinical practice.
Nevertheless, there are still controversial aspects on T-DXd that need clarification. Firstly, to date, there is no universal biomarker that enables the identification of patients who can benefit from T-DXd treatment completely independently of tumor histotype. In fact, despite the excellent results in terms of antitumor activity shown by T-DXd in the DESTINY-Pantumor studies, both studies indicate that there is still work to be done to fully understand the variables contributing to the determination of T-DXd efficacy. From DESTINY-Pantumor01, it emerges that apparently the only ERBB2 mutation in the transmembrane and juxtamembrane domain included in the study (R678Q), which is pathogenic and generally considered actionable, responds poorly to T-DXd treatment. Currently, however, the published results do not report the type of mutation in relation to the primary tumor or other factors such as prior treatments received. Therefore, it will be necessary to await the full publication to better understand if, as it seems, the type of mutation is indeed a determining factor. The results of other published studies that included tumors with ERBB2 mutations are not helpful in this regard, as neither DESTINY-Lung01 nor DESTINY-Lung02 enrolled patients with mutations in the transmembrane or juxtamembrane domains, although they were eligible according to the protocol. As for DESTINY-Pantumor02, as previously mentioned, the concern revolves around specific tumor histotypes that seem to derive lesser benefit from T-DXd despite HER2 expression. Specifically, reference is made to pancreatic adenocarcinomas (in both 3+ and 2+ cohorts) and biliary tract cancers (2+ cohort). It should be emphasized, however, that responses to T-DXd have been observed in all histotypes and that pancreatic adenocarcinomas and biliary tract tumors are generally malignancies with a poor prognosis known for refractoriness to many treatments. Nonetheless, the FDA has granted approval to T-DXd for pretreated solid tumors with high (3+) HER2 expression, and it represents a milestone, being the first agnostic approval for both an ADC and an anti-HER2 treatment.
Turning to the ongoing trials studying T-DXd in combination with other classes of drugs, it can be observed that the landscape is quite diverse. The primary concern when combining T-DXd with other potentially toxic drugs is clearly represented by pulmonary toxicity and, to a lesser extent, hematologic toxicity. Currently available clinical data are limited and still preliminary, but what emerges is that combinations with hormonal therapies (aromatase inhibitors and Selective Estrogen Receptor Degraders (SERDs)) and with anti-PD-(L)1 agents (Durvalumab and Nivolumab) do not seem to increase the risk of severe toxicity. This is an important evidence particularly for ILD, given the not common but potentially severe cases of immune-related pneumonitis caused by anti-PD(L)1 agents [Citation67]. It will be interesting to see the results of combination studies with drugs with potentially greater hematologic toxicity, such as other chemotherapeutic agents and PARP inhibitors, to understand if such combinations are sustainable for patients. Few data is available regarding the efficacy of these combinations. Some encouraging signals for the association with immunotherapy emerge looking at the MBC HR-negative/HER2 low population of DESTINY-Breast04 and BEGONIA trials. In the former, the mPFS was 8.5 months and ORR was 50%, while in the latter mPFS was 12.6 and ORR 57%. Nevertheless, BEGONIA trial was in a frontline setting, while patients in DESTINY-Breast04 were pretreated, making it impossible to compare these results. On the other side, the combinations of T-DXd with hormonal therapies will probably enter the clinical practice for HR+ MBC in the next future. The 71.4% ORR reported in combination with anastrozole in DESTINY-Breast08 trial deserves attention, and hormonal therapy may also have a role in terms of maintenance after T-DXd suspension due to toxicity.
Regarding the clinical use of T-DXd, there are contexts and settings in which there are multiple approved ADCs, such as in the case of pre-treated patients with HER2-low MBC, both HR+ and HR-, who can receive either T-DXd or Sacituzumab-govitecan, based on the results of the ASCENT and TROPiCS-02 studies [Citation68,Citation69]. Currently, there is no guide to prioritize one ADC over the other; therefore, it will be crucial to develop tools, possibly based on the expression of a panel of antigens or other biomarkers, which allow for the selection of the best ADC for the specific patient. This may become even more important after the publication of the awaited results of the DESTINY-Breast06 trial, which includes a cohort of patients with ultra-low HER2 expression that could further complicate the actual landscape. Important evidence to take a decisive step toward making ADCs a resource available for precision medicine is hoped to derive from the phase II ADC-MATCH trial (NCT06311214), expected to open for enrollment in June 2024. This trial aims precisely to evaluate the activity of three ADCs (Sacituzumab-govitecan, Enfortumab-vedotin, and T-DXd) in patients with advanced-stage solid tumors expressing the antigens against which these ADCs are directed (TROP-2, Nectin-4, and HER2, respectively).
Another contentious issue, primarily dependent on the challenging toxicity profile of T-DXd, is the duration of the treatment itself. Indeed, the studies conducted so far did not specify either a maximum treatment duration or a maximum cumulative dose, nor did they provide data on any maintenance treatments to be undertaken once T-DXd is discontinued for reasons other than disease progression. Many strategies have been proposed to reduce the toxicity of ADCs and are expected to be tested within clinical trials [Citation31]. Potentially intriguing will be the SAPPHO study, which will investigate a sequence of non-cross-resistant anti-HER2 treatments, including T-DXd, in patients with HER2-positive MBC who have not been previously treated.
Finally, another unclear aspect is represented by the treatment of patients who progress on T-DXd, as the mechanisms of resistance have not been fully elucidated. Evidence from the DAISY study suggests that reduced HER2 expression and the emergence of mutations such as SLX4 May be implicated [Citation19]. Further in vitro studies have also revealed that in some cases, resistance to the payload develops, as HER2-mutated NSCLC cell lines after progression on T-DXd maintain sensitivity to anti-HER2 tyrosine kinase inhibitors, but show resistance to camptothecins [Citation70,Citation71].
In conclusion, despite undeniable progress, there remains ample room for improvement in the use of T-DXd and ADCs in general. This improvement can be guided by the identification of biomarkers capable of predicting both treatment response and toxicity, as well as by studying resistance mechanisms to develop new combination therapies capable of overcoming it.
Article highlights
Antibody–drug conjugates are redefining the treatment paradigm of solid tumors.
Trastuzumab-Deruxtecan is the first antibody–drug conjugate to obtain agnostic approval for HER2+ tumors.
Combining Trastuzumab-Deruxtecan with other anticancer agents is gaining a great deal of interest.
Questions about patients selection, treatment duration, prioritization, and management of resistance remain.
Efforts are still required to optimize the use of Trastuzumab-Deruxtecan in clinical practice.
Declaration of interest
G Curigliano reports personal fees from Daichii Sanlyo, Astra Zeneca, Gilead, Lilly, Pfizer, Novartis, Menarini, Roche, Exact Sciences, and BluePrint outside the submitted work. E Crimini is supported by Fondazione IEO-Monzino. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank all the supporters of European Institute of Oncology through the 5 × 1000 financing.
Additional information
Funding
References
- Tarantino P, Pestana RC, Corti C, et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022 Mar;72(2):165–182. doi: 10.3322/CAAC.21705
- Ascione L, Crimini E, Trapani D, et al. Predicting response to antibody drug conjugates: a focus on antigens’ targetability. Oncologist. 2023 Nov;28(11):944–960. doi: 10.1093/ONCOLO/OYAD246
- Dowell JA, Korth-Bradley J, Liu H, et al. Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol. 2001;41(11):1206–1214. doi: 10.1177/00912700122012751
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012 Nov;367(19):1783–1791. doi: 10.1056/NEJMOA1209124/SUPPL_FILE/NEJMOA1209124_DISCLOSURES.PDF
- Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-college of American pathologists guideline update. J Clin Oncol. 2023 Aug;41(22):3867–3872. doi: 10.1200/JCO.22.02864
- André F, Hee Park Y, Kim SB, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023 May;401(10390):1773–1785. doi: 10.1016/S0140-6736(23)00725-0
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021 Mar;384(12):1125–1135. doi: 10.1056/NEJMOA2035807
- Moore KN, Angelergues A, Konecny GE, et al. Mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer. N Engl J Med. 2023 Dec;389(23):2162–2174. doi: 10.1056/NEJMOA2309169
- Shitara K, Bang Y-J, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020 Jun;382(25):2419–2430. doi: 10.1056/NEJMOA2004413
- Rugo HS, Bardia A, Marmé F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023 Oct;402(10411):1423–1433. doi: 10.1016/S0140-6736(23)01245-X
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021 Apr;384(16):1529–1541. doi: 10.1056/NEJMOA2028485
- Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021 Aug;39(22):2474–2485. doi: 10.1200/JCO.20.03489
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022 Jul;387(1):9–20. doi: 10.1056/NEJMOA2203690
- Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022 Jan;386(3):241–251. doi: 10.1056/NEJMOA2112431
- Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024 Jan;42(1):47–58. doi: 10.1200/JCO.23.02005
- Siena S, Di Bartolomeo M, Raghav K, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021 Jun;22(6):779–789. doi: 10.1016/S1470-2045(21)00086-3
- FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive solid tumors | FDA. [cited 2024 Apr 11]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2
- Tarantino P, Gupta H, Hughes ME, et al. Comprehensive genomic characterization of HER2-low and HER2-0 breast cancer. Nat Commun. 2023 Dec;14(1). doi: 10.1038/S41467-023-43324-W
- Mosele F, Deluche E, Lusque A, et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat Med. 2023 Aug;29(8):2110–2120. doi: 10.1038/S41591-023-02478-2
- Tarantino P, Modi S, Tolaney SM, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. 2021 Dec;7(12):1873–1881. doi: 10.1001/JAMAONCOL.2021.3595
- Powell CA, Camidge DR, Modi S, et al. 289P risk factors for interstitial lung disease in patients treated with trastuzumab deruxtecan from two interventional studies. Ann Oncol. 2020 Sep;31:S357–S358. doi: 10.1016/J.ANNONC.2020.08.391
- Izbicki G, Segel MJ, Christensen TG, et al. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002;83(3):111–119. doi: 10.1046/J.1365-2613.2002.00220.X
- Inaba K, Arimoto T, Hoya M, et al. Interstitial pneumonitis induced by pegylated liposomal doxorubicin in a patient with recurrent ovarian cancer. Med Oncol. 2012 Jun;29(2):1255–1257. doi: 10.1007/S12032-011-9893-0
- Jacobs C, Slade M, Lavery B. Doxorubicin and BOOP. A possible near fatal association. Clin Oncol (R Coll Radiol). 2002;14(3):262. doi: 10.1053/CLON.2002.0071
- Mazzotta M, Giusti R, Iacono D, et al. Pulmonary fibrosis after pegylated liposomal doxorubicin in elderly patient with cutaneous angiosarcoma. Case Rep Oncol Med. 2016;2016:1–5. doi: 10.1155/2016/8034832
- Yoshii N, Suzuki T, Nagashima M, et al. Clarification of clinical features of interstitial lung disease induced by irinotecan based on postmarketing surveillance data and spontaneous reports. Anticancer Drugs. 2011 Jul;22(6):563–568. doi: 10.1097/CAD.0B013E3283473F28
- Willemsen AECAB, Grutters JC, Gerritsen WR, et al. mTOR inhibitor-induced interstitial lung disease in cancer patients: comprehensive review and a practical management algorithm. Int J Cancer. 2016 May;138(10):2312–2321. doi: 10.1002/IJC.29887
- Shah RR. Tyrosine kinase inhibitor-induced interstitial lung disease: Clinical features, diagnostic challenges, and therapeutic dilemmas. Drug Saf. 2016 Nov;39(11):1073–1091. doi: 10.1007/S40264-016-0450-9
- Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017 Aug;50(2). doi: 10.1183/13993003.00050-2017
- Raschi E, Fusaroli M, Ardizzoni A, et al. Cyclin-dependent kinase 4/6 inhibitors and interstitial lung disease in the FDA adverse event reporting system: a pharmacovigilance assessment. Breast Cancer Res Treat. 2021 Feb;186(1):219–227. doi: 10.1007/S10549-020-06001-W
- Tarantino P, Ricciuti B, Pradhan SM, et al. Optimizing the safety of antibody–drug conjugates for patients with solid tumours. Nat Rev Clin Oncol. 2023 Jun;20(8):558–576. doi: 10.1038/s41571-023-00783-w
- Baselga J, Albanell J, Molina MA, et al. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001 Oct;28(5 Suppl 16):4–11. doi: 10.1016/S0093-7754(01)90276-3
- Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016 Oct;22(20):5097–5108. doi: 10.1158/1078-0432.CCR-15-2822
- Hunter FW, Barker HR, Lipert B, et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br J Cancer. 2019 Dec;122(5):603–612. doi: 10.1038/s41416-019-0635-y
- Giugliano F, Corti C, Tarantino P, et al. Bystander effect of antibody-drug conjugates: fact or fiction? Curr Oncol Rep. 2022 Jul;24(7):809–817. doi: 10.1007/S11912-022-01266-4
- Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016 Jul;107(7):1039–1046. doi: 10.1111/CAS.12966
- Li BT, Michelini F, Misale S, et al. Her2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 2020 May;10(5):674–687. doi: 10.1158/2159-8290.CD-20-0215
- Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017 Nov;18(11):1512–1522. doi: 10.1016/S1470-2045(17)30604-6
- Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019 Jun;20(6):816–826. doi: 10.1016/S1470-2045(19)30097-X
- Shitara K, Iwata H, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019 Jun;20(6):827–836. doi: 10.1016/S1470-2045(19)30088-9
- Tsurutani J, Iwata H, Krop I, et al. Targeting her2 with trastuzumab deruxtecan: a dose-expansion, phase i study in multiple advanced solid tumors. Cancer Discov. 2020 May;10(5):688–701. doi: 10.1158/2159-8290.CD-19-1014
- Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887–1896. doi: 10.1200/JCO.19.02318
- Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020 Feb;382(7):610–621. doi: 10.1056/NEJMOA1914510
- Saura C, Modi S, Krop I, et al. Trastuzumab deruxtecan in previously treated patients with HER2-positive metastatic breast cancer: updated survival results from a phase II trial (DESTINY-Breast01). Ann Oncol. 2024 Mar;35(3):302–307. doi: 10.1016/J.ANNONC.2023.12.001
- Narayan P, Osgood CL, Singh H, et al. FDA approval summary: fam-trastuzumab deruxtecan-nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin Cancer Res. 2021 Aug;27(16):4478–4485. doi: 10.1158/1078-0432.CCR-20-4557
- Pérez-García JM, Vaz Batista M, Cortez P, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. 2023 Jan;25(1):157–166. doi: 10.1093/NEUONC/NOAC144
- Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022 Sep;28(9):1840–1847. doi: 10.1038/S41591-022-01935-8
- Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023 Jan;401(10371):105–117. doi: 10.1016/S0140-6736(22)02420-5
- Narayan P, Dilawari A, Osgood C, et al. US food and drug administration approval summary: fam-trastuzumab deruxtecan-nxki for human epidermal growth factor receptor 2-low unresectable or metastatic breast cancer. J Clin Oncol. 2023 Apr;41(11):2108–2116. doi: 10.1200/JCO.22.02447
- Yamaguchi K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in anti–human epidermal growth factor receptor 2 treatment–naive patients with human epidermal growth factor receptor 2–low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J Clin Oncol. 2023 Feb;41(4):816. doi: 10.1200/JCO.22.00575
- Van Cutsem E, di Bartolomeo M, Smyth E, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023 Jul;24(7):744–756. doi: 10.1016/S1470-2045(23)00215-2
- Janjigian YY, Oh D-Y, Rha SY, et al. Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients (pts) with advanced/metastatic HER2+ gastric cancer (Gc)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. 2022 Jan;40(4_suppl):295. doi: 10.1200/JCO.2022.40.4_SUPPL.295
- Shen L, Chen P, Lu J, et al. 172P Trastuzumab deruxtecan (T-DXd) in Chinese patients (pts) with previously treated HER2-positive locally advanced/metastatic gastric cancer (GC) or gastroesophageal junction adenocarcinoma (GEJA): primary efficacy and safety from the phase II single-arm DESTINY-Gastric06 (DG06) trial. Ann Oncol. 2023 Nov;34:S1542–S1543. doi: 10.1016/j.annonc.2023.10.307
- Goto K, Goto Y, Kubo T, et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-Lung02 trial. J Clin Oncol. 2023 Nov;41(31):4852–4863. doi: 10.1200/JCO.23.01361
- FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant non-small cell lung cancer | FDA. [cited 2024 Mar 16]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-her2-mutant-non-small-cell-lung
- Raghav KPS, Siena S, Takashima A, et al. Trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-overexpressing/amplified (HER2+) metastatic colorectal cancer (mCRC): primary results from the multicenter, randomized, phase 2 DESTINY-CRC02 study. 2023 May;41(16_suppl):3501. doi: 10.1200/JCO.2023.41.16_SUPPL.3501
- Reed DR, Janeway KA, Minard CG, et al. PEPN1924, a phase 2 study of trastuzumab deruxtecan (DS-8201a, T-DXd) in adolescents and young adults with recurrent HER2+ osteosarcoma: a children’s oncology group pediatric early-phase clinical trial network study. 2023 May;41(16_suppl):11527. doi: 10.1200/JCO.2023.41.16_SUPPL.11527
- Li BT, Meric-Bernstam F, Bardia A, et al. 654O efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with solid tumors harboring specific HER2-activating mutations (HER2m): Primary results from the international phase II DESTINY-PanTumor01 (DPT-01) study. Ann Oncol. 2023 Oct;34:S459–S460. doi: 10.1016/j.annonc.2023.09.1840
- DiPeri TP, Evans KW, Raso MG, et al. Adavosertib enhances antitumor activity of trastuzumab deruxtecan in HER2-expressing cancers. Clin Cancer Res. 2023 Nov;29(21):OF1–OF14. doi: 10.1158/1078-0432.CCR-23-0103
- Hamilton EP, Shapiro CL, Boni V, et al. 162O primary analysis from DS8201-A-U105: a 2-part, open label, phase Ib trial assessing trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing advanced breast cancer. Ann Oncol. 2022 May;33:S196. doi: 10.1016/j.annonc.2022.03.181
- Jhaveri K, André F, Hamilton E, et al. Abstract RF02-03: Trastuzumab deruxtecan (T-DXd) in combination with anastrozole or fulvestrant in patients with HER2-low HR+ advanced/metastatic breast cancer: a phase 1b, open-label, multicenter, dose-expansion study (DESTINY-Breast08). Cancer Res. 2024 May;84(9_Supplement):RF02–03. doi: 10.1158/1538-7445.SABCS23-RF02-03
- Hurvitz SA, Wang LS, Chan D, et al. TRIO-US B-12 TALENT: phase II neoadjuvant trial evaluating trastuzumab deruxtecan with or without anastrozole for HER2-low, HR+ early-stage breast cancer. 2022 Jun;40(16_suppl):TPS623. doi: 10.1200/JCO.2022.40.16_SUPPL.TPS623
- Bardia A, Hurvitz S, Press MF, et al. Abstract GS2-03: GS2-03 TRIO-US B-12 TALENT: neoadjuvant trastuzumab deruxtecan with or without anastrozole for HER2-low, HR+ early stage breast cancer. Cancer Res. 2023 Mar;83(5_Supplement):GS2–03. doi: 10.1158/1538-7445.SABCS22-GS2-03
- Schmid P, Im S-A, Armstrong A, et al. BEGONIA: phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). 2021 May;39(15_suppl):1023. doi: 10.1200/JCO.2021.39.15_SUPPL.1023
- Schmid P, Wysocki P, Park YH, et al. Abstract PD11-08: PD11-08 Trastuzumab deruxtecan (T-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic hormone receptor-negative (HR−), HER2-low breast cancer: updated results from BEGONIA, a phase 1b/2 study. Cancer Res. 2023 Mar;83(5_Supplement):PD11–08. doi: 10.1158/1538-7445.SABCS22-PD11-08
- Cheema P, Hartl S, Koczywas M, et al. 695 Efficacy and safety of trastuzumab deruxtecan (T-DXd) with durvalumab in patients with non-small cell lung cancer (HER2 altered NSCLC) who progressed on anti-PD1/PD-L1 therapy (HUDSON). J Immunother Cancer. 2023 Nov;11(Suppl 1):A787–A787. doi: 10.1136/JITC-2023-SITC2023.0695
- Oliveira C, Mainoli B, Gonçalo SD, et al. Immune-related serious adverse events with immune checkpoint inhibitors: systematic review and network meta-analysis. Eur J Clin Pharmacol. 2024 Feb;80(1):1–10. doi: 10.1007/S00228-024-03647-Z
- Rugo HS, Bardia A, Marmé F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023 Oct;402(10411):1423–1433. doi: 10.1016/S0140-6736(23)01245-X
- Bardia A, Rugo HS, Tolaney SM, et al. Final results from the randomized phase III ASCENt clinical trial in metastatic triple-negative breast cancer and association of outcomes by human epidermal growth factor receptor 2 and trophoblast cell surface antigen 2 expression. J Clin Oncol. 2024 Feb;42(15):1738–1744. doi: 10.1200/JCO.23.01409
- Nilsson MB, Poteete A, Udagawa H, et al. Abstract 383: Trastuzumab deruxtecan resistance is associated with reduced responsiveness to topoisomerase inhibitors (payload resistance) but no reduction in sensitivity to HER2 tyrosine kinase inhibitors. Cancer Res. 2023 Apr;83(7_Supplement):383–383. doi: 10.1158/1538-7445.AM2023-383
- Rolfo C, Del Re M, Russo A. Empower the potential of trastuzumab deruxtecan with novel combinations. Clin Cancer Res. 2023 Nov;29(21):4317–4319. doi: 10.1158/1078-0432.CCR-23-1700
