Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a chronic liver disease that encompasses a broad spectrum of diseases with the mildest form being hepatic steatosis to the most severe form called metabolic dysfunction-associated steatohepatitis (MASH) [Citation1]. It is strongly associated with obesity, hypertension, dyslipidemia, type 2 diabetes mellitus, and metabolic syndrome [Citation2]. Accordingly, and in the light of the worldwide epidemic of obesity, the prevalence of this group of liver diseases has been rising. MASLD overall, is a very heterogenous disease and has multiple pathways that drive the disease progression. In addition, multiple environmental and genetic factors play variable roles in disease progression and for this reason finding an effective treatment and establishing a primary end point for clinical trials has been a challenging task thus far [Citation3]. Resmetirom, a thyroid hormone beta receptor agonist, is the first approved medication by the Food and Drug Association (FDA) to treat MASH in addition to weight loss and life style modification [Citation4]. On the other hand, many other agents are in clinical development including GPR119 agonists with the hope of finding more effective and safe treatment for this complex disease.
2. What medications we tried in MASH
The dual primary end point of any treatment of MASH is to improve fibrosis without worsening steatosis and to improve the steatosis without worsening the fibrosis [Citation5].Several medications have been studied over the past few years, however, many of them had failed to meet this end point. Medications with different mechanisms of action were tried, including antioxidants like vitamin E and omega-3 fatty acids that despite showing benefits in decreasing steatosis and lowering triglyceride levels respectively, did not show any significant improvement in liver fibrosis [Citation6,Citation7]. Pioglitazone as an insulin-sensitizing agent showed in PIVENS trial improvement in steatosis but did not meet the study end point as well [Citation6]. Its use in the treatment of MASH has been limited by its serious side effects. On the other hand, another antidiabetic agent which is semeglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist that enhances glucose-dependent insulin secretion, showed in a phase 2 trial compared to placebo a significant improvement MASH without statistical improvement in fibrosis compared to placebo [Citation6,Citation8]. Obeticholic acid (OCA) showed a significant improvement in liver fibrosis by activating farnesoid X receptor (FXR) and thus decreasing hepatic glucose and lipid metabolism. The REGENERATE trial, a phase 3 randomized double-blind placebo-controlled trial to show the safety and efficacy of OCA compared to placebo for MASH patients with F1-F3, showed at least 1 stage improvement in fibrosis but it did not show a significant resolution in MASH compared to placebo. For this reason, in addition to safety concern regarding pruritis and hepatic events, the FDA determined that the risk of OCA outweighs its benefits in MASH [Citation9]. Another medication that failed to show histologic improvement in phase 3 clinical trial is selonsertib, an apoptosis signal-regulating kinase (ASK) 1 inhibitor [Citation10]. A pivotal hinge point in managing MASH was the recent approval of resmetirom by the FDA based on a randomized controlled clinical trial (MAESTRO-NASH) that recruited patients with stage F1b-F3 fibrosis. The trial showed significant histological improvement in fibrosis of at least one stage in treatment groups compared to the placebo group, in addition to improvement in lipid profile with low density lipoprotein (LDL) reduction of 13.6%-16.3%, depending on the dose used, on a 52 weeks follow-up period [Citation5]. summarizes drugs tested in MASH treatment.
Table 1. Drugs tested in MASH treatment.
3. Rationale for the use of GPR119 agonists in NASH
Fatty acid metabolism is a proposed treatment target for MASH. Elevated levels of free fatty acids (FFAs) from adipose tissue increase hepatic triglyceride accumulation, augmented by increased de novo lipogenesis stimulated by hyperinsulinemia. Lipotoxicity and oxidative stress result in a proinflammatory cascade via cytokines, inflammatory molecules, and innate inflammatory cells that ultimately lead to apoptosis and activation of stellate cells which contribute further to the pathophysiology of MASH [Citation11].
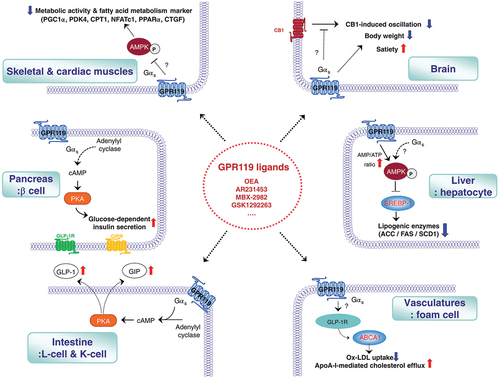
GPR119 is a G-protein coupled receptor expressed mainly in the pancreas, with variable levels of expression in the gastrointestinal tract, liver, brain, vasculature, skeletal and cardiac muscles which has multiple endogenous ligands with various signaling pathways in different tissues, summarized in [Citation12]. Activation of GPR119 receptors was shown to regulate food intake, weight loss and play a role in glucose-mediated insulin secretion and incretin release. It also acts as a fat sensor and causes suppression of the production of endogenous lipids in the setting of exogenous or dietary fat absorption [Citation12]. Studies have shown that activation of the GPR119 receptors stimulates glucagon-like peptide (GLP-1), in vitro insulin release, improvement in glucose tolerance, and increase in gastric inhibiting peptide. Moreover, GPR119 agonists were found to inhibit hepatic fat accumulation and de novo lipid synthesis hence acting as an antisteatotic agent [Citation13]. A significant finding in mice with MASH, GPR119 agonists were noticed to reduce alkaline aminotransferase (ALT), alkaline amino synthetase (AST), and cholesterol serum levels [Citation14].
Figure 1. GPR119 signaling pathways in various tissues
4. Available data on DA-1241
DA-1241 is a novel GPR119 receptor agonist that was first introduced by Kim et al in animal models as a promising treatment for diabetes mellitus and is increasingly being studied [Citation15]. This novel drug’s effect on liver steatosis was examined in different groups of mice including a combination of diet-induced, genetically modified, and chemically induced approaches to mimic the pathophysiology of MASH. Via activation of the GPR119 receptor, it was seen to cause improvement in biochemical markers that were related to steatosis and inflammation [Citation16]. In the context of hepatic inflammation, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) plays a central role by promoting the transcription of inflammatory genes following TNFα (tumor necrosis factor alpha) stimulation. DA-1241 alleviates this inflammation by preventing the nuclear translocation of the NFκB p65 subunit in hepatocyte cells. Additionally, in macrophages, DA-1241 inhibits lipopolysaccharide-induced NFκB activation and subsequent cytokine secretion. This suppression is mediated through the activation of GPR119 receptor, indicating a targeted anti-inflammatory mechanism [Citation16]. Through assessing histological findings and serological markers including AST and ALT, DA-1241 reduced hepatic inflammation and prevented the development of fibrosis with a trend to decrease the area of fibrosis by 35.8% compared to placebo [Citation16]. DA-1241 was found to enhance glucose-dependent insulin secretion and increase GLP-1 levels associated with blood glucose and triglyceride levels while at the same time preserving pancreatic beta-cell mass by preventing apoptosis, moreover, it reduced hepatic gluconeogenesis and had significantly decreased total serum cholesterol. DA-1241, administered orally, demonstrates a dose-dependent reduction in postprandial blood glucose levels in vivo [Citation15,Citation17]. This medication was tested and well tolerated in healthy volunteers, in whom medication was given orally in doses ranging from 50 to 200 milligrams daily reaching maximum plasma concentration in a few hours after administration indicating rapid absorption. The medication had a relatively long half-life of 369-590 hours depending on the given dose [Citation18]. After showing the safety of DA-1241 in healthy subjects, a phase 2 clinical trial is taking place to investigate DA-1241 role in treating MASH in humans, with the primary endpoint being the measurement of changes in ALT levels. The secondary endpoints include normalization of ALT and absolute changes in liver profile in the trial subjects [NCT06054815].
5. Conclusion
DA-1214 is a novel medication that showed promising results in improving steatosis in animal models. Preliminary studies did not show any clear effect for this drug on improving fibrosis and has no known proposed mechanism to induce fibrosis regression. Knowing MASH as a complex and challenging systemic disease, more data and studies including more animal studies on its fibrosis regression followed by early clinical trials are needed before judging the potential role for this drug in the realm of MASH treatment.
6. Expert Opinion
MASLD has been a major challenging disease with serious consequences and heavy economic burden worldwide. Lately, there is a consensus on considering fibrosis regression without worsening steatosis and steatosis resolution without fibrosis progression as proven by liver histology as an acceptable primary end point for any clinical trial. Finding an effective treatment regimen has been hindered by the complex pathophysiology of disease progression pathway and the heterogeneity in the pathway that carries the disease progression in MASH patients. There is a major consensus that any effective treatment, single or combination, should have multiple modes of action and be able to achieve the primary endpoint during not only during the treatment period but also during post trial follow up. In another word, there is a growing concern that any successful treatment including remetirom should achieve not only short-term resolution of MASH but prevent the relapse of the disease upon discontinuation of the treatment.
DA-1214 as a GPR119 receptor agonist offers a unique and a very promising mechanism of action. Theoretically, its effect on inhibiting endogenous fatty acid production and accumulation, increasing GLP-1 and improving insulin allows this drug to work on different pathways to treat MASLD. The ability of this drug to increase endogenous GLP-1 production gives this drug a unique feature. DA-1241 stands out as the sole GPR119 agonist demonstrated to effectively improve liver fibrosis in animal models, in contrast to other GPR119 agonists. None of the previously tested agents have shown any similar mechanism of action. Although, its preliminary studies showed a promising result in decreasing steatosis and inflammation, it did not show a clear result in fibrosis regression in animal model. Its mechanism of action on fibrosis repression is unclear and not well established yet. Until more data on the ability of this drug to show a significant reduction in fibrosis without worsening of steatosis, it is too early to draw a conclusion on the ability of this drug to be a major player in a very crowded field of investigative agents. Taking into consideration the disappointing reality of the failure of numerous agents in treating MASLD, the jurors are still out until DA-1214 shows a clear safety and efficacy in a well-designed phase 2 and then phase 3 trials.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
REFERENCES
- Sheka AC, Adeyi O, Thompson J, et al. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–1183. doi: 10.1001/jama.2020.2298
- Chan WK, Chuah KH, Rajaram RB, et al. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023 Sep 30;32(3):197–213.
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Alimentary Pharmacology & Therapeutics. 2011;34(3):274–285.
- Kokkorakis M, Boutari C, Hill MA, et al. Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: Trials, opportunities, and challenges. Metabolism-Clinical and Experimental. 2024. 154 155835 10.1016/j.metabol.2024.155835
- Harrison SA, Bedossa P, Guy CD, et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. New England Journal of Medicine. 2024;390(6):497–509.
- Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. New England Journal of Medicine. 2010;362(18):1675–1685.
- Scorletti E, Bhatia L, McCormick KG, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the WELCOME* study. Hepatology. 2014;60(4):1211–1221. doi: 10.1002/hep.27289
- Loomba R, Abdelmalek MF, Armstrong MJ, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023 Jun;8(6):511–522. 10.1016/S2468-1253(23)00068-7
- Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. The Lancet. 20192019/12 /14/;394(10215):2184–2196.
- Harrison SA, Wong VW-S, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. Journal of Hepatology. 20202020/07 /01/;73(1):26–39.
- Manne V, Handa P, Kowdley KV. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin Liver Dis. 2018;22(1):23–37. doi: 10.1016/j.cld.2017.08.007
- Yang JW, Kim HS, Choi YW, et al. Therapeutic application of <scp>GPR119</scp> ligands in metabolic disorders. Diabetes, Obesity and Metabolism. 2018;20(2):257–269.
- Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008 Oct 28;105(43):16767–16772. 10.1073/pnas.0808567105
- Kurtz R, Anderman MF, Shepard BD. GPCRs get fatty: the role of G protein-coupled receptor signaling in the development and progression of nonalcoholic fatty liver disease. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2021;320(3):G304–G318. doi: 10.1152/ajpgi.00275.2020
- Kim M-K, Cheong YH, Lee SH, et al. A novel GPR119 agonist DA-1241 preserves pancreatic function via the suppression of ER stress and increased PDX1 expression. Biomedicine & Pharmacotherapy. 2021;144:112324.10.1016/j.biopha.2021.112324
- Lee S-H, Park H, Yang E-K, et al. GPR119 activation by DA-1241 alleviates hepatic and systemic inflammation in MASH mice through inhibition of NFκB signaling. Biomedicine & Pharmacotherapy. 2023;166:115345.10.1016/j.biopha.2023.115345
- Kim Y, Lee SW, Wang H, et al. DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice. Diabetes & Metabolism Journal. 2022;46(2):337–348.
- KIM M-K, LEE DY, JEONG J, et al. 766-P: DA-1241, a Novel GPR119 Agonist: Data on Safety, Tolerability, and Pharmacokinetics (PK) from Part 1 of a Phase 1b Multiple Ascending Dose (MAD) Study in Healthy Volunteers (HV). Diabetes. 2021;70(Supplement_1).
