ABSTRACT
Introduction: We report an epileptic patient who experienced hallucinatory visual experiences of autobiographical memories from her past. These visual experiences were confined to the lower left quadrant of her visual field.
Methods: We carried out a single-case study that used brain-imaging, EEG and behavioural methods to study this patient.
Results: We found that this patient had an incomplete left inferior homonymous quadrantanopia due to a lesion of right occipital cortex, and also that she showed neurological abnormalities in right temporal cortex, a region that is part of the brain’s autobiographical-memory circuit.
Conclusion: We attribute the occurrence of this patient’s autobiographical-memory hallucinations to the combination of degraded visual input to right temporal cortex plus hyperexcitability of that region.
Introduction
We report here the case of a patient with left inferior homonymous quadrantanopia and ictal visual hallucinations appearing only in the left inferior visual field. These hallucinations took the form of visual autobiographical memories, a type of hallucination not previously reported in brain-injured patients. We consider the implications of this case for attempts to understand the nature of visual hallucinations accompanying visual loss.
Case report
Mrs. V, a 21-year-old female, was admitted to the National Institute of Neurology and Neurosurgery in Mexico City because of focal seizures, which had begun when she was 9 years old, probably related to perinatal hypoxia. Her ictal episodes were characterised by unresponsiveness and stereotyped sucking movements with a 1 to 2-minute duration, followed by brief postictal confusion. The frequency of these events varied over time; the frequency during the period of the neuropsychiatric consultation was 1 to 2 episodes per week, with 4 to 5 seizures per event.
Mrs. V was referred for neuropsychiatric evaluation due to depressive symptoms. She had a major depressive episode in the previous months, with a suicide attempt 4 months before evaluation, with ingestion of an unknown quantity of antiepileptic drugs. She received fluoxetine, 30 mg per day, with response, although some residual symptoms persisted: mild anhedonia, psychomotor slowing, and complaints about poor quality of sleep. On some days she experienced initial insomnia, and sometimes she suffered from intermittent awakenings at night with feelings of anguish. She described these symptoms during the neuropsychiatric consultation.
She also described the recurrent experience of brief episodes of visual complex hallucinations, which had begun 2 months before evaluation. These hallucinations occurred predominantly at night and were characterised by the sudden perception of images with colour and movement (but no sound or other type of perception). They appeared only in the left inferior visual field.
According to the patient, the images appeared unexpectedly, without any understandable relationship to the circumstances or to any actions she was carrying out at the time, and without effort or a decision of voluntary retrieval. The visual experiences disappeared spontaneously after 1 to 2 min, but not before, even if she tried to stop paying attention to the images or if she closed her eyes.
The patient referred to these scenes as being personal memories from her early years. She described a couple of scenes that appeared repeatedly in her left inferior visual field:
Almost always, a memory appears in which I enter my grandmother's room in the morning and find her sitting in her favorite chair, but her eyes are closed, as if she was tired, and I see that she has a bandaged leg.
There is also a frequent memory in which I am looking for a doll in a box with toys, but I don't see it and what I find is a light blue and white soccer ball, and a plastic truck with a missing wheel.
We are lined up in the schoolyard, and I see my classmates in their uniforms; some are laughing. To my right, there is a girl with braided hair, wearing a very green sweater which is very pretty; I notice she is wearing glasses. She looks at me too and smiles.
Apart from the depressive symptoms and the hallucinations, there were no significant abnormalities in the mental status examination, according to the psychiatric interview and to the Neurobehavioral Cognitive Status Examination (Cognistat) (Kiernan et al., Citation1987).
Mrs. V. received lamotrigine 100 mg bid and carbamazepine 500 mg a day to treat the seizures, as indicated by the Epilepsy Clinic. Bromazepam 3 mg at night was added upon report of visual hallucinations, with improvement of sleep. A total remission of these events was obtained, as well as a reduction of the frequency of seizures. Bromazepam was slowly tapered off. A six month follow up confirmed that she had no further hallucinations of autobiographical memory or depressive symptoms. Lamotrigine and Carbamazepine were maintained.
Written informed consent was obtained from the patient for this case report.
Neurological assessment
Neuro-ophthalmological evaluation revealed on kinetic perimetry an incomplete left inferior homonymous quadrantanopia (see ), with no other pathological findings on examination. The visual field defect corresponded to the area where the hallucinatory images appeared.
Figure 1. MRI and campimetry. MRI showed a loss of volume in the right occipital-temporal cortex, and a hyperintense lesion located in the cuneus gyrus of the right occipital lobe. In the left superior image, the T2 weighted MRI shows the dorsal extension of the lesion, reaching the medial parietal lobe. In the middle superior image, the T2 weighted MRI shows the lesion at the level of the dorsal occipital cortex. In the right superior image, the FLAIR sequence shows the ventral and subcortical extension of the hyperintense lesion. Below: The kinetic perimetry revealed an incomplete left inferior homonymous quadrantanopia.
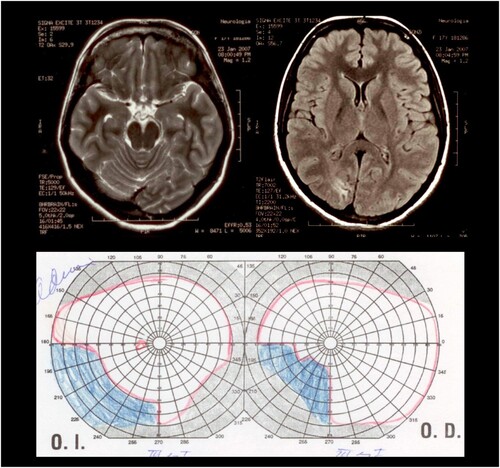
Magnetic Resonance Imaging (MRI) carried out during the period in which she had the visual hallucinations showed a marked loss of volume in the right occipital-temporal cortex, and also a hyperintense lesion located in the cuneus gyrus of the right occipital lobe. The lesion affected the dorsal aspect of the right occipital cortex, with an extension to the inferior and medial aspect of the parietal cortex in the same hemisphere, and also with an extension to the ventral subcortical white matter of the right occipital lobe (see ). The left inferior quadrantanopia that was documented by means of perimetry was consistent with the location of the right occipital lesion. Based on the clinical history, we believe that the patient had suffered perinatal hypoxia, likely to have been responsible for the occipital lobe lesion. No other cause was identified for this lesion, and the neuroradiological pattern, along with the early onset of visual problems, supports this hypothesis.
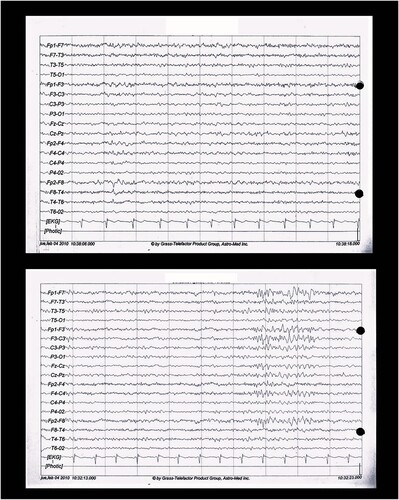
shows the EEG, which revealed focal epileptic activity in the right anterior temporal region that occasionally propagated to the left fronto-central region. Further investigation did not reveal a clear etiology for this lesion. The epileptiform activity appeared to originate from the right mesial temporal region (including the right hippocampus) through the collateral Shaffer pathway. The final relay includes the subiculum, fimbria, and neocortex of the right temporal pole.
Discussion
How might one attempt to explain the phenomena we have observed with this patient? What might we learn from these phenomena about the cognitive and neural mechanisms involved in visual hallucinations? An obvious starting point here would seem to be the Charles Bonnet Syndrome (CBS: see e.g., Ffytche et al., Citation1998; Painter et al., Citation2018; Santhouse et al., Citation2000). Patients with this syndrome experience visual hallucinations, as did our patient, and all have some form of impairment of the visual pathway from retina to occipital cortex, as did our patient.Footnote1
In some cases of CBS, the visual hallucinations are simple: just flashes of light, grid-like or branching patterns, or elementary geometric shapes; in other cases, they are complex: visual images of people, faces, objects, or whole scenes (for review, see Pang, Citation2016). There is a close correspondence between the content of a CBS patient’s hallucinations and the known functional neuroanatomy of the ventral occipital lobe: Santhouse et al. (Citation2000, p. 2055) note, “visual hallucinations were related to phasic activity within specialized visual cortex and that the location of the increases defined the type of experiences reported”. Hallucinations of colour are associated with the posterior fusiform gyrus, achromatic hallucinations with the right middle fusiform gyrus, patterned hallucinations with the collateral sulcus, hallucinations of objects with the right middle fusiform gyrus, and hallucinations of faces with the left middle fusiform gyrus (see Table 3 of Pang (Citation2016) for a summary of such findings). But despite this heterogeneity of hallucinatory types in CBS, autobiographical-memory hallucinations have not been reported in any cases of CBS.
There is a well-supported theory of CBS (see e.g., Coltheart, Citation2018; Ffytche et al., Citation1998; Painter et al., Citation2018) according to which the condition arises when two neuropathological abnormalities are jointly present. The first is some form of impairment of the visual pathway from retina to occipital cortex. The result of this impairment is that the input from occipital cortex to higher visual centres such as the face recognition, object recognition or colour recognition systems will be degraded or noisy. The second required form of neuropathology is damage to one or other of these higher visual systems, damage that makes that system hyperexcitable. The combination of degraded input to the damaged higher centre and hyperexcitability of that centre is what results in the occurrence of some form of visual hallucination.
Support for this account of CBS has been provided by Painter et al. (Citation2018). They studied eight cases of CBS (some with simple hallucinations, some with complex), in all of whom the first factor was retinal pathology (always macular degeneration). Cortical excitability was measured via electrophysiological responses to peripheral visual stimulation in these CBS patients and in a control group of patients with macular degeneration but no accompanying CBS,Footnote2 as well as in a second control group without macular degeneration.
In this study, the CBS group showed greater cortical hyperexcitability than the two control groups, which did not differ from each other. This difference was largest in visual cortical area V2 and extended to V1. However, hyperexcitability in the CBS group was also present elsewhere in occipital cortex and parietal cortex and right temporal cortex, but not frontal cortex. Painter et al. (Citation2018, p. 3477) concluded that “the CBS hyperexcitability to peripheral visual stimulation affects the earliest stages of visual cortical processing together with more distributed cortical networks”.
There is a major difference between our patient and those with CBS, namely, the nature of the visual hallucinations: No cases of CBS have been reported in which the visual hallucinations took the form of autobiographical memories, as was the case with our patient. Nevertheless, we can propose an analogous theory to account for the results we observed with our patient. First, we note that our patient did have an impairment of the visual pathway from retina to occipital cortex, and so input from there to higher brain centres would be degraded or noisy. For our patient, the relevant higher brain centre here would have to be the brain system subserving autobiographical-memory; we suggest that some component of the brain’s autobiographical-memory system was hyperexcitable in our patient (in addition to her visual-pathway impairment).
Studies of the neural basis of autobiographical memory have indicated that the autobiographical memory cognitive subsystem depends upon a widespread and bilateral network in the brain. There is evidence that this network includes right anterior temporal cortex. For example:
Based on PET activation studies (Shallice et al., 1994; Tulving et al., 1994a,b,c, 1996; Nyberg et al., 1996) and clinical data from neurological patients with selective retrograde amnesia in the episodic (Kapur et al., 1992; Markowitsch et al., 1993a,b) or semantic memory domain (De Renzi et al., 1987; Grossi et al.,1988), it has been hypothesized that the right anterior temporal and right inferolateral prefrontal cortex, structures interconnected via the ventral branch of the uncinate fascicle, are involved in episodic memory retrieval … The present findings, indicating a huge preponderance of activation in the right hemisphere, support this hypothesis for the episodic memory system in general and for autobiographical memory retrieval in particular. (Fink et al., Citation1996, p. 4280)
Conclusions
We describe an epileptic patient who reported visual hallucinations that took the form of visual autobiographical memories; these hallucinations were experienced only in her left lower visual quadrant. We propose an explanation of these results that is analogous to the explanation that is well-accepted for the Charles Bonnet Syndrome, even though autobiographical-memory hallucinations are not reported in patients with that syndrome. Our patient had, first, an impairment of the visual pathway that in her case affected only the left lower quadrant, so that visual input from this quadrant of the visual field to higher visual centres would be noisy. Additionally, the patient had neurological abnormalities of right temporal cortex, a brain region that is part of the brain’s autobiographical-memory circuit. We attribute the occurrence of her autobiographical-memory hallucinations to the combination of degraded visual input to that region and hyperexcitability of that region.
Ethical approval
The research protocol entitled “Alucinaciones complejas en pacientes con deprivación sensorial” (Complex hallucinations in patients with sensory deprivation) was approved by the Research Committee of the National Institute of Neurology and Neurosurgery of Mexico. The design was that of a case series. This case was part of the study. Additionally, the patient provided her written informed consent to the publication of her clinical, electrophysiology, and imaging data, with protection of her confidentiality.
Acknowledgements
We thank a reviewer for a detailed and constructive critique of an earlier version of this paper. J. R-B. contributed to the acquisition and interpretation of the psychopathological, neurological and brain imaging data of the case, and to the theoretical formulation. M. Y-N. contributed to the acquisition and interpretation of the psychopathological and brain imaging data of the case. I. M. J. contributed to the acquisition and interpretation of the neurological, neurophysiological and brain imaging data of the case. J. C. B. contributed to the acquisition and interpretation of the ophthalmologic data of the case. M. C. contributed to the formulation of the theory and to the writing of the paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All relevant data are reported in this paper.
Additional information
Funding
Notes
1 A large variety of etiologies of visual loss have been associated with cases of CBS: macular disease, retinal disease, neuropathic disease, amblyopia, cataract, central retinal artery occlusion, central retinal vein occlusion, cerebrovascular accident, corneal scar, nystagmus, pseudophakic bullous keratopathy, uveitis and retinitis pigmentosa (Pang, Citation2016).
2 The existence of such patients, who have an impairment of the visual pathways but do not exhibit CBS, shows that such an impairment, while necessary for the occurrence of CBS, is not sufficient. Some second factor must also be present.
References
- Coltheart, M. (2018). Charles Bonnet Syndrome: Cortical hyperexcitability and visual hallucination. Current Biology, 28(21), R1253–R1254. https://doi.org/10.1016/j.cub.2018.09.007
- Ffytche, D. H., Howard, R. J., Brammer, M. J., David, A., Woodruff, P., & Williams, S. (1998). The anatomy of conscious vision: An fMRI study of visual hallucinations. Nature Neuroscience, 1(8), 738–742. https://doi.org/10.1038/3738
- Fink, G. R., Markowitsch, H. J., Reinkemeier, M., Bruckbauer, T., Kessler, J., & Heiss, W.-D. (1996). Cerebral representation of one's own past: Neural networks involved in autobiographical memory. Journal of Neuroscience, 16(13), 4275–4282. https://doi.org/10.1523/jneurosci.16-13-04275.1996
- Fossati, P. (2013). Imaging autobiographical memory. Dialogues in Clinical Neuroscience, 15(4), 487–490. https://doi.org/10.31887/dcns.2013.15.4/pfossati
- Kiernan, R. J., Mueller, J., Langston, J. W., & Van Dyke, C. R. A. I. G. (1987). The neurobehavioral cognitive status examination: A brief but quantitative approach to cognitive assessment. Annals of Internal Medicine, 107(4), 481–485. https://doi.org/10.7326/0003-4819-107-4-481
- Painter, D. R., Dwyer, M. F., Kamke, M. R., & Mattingley, J. B. (2018). Stimulus-driven cortical hyperexcitability in individuals with Charles Bonnet hallucinations. Current Biology, 28(21), 3475–3480.e3. https://doi.org/10.1016/j.cub.2018.08.058
- Pang, L. (2016). Hallucinations experienced by visually impaired: Charles Bonnet Syndrome. Optometry and Vision Science, 93(12), 1466–1478. https://doi.org/10.1097/opx.0000000000000959
- Santhouse, A. M., Howard, R. J., & Ffytche, D. H. (2000). Visual hallucinatory syndromes and the anatomy of the visual brain. Brain, 123(10), 2055–2064. https://doi.org/10.1093/brain/123.10.2055
- Vignal, J. P., Maillard, L., McGonigal, A., & Chauvel, P. (2007). The dreamy state: Hallucinations of autobiographic memory evoked by temporal lobe stimulations and seizures. Brain, 130(1), 88–99. https://doi.org/10.1093/brain/awl329