Abstract
Purpose: Diagnostic and prognostic evaluation remains challenging in arrhythmogenic right ventricular cardiomyopathy (ARVC). We measured plasma concentration of soluble ST2 (sST2) and assessed its association with right ventricular (RV) function and ventricular arrhythmias in patients with ARVC.
Methods: We included patients with ARVC and genotype positive relatives. Soluble ST2 was determined by ELISA. We assessed myocardial function by echocardiography including strain by speckle tracking technique.
Results: We included 44 subjects (age 41 ± 15 years, 21 (48%) female). Soluble ST2 was associated with RV global strain (r = 0.44; p = 0.008), as well as with left ventricular (LV) function. Plasma levels of sST2 were higher in patients with ventricular arrhythmias than in patients without ventricular arrhythmias (35 ± 13 ng/mL vs. 26 ± 7 ng/mL, p = 0.009). The association between sST2 and ventricular arrhythmias remained significant even after adjusting for RV function (Wald = 5.2; p = 0.02).
Conclusions: Soluble ST2 is associated with RV and LV function in patients with ARVC. Soluble ST2 may aid in the determination of disease severity in ARVC.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is characterised by patchy fibrosis and fatty infiltration of the myocardium. It predominantly affects the right ventricle and is associated with a high risk of developing ventricular arrhythmias. Patients with a particularly high risk of ventricular arrhythmias are eligible for implantable cardioverter-defibrillator (ICD) therapy. However, diagnostic as well as prognostic evaluation is difficult in ARVC (Priori et al. Citation2015).
Soluble ST2 (sST2) is a decoy receptor for interleukin-33 (IL-33) (Sanada et al. Citation2007). While IL-33/ST2 signalling is involved in inflammation and immune-mediated disorders, recent data suggest that this pathway also plays an important role in heart disease (Sanada et al. Citation2007). In vitro, cardiomyocytes subjected to mechanical stress express ST2 (Weinberg et al. Citation2002). Circulating levels of sST2 are associated with outcome in patients with left heart failure (Ky et al. Citation2011, Broch et al. Citation2012, Llibre et al. Citation2016). However, elevated levels of sST2 observed in these patients may stem from endothelial release of sST2 in response to congestion (Bartunek et al. Citation2008, Demyanets et al. Citation2013, Broch et al. Citation2015). Thus, plasma sST2 may mirror not only left ventricular function, but also the degree of right ventricular failure, as observed in patients with pulmonary arterial hypertension (Carlomagno et al. Citation2013, Zheng et al. Citation2014).
We hypothesised that circulating sST2 reflects the degree of right ventricular impairment in patients with ARVC and in their genotype positive relatives. We aimed to explore if levels of circulating sST2 were associated with disease severity in the form of right and left ventricular dysfunction and the occurrence of ventricular arrhythmias in these patients.
Clinical significance
Diagnostic and prognostic evaluation is difficult in arrhythmogenic right ventricular cardiomyopathy (ARVC).
We show that the biomarker ST2 is associated with right ventricular function and ventricular arrhythmias in patients with ARVC.
Soluble ST2 is an easily obtainable biomarker that might aid decision-making in these patients.
Methods
This is a cross-sectional study comprising patients diagnosed with ARVC at Oslo University Hospital, Rikshospitalet, according to current diagnostic criteria, and their genotype positive relatives. All participants provided written, informed consent. The study complies with the Declaration of Helsinki and was approved by the Regional Committee for Medical and Health Research Ethics (REK South-East).
Patient population
We diagnosed ARVC according to current Task Force Criteria, comprising major and minor diagnostic criteria from different diagnostic categories (Marcus et al. Citation2010). We assessed myocardial structure and function by cardiac imaging (echocardiography and cardiac magnetic resonance); conduction or rhythm disturbances by electrocardiograms (ECG), late potential ECGs and 24-hour ECGs; and family history and genetic testing as previously described (Saberniak et al. Citation2017). A diagnosis of “definite” ARVC was made if the patient fulfilled at least two major criteria, one major criterion and two minor criteria, or four minor criteria covering different aspects of the disease. We made a diagnosis of “borderline ARVC” in patients with one major criterion and one minor criterion or three minor criteria, and a “possible ARVC” diagnosis was established in the presence of one major or two minor criteria. We excluded patients with clinically significant heart disease of other aetiology.
Study procedures
We obtained a thorough medical history in all the participants. All the participants underwent physical examination; blood tests including screening for known monogenic causes of ARVC; echocardiography; ambulatory 24-h electrocardiogram (ECG); and exercise testing. We defined “ventricular arrhythmia” as a history of documented sustained or non-sustained ventricular tachycardia (three or more consecutive ventricular beats >120 beats per minute), or an episode of cardiac arrest.
Biochemical analyses
Peripheral blood samples were obtained in the non-fasting state at inclusion. Blood was collected in glass tubes containing the anti-coagulant ethylenediamine tetraacetic acid, and immediately centrifuged. Plasma aliquots for the measurement of sST2 were stored at −80 °C and thawed once for analysis. Soluble ST2 was measured with the Presage® ST2 Assay (Critical Diagnostics, San Diego, CA) as described by Dieplinger and colleagues (Dieplinger et al. Citation2009). Intra- and inter-assay coefficients of variation for sST2 were <10%. The biochemical analyses were performed in duplicate, blinded to clinical and echocardiographic data.
Echocardiography
We used Vivid 7 or E9 ultrasound scanners (GE Vingmed Ultrasound, Horten, Norway), with phased array transducers. Cine loops were digitally stored and later analysed off-line using Echo-Pac (GE Vingmed). Two-dimensional parameters and conventional Doppler measurements were obtained according to current recommendations (Lang et al. Citation2015). We used left ventricular ejection fraction and global longitudinal strain as measures of left ventricular function. Left ventricular ejection fraction was obtained by Simpson’s biplane method. Longitudinal strain was measured by speckle tracking (Amundsen et al. Citation2006, Edvardsen and Haugaa Citation2011). In each of the three conventional apical imaging planes, we placed points along the endocardial border of the left ventricle. From this demarcation line, the region of interest was adjusted to encompass the entire width of the left ventricular wall. For each segment, we then measured the peak negative longitudinal strain and averaged the values over the 16 left ventricular segments (Mor-Avi et al. Citation2011). Right ventricular function was assessed by fractional area change, tricuspid annular plane systolic excursion and right ventricular global strain. Right ventricular strain was calculated as the average of peak negative longitudinal strain from six right ventricular segments (Saberniak et al. Citation2017). Measurements of right ventricular function were obtained in focussed four-chamber views (Lang et al. Citation2015). All image analyses were performed by personnel blinded to the results of the sST2 analyses.
Genetic testing
We isolated genomic DNA from peripheral blood. Genetic analyses were performed in all the participants. We sequenced the translated exons and flanking intron sequences of the genes plakophilin-2 (PKP2), desmocollin-2 (DSC2), desmoglein-2 (DSG2), and desmoplakin (DSP); and 29 of the 105 exons of the ryanodine receptor-2 (RYR2) gene. We considered mutations pathogenic if known to be associated with an ARVC phenotype. Index patients with confirmed pathogenic mutations were defined as ARVC genotype positive. In index cases with confirmed pathogenic mutations, we performed family cascade genetic screening to identify mutation-positive family members.
Statistics
We present values as mean ± standard deviation, median (interquartile range), or number (%) depending on distribution. We analysed associations between baseline characteristics and sST2 using bivariate and multivariate linear regression, with log transformed sST2 values as the dependent variable. Associations between binary variables and log-transformed sST2 levels were analysed by Students t-tests.
The association between sST2 and a history of ventricular arrhythmia was assessed by binomial regression analyses. To ensure that a possible association with ventricular arrhythmia was not a mere reflection of sST2’s ability to mirror cardiac functional impairment, we adjusted for ventricular function. In order to select the best predictor of ventricular arrhythmia among the multiple, intercorrelated measures of ventricular function obtained by echocardiography, we performed a forward conditional multiple binominal regression analysis with ventricular arrhythmia as the dependent variable, and right ventricular outflow tract dimension, fractional area change, tricuspid annular plane systolic excursion, right ventricular global strain, left ventricular ejection fraction, and left ventricular global strain as independent variables.
We performed all statistical analyses with the Statistical Package for Social Sciences version 22 software (SPSS Inc., Chicago, IL). Two-sided probability values were considered significant at p < 0.05.
Results
Patient characteristics
We included 44 patients with ARVC and mutation positive relatives (). The median sST2 value was 27.9 (range 12.5–71.6) ng/ml. Eight patients (18%) had an sST2 level above the Food and Drug Administration approved cut-point of 35 ng/ml.
Table 1. Population characteristics.
Associations between sST2 and cardiac function
The level of sST2 was higher in males than in females (30.4 [20.9 – 40.5] ng/ml vs. 25.9 [21.4 – 27.9] ng/ml; p = 0.02). The plasma concentration of sST2 was associated with patient weight and body mass index, as well as with all echocardiographic measures of left and right ventricular function (). The natural logarithm of sST2 correlated with echocardiographic parameters including left ventricular ejection fraction, left and right ventricular longitudinal strains, and tricuspid annular plane systolic movement (). In a stepwise, multiple regression analysis including body mass index, left ventricular global longitudinal strain, left ventricular ejection fraction, right ventricular longitudinal strain, right ventricular fractional area change, right ventricular outflow tract diameter, and tricuspid annular plane systolic excursion, only right ventricular longitudinal strain remained an independent predictor of sST2 in the final model (β = 0.53; p = 0.004).
Figure 1. Associations between soluble ST2 and cardiac function. Scatter plots depicting associations between logarithmically transformed soluble ST2 (lnsST2) and indices of left and right ventricular function. Panel A shows the association with left ventricular ejection fraction (LVEF); panel B the association with left ventricular global longitudinal strain (LV GLS); panel C the association with tricuspid annular plane systolic excursion (TAPSE); and panel D the association with right ventricular (RV) strain.
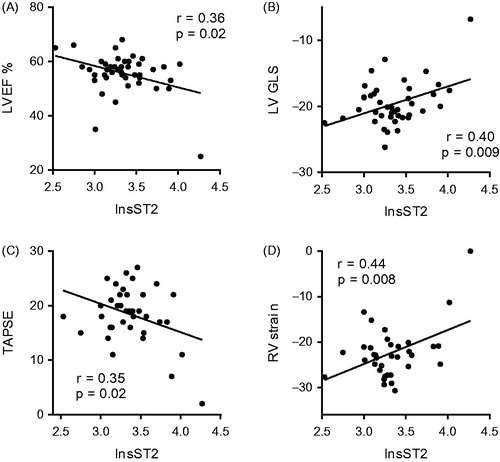
Table 2. Associations between soluble ST2 and population characteristics.
Ventricular arrhythmias
Twenty-one patients (48%) had experienced ventricular arrhythmias (). By univariate binominal regression analysis, sST2 was firmly associated with the occurrence of ventricular arrhythmia (Wald = 6.2; p = 0.01). However, arrhythmias were more prevalent in probands, and when adjusting for proband status, the association between sST2 and arrhythmias was attenuated to borderline significance (Wald = 3.5; p = 0.06). Having a history of ventricular arrhythmia was not associated with clinical variables such as body mass index (Wald = 2.11; p = 0.15), gender (X2 = 3.3; p = 0.07) or traditional echocardiographic indices such as left ventricular ejection fraction (Wald = 1.68; p = 0.20), tricuspid annular plane systolic excursion (Wald = 2.15; p = 0.14), or right ventricular outflow tract diameter (Wald = 2.60; p = 0.11). However, right and left ventricular function expressed as strains were associated with a history of ventricular arrhythmia (). Left and right ventricular strains correlated to a high degree (r = 0.82; p < 0.001), and on forward conditional binomial regression analysis, only right ventricular strain, but not left ventricular strain, remained associated with ventricular arrhythmias. Finally, sST2 and right ventricular strain were independently associated with ventricular arrhythmia when assessed by multiple binomial regression ().
Table 3. Associations between ventricular arrhythmia, and myocardial strain and soluble ST2.
Discussion
For the first time, we show that levels of sST2 are associated with right ventricular function in patients with ARVC. Moreover, circulating levels of sST2 seems to be independently associated with a history of ventricular arrhythmia in this population. Our results suggest that sST2 can be used to aid in the assessment of disease severity in patients with ARVC.
Arrhythmogenic right ventricular cardiomyopathy affects ventricular function with variable penetrance and expressivity depending on the previous amount of physical activity (Saberniak et al. Citation2014). The disease is progressive by nature, and left ventricular function is often also affected, which may lead to left ventricular systolic dysfunction and overt left ventricular failure (Haugaa et al. Citation2016). However, the most important clinical feature of ARVC is the propensity for malignant arrhythmias. The risk of developing life-threatening arrhythmias probably increases with the extent of myocyte necrosis and myocardial fibro-fatty replacement in patients with ARVC. However, due to its complex geometrical shape and its position within the chest, the right ventricle does not lend itself easily to imaging. Strain echocardiography enables improved assessment of right ventricular function, but we still need other variables to quantify disease severity in these patients.
Soluble ST2 is approved by the Food and Drug Administration for presaging outcome in patients with heart failure. While most research has focussed on the prognostic value of sST2 in patients with left ventricular failure (Ky et al. Citation2011, Broch et al. Citation2012), circumstantial evidence implies that sST2 might be a measure of congestion (Bartunek et al. Citation2008, Broch et al. Citation2015), and therefore might be associated with right ventricular failure as much as with left ventricular failure. Our results suggest that sST2 reflects right ventricular failure in patients with ARVC. Furthermore, sST2 was independently associated with potentially lethal arrhythmias in this population. Soluble ST2 is thus an easily obtainable risk marker that might allow for additional information about disease severity in patients with ARVC.
Limitations
We present results from a small cohort of patients. The study has a cross-sectional, retrospective design, which limits the ability to make causal inference. We show that there is an association between sST2 and a history of ventricular arrhythmia, but our study cannot prove that elevations in the plasma concentration of sST2 precede the onset of arrhythmias. On the other hand, due the prevalence-incidence bias (Hill et al. Citation2003), we may have underestimated the true association between sST2 and ventricular arrhythmias. In our composite definition of “ventricular arrhythmia”, we included non-sustained ventricular arrhythmias. Sustained ventricular tachycardia is a stronger predictor of sudden death, but non-sustained ventricular arrhythmia is still considered a major risk factor for adverse outcome (Corrado et al. Citation2015).
Conclusion
In patients with ARVC, plasma sST2 concentrations were associated with right and left ventricular function. Elevated levels of sST2 were independently associated with a history of ventricular arrhythmias. Our results suggest that sST2 levels may be used to aid in the evaluation of patients with ARVC and their genotype positive family members. Further research is needed to verify these results in a prospective manner, and to assess whether sST2 can be used for risk stratification in these patients.
Acknowledgements
The Presage® ST Assay was kindly provided by Critical Diagnostics, San Diego, CA.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Amundsen, B.H., et al., 2006. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. Journal of the american college of cardiology, 47, 789–793.
- Bartunek, J., et al., 2008. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. Journal of the american college of cardiology, 52, 2166–2174.
- Broch, K., et al., 2012. Soluble ST2 is associated with adverse outcome in patients with heart failure of ischaemic aetiology. European journal of heart failure, 14, 268–277.
- Broch, K., et al., 2015. Soluble ST2 reflects hemodynamic stress in non-ischemic heart failure. International journal of cardiology, 179, 378–384.
- Carlomagno, G., et al., 2013. Serum soluble ST2 and interleukin-33 levels in patients with pulmonary arterial hypertension. International journal of cardiology, 168, 1545–1547.
- Corrado, D., et al., 2015. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation, 132, 441–453.
- Demyanets, S., et al., 2013. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. Journal of molecular and cellular cardiology, 60, 16–26.
- Dieplinger, B., et al., 2009. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma-the presage ST2 assay. Clinica chimica acta, 409, 33–40.
- Edvardsen, T. and Haugaa, K.H. 2011. Imaging assessment of ventricular mechanics. Heart, 97, 1349–1356.
- Haugaa, K.H., et al., 2016. Arrhythmogenic right ventricular cardiomyopathy, clinical manifestations, and diagnosis. Europace, 18, 965–972.
- Hill, G., et al., 2003. Neyman's bias re-visited. Journal of clinical epidemiology, 56, 293–296.
- Ky, B., et al., 2011. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circulation: heart failure, 4, 180–187.
- Lang, R.M., et al., 2015. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Journal of the american society of echocardiography, 28, 1–39.
- Llibre, C., et al., 2016. The real-life value of ST2 monitoring during heart failure decompensation: impact on long-term readmission and mortality. Biomarkers, 21, 225–232.
- Marcus, F.I., et al., 2010. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation, 121, 1533–1541.
- Mor-Avi, V., et al., 2011. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. European journal of echocardiography, 12, 167–205.
- Priori, S.G., et al., 2015. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). European heart journal, 36, 2793–2867.
- Saberniak, J., et al., 2014. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. European journal of heart failure, 16, 1337–1344.
- Saberniak, J., et al., 2017. Comparison of patients with early-phase arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract ventricular tachycardia. European heart journal cardiovascular imaging, 18, 62–69.
- Sanada, S., et al., 2007. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. Journal of clinical investigation, 117, 1538–1549.
- Weinberg, E.O., et al., 2002. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation, 106, 2961–2966.
- Zheng, Y.G., et al., 2014. Plasma soluble ST2 levels correlate with disease severity and predict clinical worsening in patients with pulmonary arterial hypertension. Clinical cardiology, 37, 365–370.
