Abstract
Purpose: To assess the predictive power of the comet assay in the context of occupational exposure to pesticides.
Materials and methods: The recruited subjects completed a structured questionnaire and gave a blood sample. Exposure to pesticides was measured by means of an algorithm based on Dosemeci’s work (Agricultural Health Study). Approximately 50 images were analyzed for each sample via fluorescence microscopy. The extent of DNA damage was estimated by tail moment (TM) and is the product of tail DNA (%) and tail Length.
Results: Crude significant risks (odds ratios, ORs) for values higher than the 75th percentile of TM were observed among the exposed subjects (score > 1). The frequency of some confounding factors (sex, age and smoking) was significantly higher among the exposed workers. A significant dose–effect relationship was observed between TM and exposure score. Significant high-risk estimates (ORs), adjusted by the studied confounding factors, among exposure to pesticides and TM, % tail DNA and tail length were confirmed using unconditional logistic regression models.
Conclusions: The adjusted associations (ORs) between the comet parameters and exposure to pesticides were significant. The sensitivity of the comet test was low (41%), the specificity (89%) and the predictive positive value (0.77) were found acceptable.
Introduction
Evaluation of DNA damage by the comet assay
Direct DNA damage of single-stranded DNA or DNA strand breaks can be caused by chemical agents or their metabolites (Sies Citation1985, Barnett and Barnett Citation1998, Van Gent et al. Citation2001, Jackson Citation2002, Krejci et al. Citation2003), the processes of DNA excision, replication and recombination or by apoptosis processes (Eastman and Barry Citation1992) or the interaction of reactive oxygen species (ROS) with DNA (Møller and Wallin Citation1998). The comet assay, or single cell gel electrophoresis (SCGE), is a genotoxicity test that exploits the ability of DNA to migrate when immersed in an electric field (Lindahl and Andersson Citation1972), allowing it to cumulatively evaluate both DNA damage and alkaline-labile sites in any type of eukaryotic cell; alkaline labile sites exhibit alkylation of phosphate and are susceptible to filament rupture related to pH and alkaline exposure (Azqueta et al. Citation2014). This simple economical technique has been used for several years in various applications, such as for in vitro and in vivo tests of the potential genotoxicity of substances and preparations, biomonitoring of human exposure to mutagenic agents (Anderson et al. Citation2013), or evaluations of the efficacy of DNA repair systems (Rojas et al. Citation1999, Collins Citation2004). The combination of the standard comet assay with enzymes that recognize oxidized nucleotides and cut the DNA backbone have made this technique a valuable means to study oxidative damage of DNA (Collins Citation2014a).
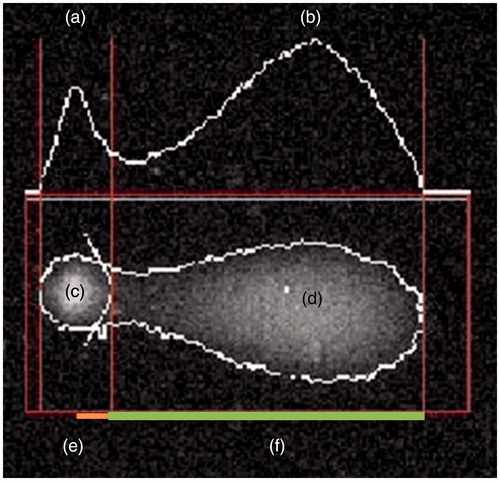
Integral DNA filaments migrate rarely and tend to concentrate in a nucleoid, while fragments generated by filament or double-strand breaks migrate in inverse proportion to their length and molecular weight (Grandi et al. Citation2006). Therefore, after electrophoresis, when viewed under a fluorescence microscope, each cell yields a comet-shaped image is obtained, the head of which consists of the integral DNA and the tail of which consists of DNA fragments of varying length that have migrated away from the nucleus. The images are analyzed using a software that identifies and delimits the areas that constitutes the head and the one constituting the tail () (Collins et al. Citation1997, Citation2001).
Figure 1. Analysis of the image of a comet. (a) Head DNA; (b) tail DNA; (c) head area; (d) tail area; (e) head radius; (f) tail length.
Similar to other genotoxicity tests, and in light of the currently available studies, the comet assay is indicative of the potential genotoxicity of exposures for individuals but is not predictive of their cancer risk.
The comet assay has proven to be a valid predictor of DNA damage due to various types of pesticides in Colombia (Varona-Uribe et al. Citation2016), Brazil (Khayat et al. Citation2013), India (Kaur et al. Citation2011) and Argentina (Simoniello et al. Citation2010), and it has been used for various crops, such as soybean (Benedetti et al. Citation2013), orchards (Kasiotis et al. Citation2012), vineyards (Kvitko et al. Citation2012) and tobacco (Da Silva et al. Citation2012, Citation2014).
Diffusion of pesticides in the study area
The study area was located in Italy between two provinces (Bari and Taranto) of the Apulia region where the most relevant agricultural production is based on vegetables, grapes and olive trees. In this area, the most used insecticides are chlorpyrifos and deltamethrin. Chlorpyrifos is an organophosphate (phosphorothioate) that undergoes oxidative desulfuration to form Chlorpyrifos-oxon, which is generally able to phosphorylate acetylcholinesterase, with a reduction in the metabolism of acetylcholine to choline and acetate. The ensuing neurotoxicant effects are currently an area of interest in terms of the oxidative DNA damage response (Thakur et al. Citation2017). Deltamethrin (Type 2 Pyrethroid) has recently been shown to have a potential carcinogen effect in mouse skin, but the underlying mechanism remains elusive (George and Shukla Citation2013). The most used herbicides were glyphosate and dimethoate. Glyphosate (a nonselective nitro-aniline compound) has only a low irritative effect, and in many recent studies, no consistent association was found with multiple myeloma (De Roos et al. Citation2005, Burstyn and De Roos Citation2016). Dimethoate (an organophosphate) affects hemoglobin contents and hematocrit, but shows no effects on total blood cell counts. It has been demonstrated that the mouse gastric tissue has the potential to become cancerous (Wang et al. Citation2013). Finally, the most diffuse fungicides were mancozeb and fosetyl-aluminum. Mancozeb (a thiocarbamate) yields a minimal irritant reaction and is a sensitizer when combined with in thiophanate. This compound is responsible for DNA damage and may be involved in the pathogenesis of diseases including cancer (Calviello et al. Citation2006). Fosetyl-aluminum (a systemic fungicide based on an aluminum salt of an organic compound) is classified as a minimal irritant, and DNA adducts were reported for treated Pacific oysters (Geret et al. Citation2013).
The main purposes of this study were to assess the association between the chronic pesticide exposure of agricultural workers operating in the provinces of Bari and Taranto and the DNA damage of peripheral lymphocytes, to evaluate the predictive positive value (PPV) of the comet assay and to assess a dose–effect relationship between the increase of the pesticide exposure score and the most relevant comet parameter [tail moment (TM)]. This is a cross-sectional study based on a group of agricultural workers exposed to various pesticides (insecticides, herbicides and fungicides) and a group of unexposed subjects.
Clinical significance
The study of oxidative stress induced in peripheral lymphocytes by aneuploidizing substances may be useful in the future to identify biological markers that predict malignancy to evaluate DNA repair. Many studies have been designed to assess the predictive power of the comet assay in the context of occupational exposure to pesticides.
The comet assay is able to estimate risks (ORs) to observe lymphocytes DNA oxidative damages parameters (TM, % tail DNA and tail length) among subjects exposed to pesticides adjusted by the studied confounding factors.
The specificity of the test was found to be acceptable (89%), and the PPV was also acceptable (77%).
Methods
The study of the comet assay, which is part of the biological studies related to a larger project, was approved by the Ethics Committee of the “Policlinico-Giovanni XXIII” University Hospital of Bari, Italy.
Study groups
The subjects involved in the study are 22 agricultural workers from the provinces of Bari and Taranto, and 24 nonexposed subjects living in the same areas. The first group was selected from agricultural enterprises in Bari and Taranto and the nonagricultural group from hematologic outpatients of the University Hospital as well as University volunteers. Overall, 2374 images were globally analyzed. The study size was of 1122 images for the exposed group and 1252 for the nonexposed group. After signing an informed consent form, each subject gave a venous blood sample. All recruited subjects completed two questionnaires: one general, aimed at gathering identification and socio-demographic data, those about schooling, family history, pathological history, habits such as smoking and alcohol, and working history, and a second questionnaire related to specific aspects of exposures during farm work. For each participant, two fresh blood cells aliquots (OECD Citation2014) were immediately prepared prior to the start of the test by treating the samples under yellow light and paying particular attention to avoiding DNA damage via direct exposure to UV light (Nowsheen et al. Citation2012).
Comet assay
The technique was conducted on the basis of the standardized comet assay methodology described by Hartmann et al. (Citation2003a, Citation2003b) and Burlinson et al. (Citation2007), according to the recommendations reported by Araldi et al. (Citation2015). The test is now recognized as a method for human biomonitoring in accordance with FDA and WHO guidelines (Singh Citation2016).
For the comet assay, we used FLUO Plus DNA stain®, a new, very sensitive nuclear fluorophore, whit high affinity to single-stranded DNA and double-stranded DNA (ssDNA and dsDNA), with virtually no background signal that proved to be very sensitive. Cell specimens were prepared using the following components: lysis solution (Lysis Sol); low melting point agarose (LMPA); disodium EDTA, 0.5 M; neutralizing buffer solution (Neutral Sol); FLUO Plus; slides; doubly distilled water; sodium hydroxide pellets; bovine serum solution (PBS), free of Ca2+ and Mg2+ ions (to avoid endonuclease activity); and dimethyl sulfoxide (DMSO).
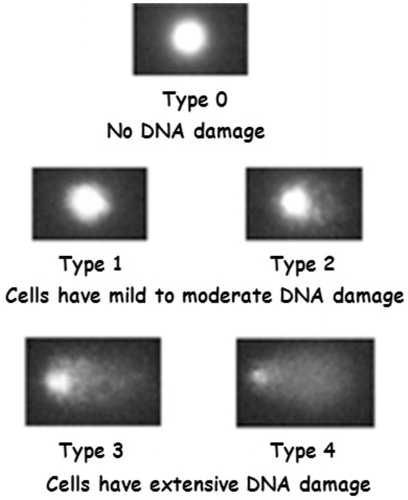
Peripheral heparin blood was diluted 1:1 with PBS. The buffy coat was separated via centrifugation on a density gradient at 800 rotations per minute (rpm) for 20 min; the buffy coat is where lymphocytes are concentrated. Cells were counted in a Neubauer chamber, and vitality was verified through trypan blue, which allows the identification of nonviable cell membranes, for which the cell membrane had become permeable. The comet assay requires that at least 75% of the cells are vital. Vital cells were counted in the Neubauer chamber prior to addition to pre-pretreated slides (Singh sandwich technique) (Singh et al. Citation1988) and were coated with layered agarose gel. On a third layer was placed the cell suspension for analysis, followed by another gel layer of agarose, which was melted at the time. For the test to be valid, the number of cells should be between 1 and 20 × 104. Counting is followed by the cell lysis (1 h, dark, 4 °C), and unwinding with alkaline solution at pH 13, which tests damage to both the single and the double filaments by electrophoresis: DNA, negatively charged, migrates to the positive pole proportionally to its damage and at an inverse speed proportional to the size of its fragments. After neutralization, DNA was stained with FLUO Plus® (excitation wave length: 490 nm, emission wave length: 535 nm), which emits green fluorescence when bound to DNA. Finally, within 48 h, a fluorescence microscope (50 randomized comets, 20–40× magnifications) was read and linked to the “Comet Assay Laboratory Universal Computer Image Analysis” software for image analysis (). For each sample, 20–25 cells or comets were randomly selected to give a total of 40–50 records per subject, for statistical analysis purposes. The interpretation of images is based on their classification into five classes: class 0 = tail-free comet, no DNA damage index; class 1 = slight damage to DNA due to the presence of a few fragments that create a single around the head of the comet; class 2 = moderate DNA damage; class 3 = extended damage; and class 4 = almost completely fragmented DNA (remarkable tail length) (). The software allows the calculation of the following parameters: length of the comet head (head length); tail length (L); fluorescence intensity, expressed as the percentage of DNA in the head (% head DNA) and the percentage of DNA present in the tail (% tail DNA, I); TM equal to the product L × I and the TM mean; and the damage index (DI), that is the sum of (% of class 1 × 1 cells) + (% of class 2 × 2 cells) + (% of class 3 × 3 cells) + (% of class 4 × 4 cells). The DI therefore has a range from 0 (if all cells are of class 0) to 400 (if all cells are of class 4). The software returns a table of useful values as an Excel file to interpret the results of the comet assay.
Figure 2. Classes of DNA damage. Source: Waters DJ et al. (Citation2007).
Retrospective evaluation of pesticide exposure
An algorithm based on the experience of Dosemeci et al. (Citation2002) within the Agricultural Research Study conducted by the National Cancer Institute in the United States was developed for the definition of an exposure intensity score and it was applied to the recruited subjects. Specifically, information was collected using dedicated questionnaire related to the use of mixing systems, pesticide application methods, the presence or absence of wet clothing, personal hygiene, the use of gloves and personal protective equipment, the repair of tanks and the use of tractors with cabins.
Using the following items is possible to calculate an intensity level score (IL):
m = MIX (conditions for product mixing) [0 = no mix; 9 = mix]; e = ENCLOSED (use of an included mixing system) [0.5 = present; 1 = nonpresent]; a = APPL (application method) [0 = none system; 1 = air application system; 2 = furrow application system; 3 = spraying bar system; 8 = shoulder pump system; 9 = motorpump system; 9 = shoulder atomizer system; 9 = towing atomizer system]; c = CAB (tractor equipped with a cabin and/or with activated carbon filter) [0.1 = cabin with filter; 0.5 = cabin without filter; 1 = no cabin no filter]; r1 = REPAR (use of PPE) [0 = Yes; 2 = No); W = WASH (equipment washing after application) [0 = no wash; 0.5 = sprinkler rinsing; 0.5 = tractor rinsing; 3 = cleaning of nozzless; 1 = tank cleaning]; p = PPE (personal protective equipment types used) [1 = non PPE use or hat; 0.8 = dust mask/facial screen/goggles/gloves/overall; 0.7 = cartridge respirators/antigas mask/resistant boots/disposable clothing; 0.6 = chemical resistant gloves]; r2 = REPL* (frequency of replacement of worn gloves) [1 = after single use; 1.1 = one time/month; 1.2 = when they are consumed]; h = HYG (types of personal hygiene, e.g., clothes-change habits, washing of arms and hands, and/or shower or bath habits) [0.2 = changes clothes immediately after/wears disposable clothing/washes arms and hands immediately after/bathes or showers immediately after/bathes or showers for lunch; 0.4 = change clothes immediately after/wears disposable clothing and/or washes arms and hands for lunch or at the end of the day; 0.8 = changes clothes the day after or bathes or showers at the end of the day; 1 = changes clothes on the weekend or washes arms and hands at the end of the day]; s = SPILL (behavior in the event of spills of products on work clothes) [1 = changes clothes immediately after; 1 = wears disposable clothing; 1.1 = changes clothes for lunch; 1.2 = changes clothes at the end of the next day; 1.4 = changes clothes at the end of the day after]. Every recruited exposed subject was assigned a variable score for IL. Every unexposed recruited subject was assigned a score of IL equal to 0 (Dosemeci et al. Citation2002).
Statistical analysis
Two slides per recruited subject and an average of 25 images for every slide were analyzed (25 images per slide [total images = 50]) based on previous experience (Grandi et al. Citation2006). The images were finalized to the increase of the power of the comet parameters measurements. Samples were taken from patients to prepare slides at a single time point, and the choice of two slides per sample was finalized to the increase certainty and give at least 25 measurements also in the case of technical mistakes. Slides were prepared just after the blood draw.
The difference in the two groups was due to difference between agricultural workers and nonagricultural workers. Unexposed people may be present among the agricultural worker group, and exposed people may be present among the nonagricultural workers.
Therefore, power was not assessed for a case-control study with outcomes such as disease or death but for a (transitional) cross-sectional study, primarily based on multiple image measurements for every participant with an overall number of 1122 images for the exposed group and 1252 for the nonexposed group. Supposing a Z α/2 = 1.96, Z β = 0.84, an expected probability of exposure among controls (л1) = 0.47 and an expected probability of exposed among Comet positive subjects (л2) = 0.57 with a difference of 0.10, this would require approximately 366 observations for the group for a power of 0.84. Thus, with 1122 observations for the exposed group and 1252 for the controls, the power is very strong.
Statistical analysis was performed using STATA 12 software. Preliminary statistical evaluation of the measured parameters was performed. All the measured parameters are quantitative variables and the exposure score is a quantitative estimate. All the measured parameters were dichotomized using a cutoff value corresponding to the 75th percentile of their cumulative distributions, because the distributions were not-normal. Then, for all the measured parameters, the risks (ORs) were calculated to determine values above the 75th percentile that were associated with pesticide exposure. The risks associated with some important individual variables were also studied, and a multivariate analysis was used to adjust the estimates for the confounding variables using an unconditional logistic regression model. Natural logarithmic transformation of all the parameters was performed, and a linear regression model was used to verify the presence of a dose–effect relationship between an increase in exposure score and the individual log transformed parameters. Finally, an evaluation of the PPV and a sensitivity and specificity assessment of the comet assay related to the pesticides exposure were also performed.
The test used for is the Pearson’s chi-square test, which has two degrees of freedom for age classes and one degree of freedom for sex and smoking habits.
Table 1. General characteristics of the two groups.
Multicollinearity was not evaluated for the linear regression model, because it was not a “multiple” regression model but only an instrument to study the linear dose–effect relationship between the TM and the exposure score.
In the multiple unconditional logistic regression, there were assessed nine different multiple models with only one measured parameter and tree different confounding variables. For specificity and sensitivity, no statistical test was used. The positive and negative likelihood ratios were calculated, but were not reported due to their irrelevance. The accuracy was also calculated. The unconditional logistic method was used due to the nonparametric distribution of the measured parameters. This model was more congruent then the conditional one because the logistic model for outcome probabilities and the related assessment of the PORs must be adapted to our cross sectional (transitional study) (Breslow and Day Citation1980). For parameter distribution testing, the skewness and kurtosis values were calculated.
Results
The studied pesticides were herbicides, fungicides and insecticides. The most important crops were vegetables (80%), grapes (10%) and olives (10%). The used insecticides were chlorpyrifos and deltamethrin. The used herbicides were glyphosate and dimetoate. The used fungicides were mancozeb and fosetyl. All the recruited subjects used individual protective devices (mask, gloves and suits), and there were reportedly at least three treatments per year.
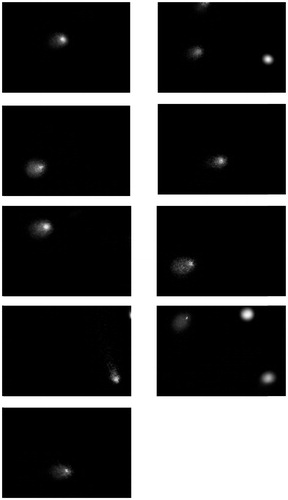
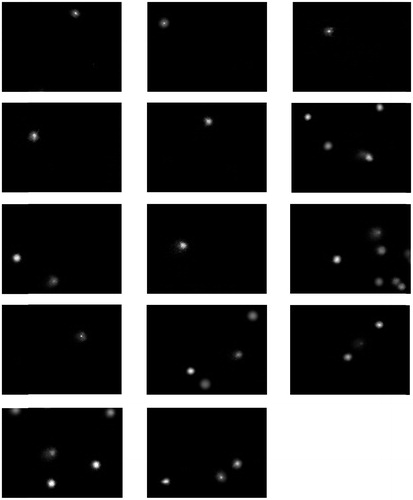
An individual exposure score (semi-quantitative evaluation) was assigned using the Dosemeci algorithm. All the subjects exposed to pesticides had scores above 1. Images of nuclei with tails, indicating greater DNA damage, were commonly observed among the pesticides-exposed workers (). In unexposed subjects, lymphocytes showed minor DNA damage or quiescent nuclei ().
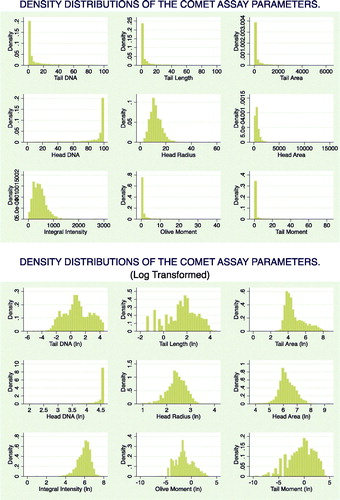
Overall, 2374 images were globally analyzed. The study size was of 1122 slides for the exposed group and 1252 for the unexposed group. Automatic reading of the recruited subjects’ slides returned comet parameters with a non-Gaussian distribution, and, thus, a logarithmic transformation that normalized the distributions was performed (). A significant dose–effect relationship between pesticide exposure (score) and the natural logarithm of the TM was also found in a significant linear regression [F = 152.29; p(F) ≤ .000; Adj R2 = 0.1307; t = 12.34; p(t) ≤ 0.00; β = 0.20] (). A correlation matrix of log transformed parameters showed significant correlations among the different measures ().
Figure 6. Regression line between Exposure increasing values of pesticides exposure and Tail Moment (Ln). (F = 152.29; p(F) = .000; Adj R2 = 0.1307; t = 12.34; p(t) = .000; β = 0.20).
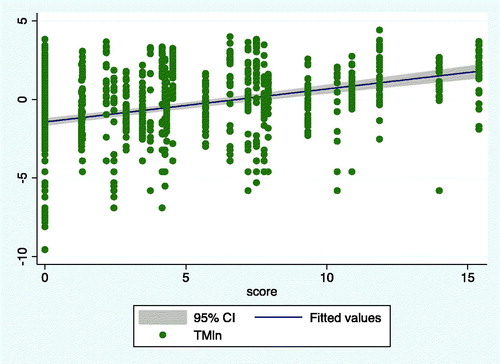
Figure 7. Correlation matrix of log transformed parameters measured by means of comet assay. TMln: tail moment[ln]; HDln: head DNA[ln]; TDln: tail DNA[ln]; OMln: olive moment[ln]; HAln: head area[ln]; IIln: Integral Intensity[ln]; HRln: head radius[ln]; TLln: tail length[ln]; TAln: tail area[ln]; SCln: score[ln].
![Figure 7. Correlation matrix of log transformed parameters measured by means of comet assay. TMln: tail moment[ln]; HDln: head DNA[ln]; TDln: tail DNA[ln]; OMln: olive moment[ln]; HAln: head area[ln]; IIln: Integral Intensity[ln]; HRln: head radius[ln]; TLln: tail length[ln]; TAln: tail area[ln]; SCln: score[ln].](/cms/asset/bd89ccdb-1c9e-4957-89aa-dd657d08551e/ibmk_a_1443513_f0007_c.jpg)
Statistical evaluation was performed based on the number of images analyzed that was 1122 in the exposed group and 1252 in the unexposed group. The frequency of subjects in older age groups (≥38 years) was significantly higher in the exposed group than in the unexposed one. Both groups were predominantly male; the frequency of smokers among the exposed group was significantly higher than among the unexposed group (). We can therefore consider these three characteristics of the groups as potential confounding variables.
To compare the two groups in terms of the different parameters of the comet assay, dichotomic variables were created using a cutoff value corresponding to the 75th percentile. The ORs of the observed parameters values categorized above the 75th percentile were significantly higher for tail length (OR = 6.36 [5.1–7.95]) and TM (OR = 5.77 [4.63–7.21]) ().
Table 2. Risk (OR) to observe values of comet parameters above the 75th percentile.
Multivariate analysis was applied to each variable that characterize the comet measures.
For the TM, the risk of observing values above the 75th percentile (0.42), adjusted for age and smoking was 4.61 [3.84–5.54]; for tail length, it was 4.61 (3.83–5.54); for tail DNA, it was 3.61 (3.02–4.30); for olive moment, it was 3.30 (2.76–3.94); and for tail area, it was 2.46 (2.06–2.94). The integral intensity was 1.35 (1.14–1.60). All of these risks are independent of the confounding factors considered while these factors have shown a differentiated influence among the various parameters. Age had an influence on TM, tail DNA, tail length and olive moment but did not affect tail area and integral intensity. Sex affected TM, integral intensity, tail length and olive moment. Smoke only affected the head DNA ().
Table 3. Adjusted risk estimates (ORs) by confounding variables (age, sex and smoking habits) by means of unconditional logistic regression models.
The specificity and the PPV of the comet test were related to the exposure to pesticides (and not to disease). The comet assay’s sensitivity to pesticides exposure (the ratio between the number of positive in the comet test for the exposure to pesticides [true positive] and all exposed to pesticides) was low (41%), and there were many false negatives among the exposed workers). The specificity was 89% with was very few false positives among unexposed subjects. The PPV of the test associated with pesticides exposure was 77% ().
Table 4. Sensitivity, specificity and predictive positive value (PPV) of the comet assay toward the pesticide exposure.
Discussion
Our study used the comet assay as a biomarker for exposure to genotoxic agents. To our knowledge, this is the first study that calculated the OR for comet assay parameters. One possible limitation of the study is the small numbers of subjects. However, many studies are performed with fewer subjects, e.g. 19 females and 12 males (Bausinger and Speit Citation2016) and 21 welders and 21 nonwelders (Villarini et al. Citation2015).
The results, in addition to confirming the genotoxicity of pesticides used by the study participants, suggest the utility of the comet assay in the biomonitoring of occupational exposure to genotoxic agents. Specifically, the TM and other parameters related to the comet “tail” (% tail DNA, tail length and tail area) that quantitatively express DNA damage showed higher values and a direct correlation to the exposure of subjects to pesticides.
Our findings agree with other studies on pesticides. Using the comet assay, Muhammad et al. (Citation2016) showed a strong correlation (R2 = 0.91) between DNA damage in terms of tail length and the blood concentration of malathion in workers involved in pesticide manufacturing industries. Agricultural workers involved in tobacco collection are regularly exposed to large quantities of pesticides; Alves et al. (Citation2016) demonstrated, through the comet assay and micronucleus test, that genetic damage and changes in oxidative balance were induced by workers’ exposure to complex mixtures of pesticides in the presence of inorganic compounds. DNA damage was investigated in the exfoliated buccal cells of workers exposed to pesticides in Guerrero, Mexico, using the comet assay and the micronucleus test: the results of the study revealed that DNA migration in the tail and the frequency of micronuclei increased significantly in the exposed group (Carbajal-Lòpez et al. Citation2016). Paddy farm workers chronically exposed to mixtures of organophosphates reported a significant increase in DNA damage, as assessed by measuring comet tail length in the exfoliated buccal mucosa (How et al. Citation2015). The comet assay technique has been used to highlight genotoxic effects in the lymphocytes of farmers exposed to pesticides, suggesting in particular the possible role of fungicides (Lebailly et al. Citation2015). The comet assay showed that both the frequency and index of DNA damaging and the index of damage were significantly higher in growers from Southern Brazil’s exposed to pesticide mixtures with genotoxic potential (Wilhelm et al. Citation2015). The comet assay showed that higher concentrations of individual pesticides (0.5–4.0 μM), but very low concentrations of pesticide mixtures caused significant DNA damage (Sultana Shaik et al. Citation2016). The comet assay was also used to confirm DNA damage in human peripheral blood lymphocytes after exposure to diazinon, an organophosphate pesticide, in agreement with the results of micronucleus (MN) and fluorescence in situ hybridization (FISH) assays (Gökalp Muranli et al. Citation2015). The comet assay is a valid test for human biomonitoring to assess the genotoxicity and carcinogenesis of various agents. In particular, the comet assay estimated that pesticide-exposed workers with the GSTP1 Ile-Ile and XRCC1399 Arg-Arg genotypes showed increased DNA damage (Saad-Hussein et al. Citation2017). The comet assay was also used to indicate that low level use of some conventional and green insecticides does not increase DNA damage (Zeljezic et al. Citation2017), while glyphosate, an important herbicide, may induce DNA damage in leucocytes (Kwiatkowska et al. Citation2017). Organophosphate and pyrethroid pesticides, in particular malondialdehyde (MDA), showed a positive correlation with DNA damage, via the comet assay, because they decrease the activity of antioxidant enzymes (Zepeda-Arce et al. Citation2017). DNA damage, as determined by the mean comet tail length, was high in women exposed to pesticides while picking cotton with bare hands, and a positive correlation of DNA damage with age and exposure time was observed (Ali et al. Citation2017). The comet assay showed that occupational exposure to pesticides was more dangerous than consumption of contaminated water, which was greater than controls; this difference between exposed and unexposed groups was not influenced by other factors, such as age or smoking or alcohol habits (Vazquez Boucard et al. Citation2017). The comet assay can also be useful in monitoring DNA damage in single cells due to exposure to chemotherapy and radiotherapy in cancer patients or to assess the cancer chemoprevention of some molecules (Santoro et al. Citation2016).
The comet assay appears to satisfy the need for a rapid, economical, noninvasive test to screen of genotoxic exposure in the workplace. The test also has many advantages in terms of ease of installation and rapid execution; it is generally very sensitive, but in our experience, it shows a low sensitivity (41%), with a high number of false negatives, and a high specificity (89%) with a low number of false positives. It can be applied to virtually any type of cell or tissue (Sasaky et al. Citation2000, Tice et al. Citation2000, Hartmann et al. Citation2003a, Citation2003b). For these reasons, numerous studies have been conducted (Neri et al. Citation2015) using the comet assay to monitor exposure to various genotoxic agents, predominantly in the workplaces (Gunasekarana et al. Citation2015), and to assess exposure to atmospheric pollution (Møller and Loft Citation2010). Jasso-Pineda et al. (Citation2015) used the comet assay to analyze DNA damage in children living in highly contaminated areas in Mexico: children exposed to high levels of polycyclic aromatic hydrocarbons and DDTs showed the highest levels of DNA damage in their blood cells.
However, age (Chen et al. Citation2007, Heuser et al. Citation2008), tobacco smoke (Al-Amrah et al. Citation2014, Rajmohan et al. Citation2015) and other factors, such as diet (Duthie et al. Citation1996, Astley et al. Citation1999), physical activity (Møller et al. Citation1996), infections (Tice et al. Citation1990), gender (Betti et al. Citation1994, Bonassi et al. Citation1995) and exposure to ultraviolet radiation (Frenzilli et al. Citation1998, Møller et al. Citation2002), may affect the test and may sometimes lead to discrepancies among the various studies. The comet assay also has limitations that must be considered for the correct interpretation of results: for example, it is not possible to detect, except at very high exposure levels, the action of genotoxic agents such as aneugenes, which are compounds capable of inducing aneuploidy through interactions with the mitotic melt; it is not possible to detect agents that reduce the availability of nucleotides for synthesis or the shelter of nucleic acids using the comet assay, nor can it detect so-called cross-linkers, which form cross-linked links between DNA and proteins or within DNA at the single strand level (intra-strand) or between the two filaments inter-strand (Grandi et al. Citation2006).
Another limitation of this test concerns cytotoxicity-related influences that result in DNA fragmentation in cells during necrosis or apoptosis, leading to the formation of comet-headed comets, called “ghost cells” that are routinely excluded from the analysis (Grandi et al. Citation2006). Finally, in the choice of biological substrates, monocytes exhibit a faster DNA damage kinetics than lymphocytes (Knudsen et al. Citation1992). The International Program on Chemical Safety (IPCS) has proposed guidelines for the use of the comet assay in the biomonitoring of populations exposed to occupational or environmental genotoxicity (Albertini et al. Citation2000). Reaffirming that the test cannot be considered predictive of the individual risk of manifesting neoplastic disease, the multiplicity of mechanisms that can induce genotoxicity makes fundamental definitions of exposure through environmental and/or biological monitoring and the possible confounding factors. In particular, for DNA repair mechanisms, special attention should be paid to the effects on Comet Assay results of DNA repair mechanisms inhibitors and to the analysis of genetic polymorphisms (Albertini et al. Citation2000). Other technical aspects that could modulate the results of the comet assay include the uptake times for biological samples and the test run in relation to the work shift (start of turn, at regular intervals during the turn or at the end of the turn) and the timing during the work week (beginning or end of the week) (Grandi et al. Citation2006). It was also noted that the inter-laboratory variation in DNA damage measurements via the comet assay was greater than intra-laboratory variation (Frenzilli et al. Citation1998). Analysis of the effect of all these variables on the results of the comet assay has allowed standardization of the protocol and its further validation (Hartmann et al. Citation2003a, Citation2003b; Burlinson et al. Citation2007; Valverde and Rojas Citation2009; Collins et al. Citation2014b; OECD Citation2014; Araldi et al. Citation2015; Neri et al. Citation2015).
Conclusion
In conclusion, in our experience, the comet assay is a quick, reliable, easy-to-use test of genotoxicity, with high specificity and a good PPV. In our experience, the test is not predictive of individual cancer risk, but is predictive of chronic exposure to pesticides. Validation of comet assay in the biomonitoring of cancer diseases could be interesting for future studies and should be considered in the design of large multi-centric studies with large sample size, various routes of exposure and analytical characterization of confounding factors.
Disclosure assessment
No potential conflict of interest was reported by the authors.
References
- Al-Amrah, H.J., et al., 2014. Genotoxicity of waterpipe smoke in buccal cells and peripheral blood leukocytes as determined by comet assay. Inhalation toxicology, 26 (14), 891–896.
- Albertini, R.J., et al., 2000. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans: international Programme of Chemical Safety. Mutation research, 463, 111–172.
- Ali, T., et al., 2017. Pesticide genotoxicity in cotton picking women in Pakistan evaluated using comet assay. Drug and chemical toxicology, 19, 1–8.
- Alves, J.S., et al., 2016. Investigation of potential biomarkers for the early diagnosis of cellular stability after the exposure of agricultural workers to pesticides. Annals of the Brazilian academy of sciences, 88 (1), 349–360.
- Anderson, D., et al., 2013. The comet assay in human biomonitoring. Methods in molecular biology, 1044, 347–362.
- Araldi, R.P., et al., 2015. Using the comet and micronucleus assays for genotoxicity studies: a review. Biomedicine and pharmacotherapy, 72, 74–82.
- Astley, S., et al., 1999. Vitamin E supplementation and oxidative damage to DNA and plasma LDL in type 1 diabetes. Diabetes care, 22, 1626–1631.
- Azqueta, A., et al., 2014. Comet assay to measure DNA repair: approach and applications. Frontiers in Genetics, 5, 288–288.
- Barnett, Y.A., and Barnett, C.R., 1998. DNA damage and mutation: contributors to the age-related alterations in T cell-mediated immune responses? Mechanisms of ageing and development, 102, 165–175.
- Bausinger, J., and Speit, G., 2016. The impact of lymphocyte isolation on induced DNA damage in human blood samples measured by the comet assay. Mutagenesis, 31 (5), 567–572.
- Benedetti, D., et al., 2013. Genetic damage in soybean workers exposed to pesticides: evaluation with the comet and buccal micronucleus cytome assays. Mutation research, 752 (1–2), 28–33.
- Betti, C., et al., 1994. Microgel electrophoresis assay (comet test) and SCE analysis in human. Mutation research, 307, 323–333.
- Bonassi, S., et al., 1995. Influence of sex on cytogenetic end point: evidence from a large human sample and review of the literature. Cancer epidemiology, biomarkers & prevention, 4, 671–679.
- Breslow, N.E., and Day, N.E., 1980. Adaptation of the logistic model to case-control studies. In: Statistical methods in cancer research: volume 1. The analysis of case-control studies. Lyon: IARC Scientific Publications, International Agency for Research on Cancer. ISBN: 92 832 0132 9.
- Burlinson, B., et al., 2007. Fourth International Workgroup on Genotoxicity testing: results of the in vivo comet assay workgroup. Mutation research, 627, 31–35.
- Burstyn, I., and De Roos, A.J., 2016. Visualizing the heterogeneity of effects in the analysis of associations of multiple myeloma with glyphosate use. Comments on Sorahan, T. Multiple Myeloma and Glyphosate Use: a re-analysis of US Agricultural Health Study (AHS) Data. International journal of environmental research and public health, 12, 1548–1559.
- Calviello, G., et al., 2006. DNA damage and apoptosis induction by the pesticide Mancozeb in rat cells: involvement of the oxidative mechanism. Toxicology and applied pharmacology, 211 (2), 87–96.
- Carbajal-López, Y., et al., 2016. Biomonitoring of agricultural workers exposed to pesticide mixtures in Guerrero state, Mexico, with comet assay and micronucleus test. Environmental science and pollution research, 23, 2513–2520.
- Chen, J., et al., 2007. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic acids research, 35, 7417–7428.
- Collins, A.R., et al., 1997. The comet assay: what can it really tell us? Mutation research, 375, 183–193.
- Collins, A.R., et al., 2001. Interindividual differences in repair of DNA base oxidation, measured in vitro with the comet assay. Mutagenesis, 16, 297–301.
- Collins, A.R., 2004. The comet assay for DNA damage and repair: principles, applications, and limitations. Molecular biotechnology, 26, 249–261.
- Collins, A.R., 2014a. Measuring oxidative damage to DNA and its repair with the comet assay. Biochimica Et Biophysica Acta, 1840 (2), 794–800.
- Collins, A., et al., 2014b. The comet assay as a tool for human biomonitoring studies: the ComNet project. Mutation research/reviews in mutation research, 759, 27–39.
- Da Silva, F.R., et al., 2012. Genotoxic biomonitoring of tobacco farmers: biomarkers of exposure, of early biological effects and of susceptibility. Journal of hazardous materials, 225–226, 81–90.
- Da Silva, F.R., et al., 2014. Genotoxic assessment in tobacco farmers at different crop times. The science of the total environment, 490, 334–341.
- De Roos, A.J., et al., 2005. Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study. Environmental health perspectives, 113 (1), 49–54.
- Dosemeci, M., et al., 2002. A quantitative approach for estimating exposure to pesticides in the agricultural health study. The annals of occupational hygiene, 46 (2), 245–260.
- Duthie, S.J., et al., 1996. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer research, 56, 1291–1295.
- Eastman, A., and Barry, M.A., 1992. The origins of DNA breaks: a consequence of DNA damage, DNA repair, or apoptosis? Cancer investigation, 10, 229–240.
- Frenzilli, G., et al., 1998. Comet assay on children’s leukocytes 8 years after the Chernobyl disaster. Mutation research, 415, 151–158.
- George, J., and Shukla, Y., 2013. Early changes in proteome levels upon acute deltamethrin exposure in mammalian skin system associated with its neoplastic transformation potential. The journal of toxicological sciences, 38 (4), 629–642.
- Geret, F., et al., 2013. Effects of low-dose exposure to pesticide mixture on physiological responses of the Pacific oyster, Crassostrea gigas. Environmental toxicology, 28 (12), 689–699.
- Gökalp Muranli, F.D., et al., 2015. Genotoxic effects of diazinon on human peripheral blood lymphocytes. Arhiv Za Higijenu Rada i Toksikologiju, 66, 153–158.
- Grandi, C., et al., 2006. Impiego del comet test in medicina del lavoro e tossicologia industriale: considerazioni e prospettive. Giornale Italiano Di Medicina Del Lavoro Ed Ergonomia, 28 (1), 5–13.
- Gunasekarana, V., et al., 2015. A comprehensive review on clinical applications of comet assay. Journal of clinical and diagnostic research, 9 (3), GE01–GE05.
- Hartmann, A., et al., 2003a. Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis, 18 (1), 45–51.
- Hartmann, A., et al., 2003b. Comparative study with the alkaline comet assay and the chromosome aberration test. Mutation research, 536, 27–38.
- Heuser, V., et al., 2008. Influence of age and sex on the spontaneous DNA damage detected by micronucleus test and comet assay in mice peripheral blood cells. Cell biology international, 32 (10), 1223–1229.
- How, V., et al., 2015. Characterization of risk factors for DNA damage among paddy farm worker exposed to mixtures of organophosphates. Archives environmental & occupational health, 70 (2), 102–190.
- Jackson, S.P., 2002. Sensing and repairing DNA double-strand breaks. Carcinogenesis, 23, 687–696.
- Jasso-Pineda, Y., et al., 2015. DNA damage in Mexican children living in high-risk contaminated scenarios. Science of the total environment, 518–519, 38–48.
- Kasiotis, K.M., et al., 2012. Monitoring of systemic exposure to plant protection products and DNA damage in orchard workers. Toxicology letters, 210 (2), 182–188.
- Kaur, R., et al., 2011. Evaluation of DNA damage in agricultural workers exposed to pesticides using single cell gel electrophoresis (comet) assay. Indian journal of human genetics, 17 (3), 179–187.
- Khayat, C.B., et al., 2013. Assessment of DNA damage in Brazilian workers occupationally exposed to pesticides: a study from Central Brazil. Environmental science and pollution research, 20 (10), 7334–7340.
- Knudsen, L.E., et al., 1992. Induction of DNA repair synthesis in human monocytes/B-lymphocytes compared with T-lymphocytes after exposure to N93 acetoxy-N-acetylaminofluorene and dimethylsulfate in vitro. Carcinogenesis, 13, 1285–1287.
- Krejci, L., et al., 2003. Mending the break: two DNA double-strand break repair machines in eukaryotes. Progress in nucleic acid research and molecular biology, 74, 159–201.
- Kvitko, K., et al., 2012. Susceptibility to DNA damage in workers occupationally exposed to pesticides, to tannery chemicals and to coal dust during mining. Genetics and molecular biology, 35 (4 suppl), 1060–1068.
- Kwiatkowska, M., et al., 2017. DNA damage and methylation induced by glyphosate in human peripheral blood mononuclear cells (in vitro study)). Food and chemical toxicology: an international journal published for the British industrial biological research association, 105, 93–98.
- Lebailly, P., et al., 2015. DNA damage in B and T lymphocytes of farmers during one pesticide spraying season. International archives of occupational and environmental health, 88, 963–972.
- Lindahl, T., and Andersson, A., 1972. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry, 11, 3618–3623.
- Møller, P., et al., 1996. Oxidative stress associated with exercise, psychological stress and life-style factors. Chemico-biological interactions, 102, 17–36.
- Møller, P., and Wallin, H., 1998. Adduct formation, mutagenesis and nucleotide excision repair of DNA damage produced by reactive oxygen species and lipid peroxidation products. Mutation research, 410, 271–290.
- Møller, P., et al., 2002. Sunlight-induced DNA damage in human mononuclear cells. Faseb journal, 16 (1), 45–53.
- Møller, P., and Loft, S., 2010. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environmental health perspectives, 118 (8), 1126–1136.
- Muhammad, A., et al., 2016. Biomonitoring of toxic effects of pesticides in occupationally exposed individuals. Safety and health at work, 7, 156–160.
- Neri, M., et al., 2015. Worldwide interest in the comet assay: a bibliometric study. Mutagenesis, 30 (1), 155–163.
- Nowsheen, S., et al., 2012. Assaying DNA damage in hippocampal neurons using the comet assay. Journal of visualized experiments, 70, e50049.
- OECD. 2014. Test No. 489: In vivo mammalian alkaline comet assay. http://dx.doi.org/10.1787/9789264224179-en.
- Rajmohan, M., et al., 2015. In vivo autofluorescence spectroscopic study and evaluation of DNA damage by comet assay in smokers. Journal of clinical and diagnostic research, 9 (5), ZC16–ZC19.
- Rojas, E., et al., 1999. Single cell gel electrophoresis assay: methodology and applications. Journal of chromatography: B, biomedical sciences and applications, 722, 225–254.
- Saad-Hussein, A., et al., 2017. GSTP1 and XRCC1 polymorphisms and DNA damage in agricultural workers exposed to pesticides. Mutation research, 819, 20–25.
- Santoro, R., et al., 2016. Comet assay in cancer chemoprevention. Methods in molecular biology, 1379, 99–105.
- Sasaky, Y.F., et al., 2000. The comet assay with multiple mouse organs: comparison of comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and US NTP Carcinogenicity database. Critical reviews in toxicology, 30, 629–799.
- Sies, H., 1985. Oxidative stress: introductory remarks. London: Academic Press; 1, 8.
- Simoniello, M.F., et al., 2010. Biomarkers of cellular reaction to pesticide exposure in a rural population. Biomarkers, 15 (1), 52–60.
- Singh, N.P., et al., 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental cell research, 175 (1), 184–191.
- Singh, N.P., 2016. The comet assay: reflections on its development, evolution and applications. Mutation research - reviews in mutation research, 767, 23–30.
- Sultana Shaik, A., et al., 2016. Evaluation of cytotoxicity and genotoxicity of pesticide mixtures on lymphocytes. Toxicology mechanisms and methods, 26 (8), 588–594.
- Thakur, S., et al., 2017. APE1 modulates cellular responses to organophosphate pesticide-induced oxidative damage in non-small cell lung carcinoma A549 cells. Molecular and cellular biochemistry, [Epub ahead of print]. doi: 10.1080/19338244.2014.910489.
- Tice, R.R., et al., 1990. The single cell gel assay: a sensitive technique for evaluating intercellular differences in DNA damage and repair. Basic life science, 53, 291–301.
- Tice, R.R., et al., 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environmental and molecular mutagenesis, 35, 206–221.
- Valverde, M., and Rojas, E., 2009. Environmental and occupational biomonitoring using the comet assay. Mutation research, 681 (1), 93–109.
- Van Gent, D.C., et al., 2001. Chromosomal stability and the DNA double-stranded break connection. Nature reviews genetics, 2, 196–206.
- Varona-Uribe, M.E., et al., 2016. Exposure to pesticide mixtures and DNA damage among rice field workers. Archives of environmental & occupational health, 71 (1):3–9.
- Vazquez Boucard, C., et al., 2017. A study of DNA damage in buccal cells of consumers of well- and/or tap-water using the comet assay: assessment of occupational exposure to genotoxicants. Environmental and Molecular Mutagenesis, 58 (8), 619–627.
- Villarini, M., et al., 2015. Primary DNA damage in welders occupationally exposed to extremely-low-frequency magnetic fields (ELF-MF). Ann ig, 27 (3), 511–519.
- Wang, Q.L., et al., 2013. Risk assessment of mouse gastric tissue cancer induced by dichlorvos and dimethoate. Oncology letters, 5 (4), 1385–1389.
- Waters, D.J., et al. 2007. Non-invasive prediction of prostatic DNA damage by oxidative stress challenge of peripheral blood lymphocytes. Cancer epidemiology biomarkers & prevention, 16(9), 1906–1910.
- Wilhelm, C.M., et al., 2015. Assessment of DNA damage in floriculturists in southern Brazil. Environmental science and pollution research international, 22, 8182–8189.
- Zeljezic, D., et al., 2017. The effect of insecticides chlorpyrifos, α-cypermethrin and imidacloprid on primary DNA damage, TP 53 and c-Myc structural integrity by comet-FISH assay. Chemosphere, 182, 332–338.
- Zepeda-Arce, R., et al., 2017. Oxidative stress and genetic damage among workers exposed primarily to organophosphate and pyrethroid pesticides. Environmental toxicology, 32 (6), 1754–1764.