Abstract
Purpose: Nonconventional vapor products (NVP), designed to reduce exposure to cigarette smoke toxicants (CSTs), could cause changes in biomarkers of potential harm (BoPH). Although, NVPs reduced CSTs exposure compared to conventional cigarettes (CC), the changes in the BoPH values varied among the studies. Hence, further information on BoPH using NVPs is needed.
Material and methods: The data of two similarly designed studies using a kind of NVP, a noncombustion and nonheating inhaler type of smokeless tobacco product (NCIT) used under 31-day confinement, were pooled, and the differences in 15 BoPH between smokers and nonsmokers at baseline and between the 1 mg tar CC (CC1) group and NCIT group at Day 28/29 were analyzed.
Results: At baseline, the levels of eight BoPH (red blood cells, white blood cells, 8-epi-prostaglandin F2α, 8-hydroxy-2′-deoxyguanosine, malondialdehyde, 11-dehydrothromboxane B2, total cholesterol and glucose) were significantly different between smokers and nonsmokers. At Day 28/29, the levels of six BoPH were significantly different between NCIT and CC1 (8-epi-prostaglandin F2α, malondialdehyde, 11-dehydrothromboxane B2: CC1 > NCIT, total bilirubin, low-density lipoprotein cholesterol and total cholesterol: CC1 < NCIT).
Conclusions: Reduced exposure to CSTs has favorable effects on BoPH associated with oxidative stress, antioxidant capacity and platelet activation/coagulation but not in lipid metabolism.
Introduction
It has been reported that cigarette smoking is associated with an increased risk of several diseases such as cancers in the oral cavity, stomach and lungs, pulmonary diseases and cardiovascular diseases (CVD) (Office of the Surgeon et al. Citation2004). In particular, the pathophysiology underlying atherosclerosis associated with cigarette smoking has been well documented; it is believed that the changes in endothelial functions, cholesterol metabolism and platelet functions and the induction of oxidative stress and inflammation are involved (Howard et al. Citation1998, Ambrose and Barua Citation2004).
Some companies, including tobacco companies, have been developing a nonconventional vapor product (NVP) such as heat-not burn tobacco products and electric cigarette with the aim of reducing the risks associated with cigarette smoking, according to the reports of the regulatory authorities (IOM Citation2001, U.S. Family Smoking Prevention and Tobacco Control Act). The FDA (Citation2012a) recommended that human studies should be conducted to assess the human health risks related to the use of the tobacco product, including exposure to cigarette smoke constituents (e.g. biomarkers of exposure [BoE]) and health outcomes (e.g. disease incidence or mortality).
In order to assess health outcomes, studies to identify biomarkers of potential harm (BoPH) associated with oxidative stress, inflammation, lipid metabolism, glucose metabolism and pulmonary functions have been conducted using smokers (SM) and nonsmokers (NS) (Burchfiel et al. Citation1995, Prieme et al. Citation1998, Eliasson et al. Citation2001, Minami et al. Citation2002, Polidori et al. Citation2003, Flores et al. Citation2004, Wannamethee et al. Citation2005, Halvorsen et al. Citation2007, Ludicke et al. Citation2015, Ogden et al. Citation2015). Although, there are many clinical studies on BoE using NVPs, there are few studies on BoPH associated with smoking using NVPs (Roethig et al. Citation2008, Ogden et al. Citation2015, Ludicke et al. Citation2018). In addition, although, some studies have found that exposure to cigarette smoke toxicants (CSTs) with NVPs were significantly reduced compared to conventional cigarettes (CC), the changes in BoPH varied among the studies. One reason for the inconsistency between the reduced BoE and the change in BoPH might be due to the ambulatory setting that could have influenced BoPH levels. The ambulatory setting brings various factors into play such as diet, exercise and environment. The confinement study design eliminates any concerns regarding compliance with assigned product use and environmental impact, thus, this approach is preferable when assessing NTVs in terms of the effect of reduced cigarette smoke exposure on BoPH.
The novel noncombustion and nonheating inhaler type of smokeless tobacco product (NCIT), a kind of NVP consisting of a tapered mouthpiece and cartridge filled with finely cut tobacco leaves, has been developed. The flavor components, including nicotine, can be delivered from the tobacco leaves not by burning or heating but just by the air flow when users inhale through its mouthpiece. Chemical analysis of NCIT vapor found that most measured cigarette smoke constituents except nicotine were below the quantifiable level. We previously conducted two confinement clinical studies (Study 1: UMIN000004334 and Study 2: UMIN000006215) in which healthy adult Japanese smokers switched from smoking 1 mg tar CC (CC1) to NCIT use during the study. The results showed that switching to NCIT use significantly reduced some BoEs selected as harmful and potentially harmful constituents (HPHCs) in CC smoke (FDA Citation2012b), and mutagens detected by the urine mutagenicity testing in the NCIT group were similar to those in the NS group (Kakehi et al. Citation2012, Ohara et al. Citation2014, Miura et al. Citation2015). Although, the exposure level other than nicotine in NCIT use is similar to that of NS, switching from smoking CC1 to NCIT use did not show marked effects on BoPH levels in either study.
In this article, the effect of reduced exposure to cigarette smoke on BoPH by using NCIT for 29 days with the statistical power enhanced by integrating the data of two clinical studies (Studies 1 and 2) under a clinically controlled setting is presented. We also compared the BoPH levels for smokers who switched to NCIT use with those of nonsmokers. In addition, we present a comparison between SM and NS at baseline.
In the current study, we analyzed plasma cotinine and carboxy-hemoglobin (CoHb) as primary BoE. We also analyzed the commonly used BoPH which are related to the aforementioned pathophysiology of arteriosclerosis: hematology (red blood cell: RBC, white blood cell: WBC), oxidative stress (urine 8-iso-prostaglandin F2α: 8-epi-PGF2α, 8-hydroxy-2′-deoxyguanosine: 8-OHdG and malondialdehyde: MDA), antioxidant capacity (total bilirubin: T-bil), inflammation (fibrinogen: FIB, and high sensitive-C reactive protein: hs-CRP), platelet activation/coagulation (urine 11-dehydrothromboxane B2: 11-DHTXB2), endothelial function (soluble intercellular adhesion molecule-1: sICAM-1), lipoprotein metabolism (high-density lipoprotein-cholesterol: HDL-C, low-density lipoprotein: LDL-C), total cholesterol: TC and glucose metabolism (Glucose: GLU, hemoglobin A1C: HbA1c).
Clinical significance
Although, NVPs showed reduced exposure to CSTs, the changes in BoPH varied among the studies.
This study was conducted under confinement to exclude as much as possible subject compliance bias in the use of the assigned product and other factors considered to influence BoPH levels.
The comparison of SM and NS at baseline BoPH levels basically agreed with the findings of our previous study and other reports.
Switching to NCIT use for 29 days, it was shown that the levels of some BoPH associated with oxidative stress, antioxidant capacity and platelet activation/coagulation approached NS levels.
Material and methods
Study design
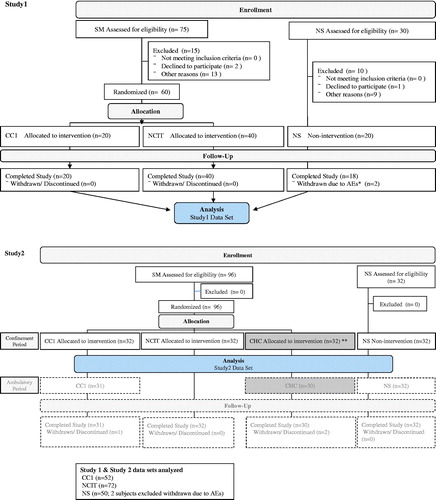
These two studies were controlled (31-day confinement), open-label studies and were conducted at four clinical research centers in a randomized manner for SM and in a nonrandomized manner for NS: Studies 1 and 2 at Hakata Hospital (Fukuoka, Japan), the OCROM Clinic (Osaka, Japan), Maruyama Hospital (Shizuoka, Japan) and Sumida Hospital (Tokyo, Japan). All studies were performed in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki; participating volunteers gave written informed consent and the studies were approved by each institutional ethics committee.
Study 1: A randomized (nonrandomized for NS), controlled, 31-day confinement study (smoked or used the designated tobacco products for 29 days) with three arms consisting of a menthol type of NCIT, CC1 and NS. (Number of subjects: SM 60 [NCIT 40, CC1 20], NS 20)
Study 2: A randomized 31-day confinement study (smoked or used the designated tobacco products for 29 days), and a further 29-day ambulatory period with four arms consisting of a regular type of NCIT, carbon-heated cigarette (CHC), CC1 and NS. (Number of subjects: SM 96 [NCIT 32, CHC 32, CC1 32], NS 32). The CHC data and the data from the subsequent 29-day ambulatory period in Study 2 were not used in this combined analysis.
NCIT group and CC1 group were confined in different areas to exclude potential for secondhand CS exposure to NCIT group.
Subjects
The CONSORT flow diagram shows the progress through the phases of the study. In both studies, healthy Japanese males (SM and NS), aged 21–49 years and having a body mass index (BMI) in the range of 18.5–25.0 kg/m2 were enrolled. SMs who reported smoking at least 20 cigarettes (1 mg tar value, printed on the package: Study 1 non-menthol or menthol cigarette; Study 2 non-menthol cigarette) per day for more than one year, and the same brand for at least eight weeks preceding the screening period were recruited. A total of 233 male subjects were enrolled, and 203 subjects completed the studies.
Procedure
Marker measurements
All measurements were conducted by the LSI Medience Corporation (Tokyo, Japan) using validated methods.
BoE: COHb levels in whole blood were measured by the cyanmethemoglobin method using a microplate reader (Okuzono et al. Citation1976). Plasma cotinine levels were measured by liquid chromatography–tandem mass spectrometry (LC–MS/MS) (Miura et al. Citation2013). COHb was measured on Day 0 and Day 28, and plasma cotinine was measured on Day 1 and Day 29.
BoPH: Routine hematology (WBC and RBC) and clinical chemistry (HbA1c, hs-CRP, FIB, GLU, HDL-C, LDL-C, TC and T-bil) were analyzed using validated analytical methods with appropriate quality controls. Urine MDA levels were measured by high-performance liquid chromatography according to the method of Korchazhkina et al. (Citation2003). Urine 8-epi-PGF2α, 8-OHdG, 11-DHTXB2, plasma sICAM-1 levels were determined by microplate enzyme immunoassays using their respective kits: 8-isoprostane EIA kit (Cayman Chemical, Ann Arbor, MI, USA); New 8-OHdG Check ELISA kit (Japan Institute for the Control of Aging, Fukuroi, Japan); 11-dehydro-Thromboxane B2 Enzyme Immunoassay Kit (Assay designs, Enzo Life Sciences, Inc, NY, USA); human sICAM-1 ELISA. Kit (Bender MedSystems, Vienna, Austria). BoPH was measured on Day 0, 1, 28, 29.
Test tobacco products
The CC1 for both Studies 1 and 2 is one of the most popular brands in Japan. It has package-printed values of 1.0 mg tar and 0.1 mg nicotine, which are based on the measurement of tar and nicotine under ISO machine smoking conditions (International Organization for Standardization Citation2000).
NCIT for both Study 1 (mint flavor) and Study 2 (regular flavor) were shown to contain 0.0006 mg of nicotine per puff based on an average of 1920 puffs per one cartridge under a machine smoking regimen, which was set referring to the Health Canada Intense method for cigarette machine smoking conditions (Health Canada Citation1999): 55 ml puff volume, 2-s duration and 30-s interval. Tar was not detected under this condition. Detailed results of the chemical measurement were provided in the previous report (Ohara et al. 2014, Miura et al. Citation2015).
Statistical analysis
The data from the 31-day confinement studies (smoked or used the designated tobacco products for 29 days) from two similarly designed studies were pooled to increase statistical power. The population for statistical analysis included the 174/176 (98.8%) subjects from the two clinical studies with a similar design who had complete data at all assessment points.
Differences between CC1 and NCIT for COHb on Day 28 and plasma cotinine on Day 29 were performed using nonparametric Wilcoxon rank sum test. For BoPH analysis, the average of Day 0 and Day 1 per group was used as baseline values for comparison between SM and NS. The differences at baseline between SM and NS were evaluated by performing an analysis of variance (ANOVA). The ANOVA model included Study, Group and Study*Group interaction. The average of Day 28 and Day 29 (Day 28/29) per group was used for comparison between NCIT group and CC1 group. The differences at Day 28/29 were evaluated by performing an analysis of covariance (ANCOVA). The ANCOVA model included Study, Group, Study*Group interaction and Baseline as covariate. If the Study*Group interaction was not statistically significant in these models, the interactions from both analyses were pooled.
Statistical significance was based on a 2-sided significance level of 0.05. SAS for Windows (SAS Institute, Cary, NC, USA) was used for conducting the statistical analyses.
Results
Study population
Among the 233 healthy males enrolled in Studies 1 and 2, 124 SM (CC1 group and NCIT group) and 50 NS were eligible for this data analysis (CONSORT flow diagram). The demographics and baseline characteristics at screening are shown in . The demographics of age, BMI, smoking duration and the daily cigarette consumption are generally similar between CC1 group and NCIT group in SM. The age of SM was generally similar to that of NS and the BMI of SM was slightly lower than that of NS.
Table 1. Demographics and baseline characteristics at screening, according to group.
Biomarkers
Biomarkers of exposure
COHb and plasma cotinine levels are shown in . At baseline, SM (before being assigned to the NCIT group or CC1 group) smoked their usual brand of cigarettes with 1 mg ISO tar delivery.
Table 2. Carboxyhemoglobin and plasma cotinine, according to group.
Although, the median level of COHb was higher in SM compared to NS, there was no marked difference between the CC1 and NCIT groups at baseline (). At Day 28, the median level of COHb in the NCIT group was significantly lower than that in the CC1 group (CC1: 4.86%, NCIT: 1.43%; p < 0.0001), which was close to that of the NS group (NS: 0.86%).
Although, the median level of plasma cotinine was higher in SM compared to NS, there was no marked difference between the CC1 group and the NCIT group at baseline (). At Day 29, the median level of plasma cotinine in the NCIT group was significantly lower than that in the CC1 group. The median level of plasma cotinine in the NCIT group was clearly higher than that in the NS group (NCIT: 9.92 ng/ml, NS: 0.25 ng/ml).
Biomarkers of potential harm
BoPH levels at baseline in SM compared with NS are shown in . BoPH levels between CC1 and NCIT were similar at baseline (). Comparison of BoPH between the NCIT group and the CC1 group at Day 28/29 are shown in .
Table 3. Hematology biomarkers and biomarkers related to cardiovascular diseases (Arithmetic means [SE] for baseline).
Table 4. Hematology biomarkers and biomarkers related to cardiovascular diseases, according to group (Arithmetic means [SE] for baseline and Day 28/29).
Hematology (RBC and WBC) results for all participants were within the normal reference range for males in the age range of this study. The RBC level was significantly lower in SM compared to NS at baseline (p = 0.0373; ). At Day 28/29, no difference was observed between the NCIT group and the CC1 group (). At baseline, the WBC level was significantly higher in SM compared to NS (p < 0.0001; ). The WBC levels in the NCIT and CC1 groups decreased at Day 28/29, and the difference in the levels between them did not reach the significance level ().
The levels of urine 8-epi-PGF2α, 8-OHdG and MDA were significantly higher in SM compared to NS at baseline (p < 0.0001, p = 0.0002, p = 0.0011, respectively) (). At Day 28/29, the level of 8-OHdG was not significantly different between the NCIT group and CC1 group (p = 0.4914), but the levels of 8-epi-PGF2α and MDA were significantly lower in the NCIT group compared to the CC1 group (p = 0.0484, p = 0.0369, respectively), and the levels of 8-epi-PGF2α and MDA in the NCIT group were similar to those seen in the NS group at Day 28/29 ( and ).
Figure 1. Biomarkers related to cardiovascular diseases according to group (mean ± SE). CC1: smoke 1 mg ISO tar conventional cigarette; NCIT: use noncombustion inhaler type of tobacco product; NS: nonsmokers; SE: standard error.
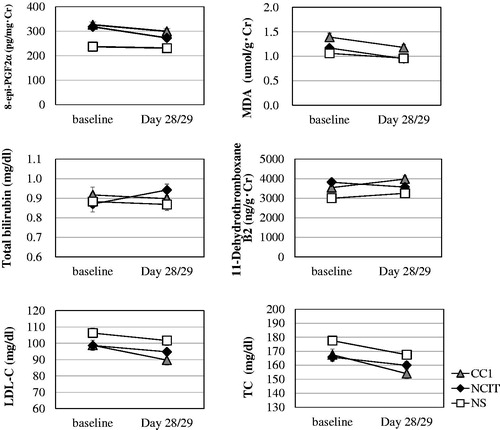
The level of total bilirubin (T-bil) did not differ significantly between SM and NS at baseline (p = 0.8473; ). The level of T-bil was significantly higher in the NCIT group compared to the CC1 group (p = 0.0375), and that in the NCIT group was higher than that of NS at Day 28/29 ( and ).
There was no difference in levels of hs-CRP and FIB, either between SM and NS at baseline (p = 0.4225 and 0.647, respectively) () or between the NCIT and CC1 groups at Day 28/29 (p = 0.2118, p = 0.9695, respectively; ).
The level of urine 11-DHTXB2 was significantly higher in SM compared to NS at baseline (p < 0.0001; ). There was a significant difference between the NCIT group and the CC1 group (p < 0.0001) at Day 28/29 (). Moreover, the level in the NCIT group was similar to that in the NS group at Day 28/29 ().
There was no significant difference in the sICAM-1 level, either between SM and NS at baseline (p = 0.3235; ) or between the NCIT and CC1 groups at Day 28/29 (p = 0.2332; ).
The level of HDL-C did not differ significantly between SM and NS at baseline, but tended to be higher in NS compared to SM (p = 0.0512; ). It was not significantly different between the NCIT group and the CC1 group at Day 28/29 (p = 0.4293; ). The level of LDL-C was not significantly different between SM and NS at baseline (p = 0.1002; ). It was significantly higher in the NCIT group compared to the CC1 group at Day 28/29 (p = 0.0286), and the level in the NCIT group was similar to that of the NS group (, ). The level of TC was significantly lower in SM compared to NS at baseline (p = 0.0174; ), but was significantly higher in the NCIT group compared to the CC1 group (p = 0.0110) at Day 28/29 (, ). The level in the NCIT group approached that of the NS group at Day 28/29.
The levels of GLU were significantly lower in SM compared to NS at baseline (p = 0.003; ), but there was no difference between the NCIT and CC1 groups at Day 28/29 (p = 0.932; ). The level of HbA1c did not differ between SM and NS at baseline (p = 0.4436; ) or between the NCIT and CC1 groups (p = 0.1611) at Day 28/29 ().
Discussion
Nonconventional vapor products (NVP), such as heat-not burn tobacco products and electric cigarette, are designed to reduce exposure to CSTs. NCIT, a kind of NVP, is a new form of smokeless (noncombustion and nonheating) tobacco product that consists of a tapered mouthpiece and cartridge filled with finely cut tobacco leaves. In previous studies, NCIT has been shown to reduce exposure to cigarette smoke constituents, and the exposure levels to some cigarette smoke constituents except nicotine in the NCIT group were found to be similar to those in the NS group (Ohara et al. 2014, Miura et al. Citation2015). In other words, the exposure level to cigarette smoke constituents by NCIT use is considered to be close to the level of smoking cessation compared with that of other NVPs that need to heat tobacco leaves.
Differences in BoPH between SM and NS at baseline
At baseline, the levels of RBC, WBC, 8-epi-PGF2α, 8-OHdG, MDA, 11-DHTXB2, TC and GLU were significantly different between SM and NS. These findings are consistent with many reports (Harman et al. Citation2003, Garg et al. Citation2006, Kawada et al. Citation2006, Calapai et al. Citation2009, Frost-Pineda et al. Citation2011, Ludicke et al. Citation2015). We previously conducted a comparison study between SM and NS in 396 Japanese subjects (UMIN000021921), and found a statistically significant difference in RBC (SM: 455 × ^410/μl, NS: 466 × ^410/μl), WBC (SM: 64.1 × ^210/μl, NS: 51.7 × ^210/μl), urine 8-epi-PGF2α (SM 370 pg/mg·cre, NS: 299 pg/mg·cre), urine 8-OHdG (SM: 7.55 ng/mg·cre, NS: 6.76 ng/mg·cre), urine MDA (SM: 1.10 μmol/g·cre, NS: 0.821 μmol/g·cre), serum T-bil (SM: 0.652 mg/dl, NS: 0.818 mg/dl), urine 11-DHTXB2 (SM: 2484 ng/g·cre, NS: 1739 ng/g·cre), sICAM-1 (SM: 419 ng/ml, NS: 324 ng/ml), HDL-C (SM: 65.4 mg/dl, NS: 72.4 mg/dl), TC (SM: 183 mg/dl, NS: 192 mg/dl) and HbA1c (SM: 4.70%, NS: 4.76%), whereas hs-CRP (SM: 0.0513 mg/dl, NS: 0.0460 mg/dl; p = 0.184); FIB (SM: 309 mg/dl, NS: 296 mg/dl; p = 0.087); LDL-C (SM: 106 mg/dl, NS: 111 mg/dl; p = 0.057) were statistically insignificant (Bito et al. Citation2011).
In the present study, there was no significant difference in the levels of hs-CRP, FIB, sICAM-1, LDL-C, HDL-C, HbA1c and T-bil in SM and NS at baseline and significant differences in hs-CRP, FIB, LDL-C, sICAM-1 and HDL-C have been reported in some studies including our previous comparison study (Calapai et al. Citation2009, Bito et al. Citation2011, Frost-Pineda et al. Citation2011, Ludicke et al. Citation2015, Ogden et al. Citation2015), the reason for the poor reproducibility of these BoPH when SM and NS are compared is currently not clear. However, from the results obtained, the levels of many BoPH in the present study are consistent with the findings of our previous study and other reports. These biomarkers could be major indicators of BoPH associated with smoking.
Differences in BoPH at Day 28/29
The level of HDL-C was not significantly different between the NCIT group and the CC1 group (p = 0.4293). Although, three previous-reported clinical studies showed that there was a statistically significant increase of HDL-C in the NVP (Electrically Heated Cigarette Smoking System (EHCSS/EHCSS-K6/electric cigarette) group compared with the CC group (Roethig et al. Citation2008, Cravo et al. Citation2016, Ludicke et al. Citation2018), other studies did not show this (Ogden et al. Citation2015, Walele et al. Citation2018). Therefore, the changes in HDL-C levels after switching to NVPs were not consistent. A model developed by Frost-Pineda et al. (Citation2011) showed that the most important factor influencing HDL-C is BMI. In the present study, BMI values decreased in all groups (CC1, NCIT, NS) during the study period (from baseline to Day 28/29; CC1: from 21.0 kg/m2 to 20.8 kg/m2, NCIT: from 20.9 kg/m2 to 20.6 kg/m2, NS: from 22.0 kg/m2 to 21.5 kg/m2). Therefore, BMI reduction brought about by changes in diet (2300 kcal/day) in a controlled setting might have hidden the change in HDL-C.
It is well known that higher LDL-C and/or TC level in blood is associated with an increased risk for CVDs (Sharrett et al. Citation2001, U.S. Department of Health and Human Service Citation2010). In the current study, the levels of TC and LDL-C were significantly higher in the NCIT group compared to the CC1 group at Day 28/29. In other clinical studies carried out in an ambulatory setting using NVPs, a significant change in LDL-C and TC has not been reported in NVP groups (Cravo et al. Citation2016, Ludicke et al. Citation2018, Walele et al. Citation2018). It should be noted that these levels in the NS group were higher than those of the SM group at baseline, and the higher TC level in NS compared with SM is consistent with the results of our previous comparison study (UMIN000021921) (Bito et al. Citation2011). Additionally, the levels of LDL-C and TC in the NCIT group at Day 28/29 approached those of NS (LDL-C; CC1: 89.71 mg/dl, NCIT: 94.75 mg/dl, NS: 101.69 mg/dl, TC; CC1: 154.00 mg/dl, NCIT: 159.99 mg/dl, NS: 167.50 mg/dl) (), but the levels of LDL-C and TC in all groups (CC1, NCIT and NS) remained within the normal clinical reference range. This study and our previous study (UMIN000021921) (Bito et al. Citation2011) revealed that at least for Japanese SM, the level of HDL-C shows a lower or lower tendency and the levels of LDL-C and TC show higher or higher tendency than NS. In this study, the level of TC decreased when taking a ruled meal and being under confinement, and the levels of LDL-C and HDL-C decreased with decreasing in the level of TC. Thus, the levels of cholesterol related markers would be affected by diet and other living conditions, so it is necessary to pay sufficient attention when using them as BoPH.
In the controlled clinical study using NVP (EHCSS-K6), a significant reduction in the WBC count was observed within three days (Roethig et al. Citation2010). Although, the present study was also a controlled study, the WBC level was not significantly different between the NCIT group and CC1 group at Day 28/29. The inconsistency with the other controlled study might be associated with the tar content of the CC used. Subjects who regularly smoked CC1, which has the lowest level tar in commercially available CC in Japan, were recruited in this study. Our previous study showed that the WBC level was affected by the tar content in the cigarette that the subject smoked regularly (p = 0.020) (UMIN000021921) (Bito et al. Citation2011). By conducting a multiple regression analysis, that study showed the effect of personal characteristics on the WBC level as follows: p value; sex: 0.709; age: 0.571; BMI: 0.013; high-fat food intake score: 0.747; caffeine intake score: 0.7222; alcohol intake score: 0.879; number of cigarettes smoked per day <0.001; tar content in the cigarettes that the subject smoked regularly; 0.020. Moreover, Frost-Pineda et al. (Citation2011) indicated that factors other than smoking history such as BMI, race, age and gender significantly influenced the WBC count, and among them, the most important factor was BMI. On the other hand, in an ambulatory clinical study in which smokers switched to NVPs (Eclipse/EHCSS/EHCSS-K6/electric cigarette), no consistent change in the WBC count was observed (Roethig et al. Citation2008, Martin Leroy et al. Citation2012, Cravo et al. Citation2016, Ludicke et al. Citation2018). A literature search of 15 smoke cessation studies by Scherer (Citation2018) estimated that the period required for the WBC to recover to the NS level was almost two years after quitting smoking. Our result and the other reports suggest that reduced cigarette smoke exposure may have effects on the change in the WBC count, but the full recovery of WBC to NS levels takes a significant period of time.
In the present study, there was no significant difference in the sICAM-1 level between SM and NS at baseline nor between the NCIT group and CC1 group at Day 28/29. In the previous studies comparing SM and NS, the levels of sICAM-1 were significantly different between SM and NS (Bito et al. Citation2011, Ludicke et al. Citation2015, Ogden et al. Citation2015). In an ambulatory clinical study in which SM switched to NVPs (Eclipse/menthol Tobacco Heating System [mTHS]), a significant reduction in sICAM-1 was observed within three month in two studies (Ogden et al. Citation2015, Ludicke et al. Citation2018). A literature search of seven smoking cessation studies performed by Scherer (Citation2018) estimated that the time needed for the sICAM-1 level to recover to the NS level was about eight months after quitting smoking. The inconsistency between this study result and the previous studies comparing SM and NS and the other studies using NVPs are thought to be due to the difference in the CC tar content of the CC used and/or number of CC smoked per day in these studies, similar to the results obtained for WBC. Thus, whether sICAM-1 qualifies as a BoPH requires further study.
According to some reports (Roethig et al. Citation2008, Martin Leroy et al. Citation2012, Ogden et al. Citation2015, Ludicke et al. Citation2018), the levels of hs-CRP and FIB did not change significantly when SM switched to NVPs, which was consistent with the results obtained in the present study.
8-epi-PGF2α is one of the lipid peroxidation products formed from arachidonic acid by a nonenzymatic, free-radical-initiated process and is not only an indicator of oxidative stress and lipid peroxidation but is also a biologically active substance (Janssen Citation2001, Cracowski et al. Citation2002). MDA is also a lipid peroxidation product formed from polyunsaturated fatty acids, such as arachidonic acid, and, is regarded as an indicator of oxidative stress (Tsikas Citation2017). In the present study, concerning the levels of 8-epi-PGF2α and MDA, significant changes in SM switched to NCIT use were observed and these levels approached the levels of NS. The change of 8-epi-PGF2α was somewhat controversial; in three studies (Roethig et al. Citation2008, Ogden et al. Citation2015, Ludicke et al. Citation2018), a significant decrease after switching to NVPs (EHCSS/THS2.2) was found, while in two other studies (Martin Leroy et al. Citation2012, Ogden et al. Citation2015), it was not significant. The reason for the inconsistency is unclear because there was a lack of uniformity in the test conditions (e.g. study duration, confinement/ambulatory condition). In a literature review, Scherer (Citation2018) stated that there was uncertainty regarding the recovery time of 8-epi-PGF2α after quitting smoking. Moreover, another literature search by Peck et al. (Citation2018) indicated that there was no substantial evidence of a strong direct link between 8-iso-PGF2a and smoking cessation except where a long period of time had elapsed. Our results and the other reports suggest that a reduction in cigarette smoke exposure affects the level of 8-epi-PGF2α, but the recovery time to NS levels has still not been clarified. The change in the level of MDA after switching to NCIT was statistically significant in the present study. Although, there are few reports on MDA using NVPs, one study showed a significant change after 28 days of NVP use (Ikonomidis I et al. Citation2018). Therefore, our results and the 28-day NVP use study suggest that reduced cigarette smoke exposure, a condition close to the smoking cessation level, have effects on the change in MDA.
Bilirubin is a potent antioxidant (Stocker et al. Citation1987), and concentrations have been found to have an inverse association with the risk of CVD, stroke and metabolic syndrome (Kimm et al. Citation2009, Lin et al. Citation2009). Although, studies on T-bil comparing SM and NS, including our previous comparison study, showed that the levels were statistically significantly lower in the SM group compared to the NS group (Van Hoydonck et al. Citation2001, Bito et al. Citation2011, Frost-Pineda et al. Citation2011), we could not find the same results in this study. The level of serum T-bil was significantly higher in the NCIT group compared to the CC1 group at Day 28/29. In a 12-month clinical study, there were no statistically significant changes in T-bil in subjects who switched to NVP (EHCSS) compared to those continuing to smoke CC at the end of study (Roethig et al. Citation2008). Although, there are few smoking cessation reports, one six-week cessation study showed that a significant increase in T-bil in subjects who continuously abstained from smoking compared to those who did not (p = 0.037) (O'Malley et al. Citation2014). Therefore, our results and those of the six-week cessation study suggest that reduced cigarette smoke exposure close to the smoking cessation condition have effects on the change of T-bil.
The decrease in the level of 11-DHTXB2 after switching to NCIT was statistically significant in the present study. Similar findings have been reported (Roethig et al. Citation2008, Martin Leroy et al. Citation2012), but negative results have also been reported (Ludicke et al. Citation2018). Two smoking cessation studies reported on 11-DHTXB2, and these studies provided evidence that there was statistically significant decrease in the 11-DHTXB2 level after stopping smoking for three days or 14 days (Rangemark et al. Citation1993, Saareks et al. Citation2001, respectively). Therefore, our results and the findings in the two cessation studies suggest that reduction in cigarette smoke exposure approaching that of the smoking cessation level can cause a decrease in the 11-DHTXB2 level.
The RBC count was significantly lower in SM compared to NS at baseline, and this result is consistent with our previous comparison study (UMIN000021921) (Bito et al. Citation2011) and another study (Kawada et al. Citation2006). Kawada et al. (Citation2006) conducted a study that included 8751 males (46.4 ± 8.0 years) in the community and occupational field and found that the RBC count was lower in SM than in NS. In contrast, in a study conducted by Fujitani et al. (Citation2003) that included 190 males and 159 females, there was no significant difference between SM and NS. In the present study, the difference at Day 28/29 in the RBC count between the NCIT group and the CC group was not significantly different. Similar findings were noted previously in a study where smokers of CCs switched to NVP (electric cigarette) over three months (Cravo et al. Citation2016). On the other hand, two clinical studies of 12 months and 1 month, respectively, showed a decrease in the RBC count in SM switched from CC to NVP (EHCSS) (Roethig et al. Citation2008, Martin Leroy et al. Citation2012). Thus, at the present time, there is no clear conclusion that the RBC count is affected by habitual smoking.
The levels of GLU at baseline in SM are significantly lower than in NS in this study, a finding which is opposite to that reported by Kawada et al. (Citation2006), who compared the difference between SM and NS. They showed that the GLU level was significantly higher in SM compared to NS. On the other hand, a meta-analysis of 16,886 males and 18,539 females reported no significant difference between current and never-smokers but GLU was higher in ex-smokers (Soulimane et al. Citation2014). In the present study at baseline, the level of HbA1c, which is a long-term marker of blood GLU level, was not different between SM and NS. At baseline, BMI in SM was significantly lower than that of NS (SM: 20.9 kg/m2, NS: 21.9 kg/m2; p = 0.002) in this study, and a positive relationship between BMI and GLU level has been reported (Moriuchi et al. Citation2010). Therefore, the fact that GLU levels were lower in SM than in NS in the present study might be due to the difference in BMI between the two groups in this particular population.
The strength of this research is that the study was conducted under the condition of 31-day confinement which completely eliminated compliance bias and ensured that the subjects received only the assigned product. Similarly, the other environmental factors including diet and, exercise, which are thought to influence BoPH levels, were also minimized. Moreover, the study was conducted using NCIT (one of novel NVP), which does not heat or burn tobacco leaves, and a variety of BoPH were analyzed. Since the level of exposure to cigarette smoke constituents by NCIT use is considered to be equivalent to that of smoking cessation, the direction of change in BoPH achieved through smoking cessation could be reproduced. Therefore, the present findings provide new information that clarifies the health risks associated with smoking cigarettes. On the other hand, it should be noted that smokers recruited to take part in this clinical trial were Japanese males who smoked a cigarette with 1 mg tar, which is the lowest tar value in Japan. Because the tar content of CC is likely to affect BoPH levels, the magnitude of changes in BoPH in response to reduced exposure to cigarette smoke might be relatively small in this study.
Conclusion
The study results from the two combined studies showed changes in six BoPH in SM who switched to NCIT use for 29 days under confinement (8-epi-prostaglandin F2α, malondialdehyde, 11-dehydrothromboxane B2: CC1 > NCIT, total bilirubin, low-density lipoprotein cholesterol and total cholesterol: CC1 < NCIT). Additionally, the levels of BoPH using NCIT at Day 28/29 except for T-bil approached those of the NS group. These results suggest that reduced exposure to cigarette smoke approaching that of smoking cessation levels has favorable effects on changes in BoPH believed to be associated with oxidative stress, antioxidant capacity and platelet activation/coagulation but not in lipid metabolism. We believe that our results using NCIT, one of novel NVP, in a combined analysis that enhanced statistical power, provide new useful information for a better understanding of the health risks associated with cigarette smoking. However, in order to clarify the health risks associated with smoking, further studies including clinical studies using an appropriate sample size with meaningful statistical power and SM having various smoking backgrounds (such as number of CC smoked per day, tar value of regularly smoked CC and smoking duration) will be necessary
Acknowledgements
The authors are very grateful to Hakuo Takahashi, M.D., Ph.D., Kunio Iwata, Ph.D., Ms. Aoi Kahehi, and employees of JT Scientific & Regulatory Affairs Division and R& D Group Scientific Product Assessment Center
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ambrose, J.A. and Barua, R.S., 2004. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American college of cardiology, 43 (10), 1731–1737.
- Bito, R., et al., 2011. Exploratory study of smoking-specific biomarkers of effect in healthy adult smokers and non-smokers. The 32th annual scientific meeting of Japanese society of clinical pharmacology and therapeutics. (poster presentation; in Japanese). Hamamatsu: Japanese society of clinical pharmacology and therapeutics.
- Burchfiel, C.M., et al., 1995. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. American journal of respiratory and critical care medicine, 151 (6), 1778–1785.
- Calapai, G., et al., 2009. A cross-sectional investigation of biomarkers of risk after a decade of smoking. Inhalation toxicology, 21 (13), 1138–1143.
- Health Canada, 1999. Determination of “tar,” nicotine and carbon monoxide in mainstream tobacco smoke-official method. Ottawa, Canada: Health Canada (SOR-2000-272).
- Cracowski, J.-L., et al., 2002. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends in pharmacological sciences, 23 (8), 360–366.
- Cravo, A.S., et al., 2016. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regulatory toxicology and pharmacology, 81 (Suppl 1), S1–S14.
- Eliasson, B., et al., 2001. Effect of smoking reduction and cessation on cardiovascular risk factors. Nicotine & tobacco research, 3 (3), 249–255.
- FDA (Food and Drug Administration), 2012a. Guidance for industry — modified risk tobacco product applications—DRAFT GUIDANCE. Available from: https://www.fda.gov/media/83300/download
- FDA (Food and Drug Administration), 2012b. Guidance for industry – reporting harmful and potentially harmful constituents in tobacco products and tobacco smoke under section 904(a)(3) of the federal food, drug, and cosmetic act-draft guidance. Available from: https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm297752.htm.
- Flores, L., et al., 2004. The effects of smoking and its cessation on 8-epi-PGF2alpha and transforming growth factor-beta 1 in Type 1 diabetes mellitus. Diabetic medicine, 21 (3), 285–289.
- Frost-Pineda, K., et al., 2011. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine & tobacco research, 13 (3), 182–193.
- Fujitani, T., et al., 2003. Effects of smoking on hematological parameters. Tokyo metropolitan institute of public health, 54, 370–375 [in Japanese].
- Garg, N., et al., 2006. Levels of lipid peroxides and antioxidants in smokers and nonsmokers. Journal of periodontal research, 41 (5), 405–410.
- Halvorsen, B., et al., 2007. Effect of smoking cessation on markers of inflammation and endothelial cell activation among individuals with high risk for cardiovascular disease. Scandinavian journal of clinical and laboratory investigation, 67 (6), 604–611.
- Harman, S.M., et al., 2003. Urinary excretion of three nucleic acid oxidation adducts and isoprostane F(2)alpha measured by liquid chromatography-mass spectrometry in smokers, ex-smokers, and nonsmokers. Free radical biology & medicine, 35 (10), 1301–1309.
- Howard, G., et al., 1998. Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. JAMA, 279 (2), 119–124.
- Ikonomidis, I., et al., 2018. Electronic cigarette smoking increases arterial stiffness and oxidative stress to a lesser extent than a single conventional cigarette: an acute and chronic study. Circulation, 137 (3), 303–306.
- [IOM] Institute of Medicine Committee to Assess the Science Base for Tobacco Harm Reduction, 2001. Clearing the smoke: assessing the science base for tobacco harm reduction. Washington, DC: National Academies Press. Copyright 2001 by the National Academy of Sciences. All rights reserved
- International Organization for Standardization, 2000. Routine analytical cigarette smoking machine, ISO3308. Available from: https://www.iso.org/standard/28325.html
- Janssen, L.J., 2001. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. American journal of physiology. Lung cellular and molecular physiology, 280 (6), L1067–L1082.
- Kakehi, A., et.al., 2012. Exposure to cigarette smoke constituents in adult Japanese smokers who switched to a non combustion inhaler type of tobacco. 2012 American College of Clinical Pharmacology Meeting. Sheraton San Diego Hotel & Marina.
- Kawada, T., et al., 2006. Study on characteristics of laboratory test value in smokers. Smoking research foundation [in Japanese].
- Kimm, H., et al., 2009. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke, 40 (11), 3422–3427.
- Korchazhkina, O., et al., 2003. Measurement by reversed-phase high-performance liquid chromatography of malondialdehyde in normal human urine following derivatisation with 2,4-dinitrophenylhydrazine. Journal of chromatography. B, analytical technologies in the biomedical and life sciences, 794 (2), 353–362.
- Lin, L.-Y., et al., 2009. Serum bilirubin is inversely associated with insulin resistance and metabolic syndrome among children and adolescents. Atherosclerosis, 203 (2), 563–568.
- Ludicke, F., et al., 2015. A Japanese cross-sectional multicentre study of biomarkers associated with cardiovascular disease in smokers and non-smokers. Biomarkers, 20, 411–421.
- Ludicke, F., et al., 2018. Effects of switching to the menthol tobacco heating system 2.2, smoking abstinence, or continued cigarette smoking on clinically relevant risk markers: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (part 2). Nicotine & tobacco research, 20, 173–182.
- Martin Leroy, C., et al., 2012. Reduced exposure evaluation of an electrically heated cigarette smoking system. Part 7: a one-month, randomized, ambulatory, controlled clinical study in Poland. Regulatory toxicology and pharmacology , 64 (2 Suppl), S74–S84.
- Minami, J., et al., 2002. Effects of smoking cessation or alcohol restriction on metabolic and fibrinolytic variables in Japanese men. Clinical science (London, England: 1979), 103 (2), 117.
- Miura, N., et al., 2013. Pharmacokinetic analysis of nicotine when using non-combustion inhaler type of tobacco product in Japanese adult male smokers. Regulatory toxicology and pharmacology , 67 (2), 198–205.
- Miura, N., et al., 2015. A study to investigate changes in the levels of biomarkers of exposure to selected cigarette smoke constituents in Japanese adult male smokers who switched to a non-combustion inhaler type of tobacco product. Regulatory toxicology and pharmacology , 71 (3), 498–506.
- Moriuchi, T., et al., 2010. Diabetes progression from "high-normal" glucose in school teachers. Internal medicine, 49 (13), 1271–1276.
- Office of the Surgeon G., et al., 2004. Reports of the surgeon general. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention.
- Ogden, M.W., et al., 2015. Switching from usual brand cigarettes to a tobacco-heating cigarette or snus: part 3. Biomarkers of biological effect. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals, 20 (6–7), 404–410.
- Ohara, H., et.al., 2014. A study of the changes of biomarkers for various types of tobacco in healthy adult male smokers. The 35th annual scientific meeting of Japanese society of clinical pharmacology and therapeutics (Poster Presentation; in Japanese). Ehime.
- Okuzono, H., et al., 1976. A spectrophotometric method for the determination of carboxyhemoglobin in blood, and some clinical reports (author's transl.). Rinsho byori the japanese journal of clinical pathology, 24, 616–621.
- O'Malley, S.S., et al., 2014. Smoking cessation is followed by increases in serum bilirubin, an endogenous antioxidant associated with lower risk of lung cancer and cardiovascular disease. Nicotine & tobacco research, 16, 1145–1149.
- Peck, M.J., et al., 2018. Review of biomarkers to assess the effects of switching from cigarettes to modified risk tobacco products. Biomarkers, 23(3), 213–244.
- Polidori, M.C., et al., 2003. Cigarette smoking cessation increases plasma levels of several antioxidant micronutrients and improves resistance towards oxidative challenge. British journal of nutrition, 90 (1), 147–150.
- Prieme, H., et al., 1998. Effect of smoking cessation on oxidative DNA modification estimated by 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion. Carcinogenesis, 19 (2), 347–351.
- Rangemark, C., et al., 1993. Excretion of thromboxane metabolites in healthy women after cessation of smoking. Arteriosclerosis, thrombosis, and vascular biology, 13, 777–782.
- Roethig, H.J., et al., 2008. A 12-month, randomized, controlled study to evaluate exposure and cardiovascular risk factors in adult smokers switching from conventional cigarettes to a second-generation electrically heated cigarette smoking system. Journal of clinical pharmacology, 48 (5), 580–591.
- Roethig, H.J., et al., 2010. Short term effects of reduced exposure to cigarette smoke on white blood cells, platelets and red blood cells in adult cigarette smokers. Regulatory toxicology and pharmacology, 57 (2–3), 333–337.
- Saareks, V., et al., 2001. Effects of smoking cessation and nicotine substitution on systemic eicosanoid production in man. Naunyn-Schmiedeberg's archives of pharmacology, 363 (5), 556–561.
- Scherer, G., 2018. Suitability of biomarkers of biological effects (BOBEs) for assessing the likelihood of reducing the tobacco related disease risk by new and innovative tobacco products: a literature review. Regulatory toxicology and pharmacology , 94, 203–233.
- Sharrett, A.R., et al., 2001. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions. The Atherosclerosis Risk in Communities (ARIC) Study. Circulation, 104 (10), 1108–1113.
- Soulimane, S., et al., 2014. HbA1c, fasting and 2 h plasma glucose in current, ex- and never-smokers: a meta-analysis. Diabetologia, 57 (1), 30–39.
- Stocker, R., et al., 1987. Bilirubin is an antioxidant of possible physiological importance. Science, 235 (4792), 1043.
- Tsikas, D., 2017. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Analytical biochemistry, 524, 13–30.
- U.S. Department of Health and Human Services, 2010. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53017/pdf/Bookshelf_NBK53017.pdf
- Van Hoydonck, P.G., et al., 2001. Serum bilirubin concentration in a Belgian population: the association with smoking status and type of cigarettes. International journal of epidemiology, 30 (6), 1465–1472.
- Walele, T., et al., 2018. Evaluation of the safety profile of an electronic vapour product used for two years by smokers in a real-life setting. Regulatory toxicology and pharmacology, 92, 226–238.
- Wannamethee, S.G., et al., 2005. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. European heart journal, 26 (17), 1765–1773.