Abstract
Background
Whether Copeptin combined with high sensitivity troponin below the respective decision cut-offs improves rule-out of NSTEMI and may predict all-cause death at 30-day is under debate.
Methods
Data on 10,329 patients from 5 trials were pooled to evaluate the diagnostic and prognostic performance of an initial Copeptin below decision cut-off in combination with a (hs)-cTn below the uppler limit of normal (ULN) compared to a) the initial (hs)-cTn alone in the standard serial sampling strategy based on the 99th percentile and b) a single marker strategy (SMS) based on hs-cTn < limit of detection. Endpoints were sensitivities and negative predictive values (NPV) for rule-out of NSTEMI, 30-day all-cause mortality and rates of eligibility for DMS or SMS.
Results
NPV for NSTEMI was higher for DMS than for the initial cTn, regardless assay sensitivity. The highest NPVs were observed with DMS vs. hs-cTn (99.4% [95% CI: 99.0%–99.6%] vs. 98.8% [98.4%–99.1%],) , and improved performance was consistent across all important subgroups including presentation <3 h, again irrespective of assay sensitivity. The point estimate of all NPVs for all-cause death exceeded 99.75%. In the label populations, DMS versus SMS demonstrated comparably high NPVs for rule-out of NSTEMI (99.4% [99.0%–99.6%] vs. 99.9% [99.2%–100.0%]), very low mortality after rule-out (0.1% [0.0%–0.4% vs. 0.0% [0.0%–1.2%]), but eligibility for rule-out was 2.4-fold higher (61.4% [59.9%–62.9%] vs. 25.3% [23.7%–26.9%]) with DMS than SMS.
Conclusion
Findings from a large pooled cohort corroborate the safety of the dual marker strategy for instant rule-out of NSTEMI, extending evidence to hs-cTn. Copeptin below cut-off in combination with hs-cTn below ULN may be used in more than 2.4-times more patients presenting with suspected ACS than a single marker strategy based on very low hs-cTn, without the need to exclude very early presenters or other important subgroups.
Introduction
Earlier findings from clinical trials (Reichlin et al. Citation2009, Keller et al. Citation2010, Maisel et al. Citation2013) and from three meta-analyses (Lipinski et al. Citation2014, Raskovalova et al. Citation2014, Shin et al. Citation2018) have conferred substantial evidence for the added value of Copeptin measured together with conventional or contemporary sensitive cardiac troponin (cTn). The intended use of this dual marker strategy (DMS) is to rule-out NSTEMI with a single blood draw at presentation. Accordingly, a NSTEMI is excluded if Copeptin is below the decision cut-off and cTn or hs-cTn is normal, i.e. below the upper limit of normal (ULN). Other combinations of Copeptin and cTn are disregarded. More recent findings from the Biomarkers-in-Cardiology-8 (BIC-8) trial (Möckel et al. Citation2015), a large randomized interventional trial, and from the international multicenter Pro-Core registry (Giannitsis et al. Citation2019) add evidence that earlier discharge guided by DMS is at least as safe and more cost effective (Reinhold et al. Citation2018) than the standard cTn algorithm that is based on serial measurements.
DMS is attractive as a single blood draw does not only allow the rule-out of NSTEMI, obviating the need for a second venous puncture and blood processing, but also allows identification of a substantial higher number of eligible low risk subjects with suspected ACS who qualify for an early and safe discharge from the ED (Mueller-Hennessen et al. Citation2019). In the past, data on the safety of early rule-out and discharge in patients with suspected ACS were almost exclusively derived from observational trials where physicians were blinded to the results of experimental hs-cTn assays and protocols, and where patients were treated according to local standards (Reichlin et al. Citation2012, Mokhtari et al. Citation2016, Mueller et al. Citation2016, Neumann et al. Citation2016). In contrast, findings from randomized interventional trials (Mahler et al. Citation2015, Möckel et al. Citation2015, Than et al. Citation2016, Body et al. Citation2017, Chew et al. Citation2019) and prospective registries (Giannitsis et al. Citation2019, Twerenbold et al. Citation2019, Stoyanov et al. Citation2020) on safety of early discharge using rapid algorithms based on hs-cTn are sparse.
More recently a debate started about the performance of DMS when high sensitivity cardiac troponin (hs-cTn) and fast protocols are being used (Wildi et al. Citation2019). However, evidence is limited since other comparative studies (Boeddinghaus et al. Citation2017, Chapman et al. Citation2017) and a collaborative meta-analysis (Pickering et al. Citation2017) on the diagnostic or prognostic performance of serial or single very low hs-cTn concentrations did not evaluate the performance of DMS at all. In addition, non-exclusion of high risk patients (Mueller-Hennessen et al. Citation2019), a wide range of troponin assay types and Copeptin cut-offs as well as statistical issues further complicate the interpretation of inconsistent study findings (Meune et al. Citation2011, Sebbane et al. Citation2013, Lipinski et al. Citation2014, Raskovalova et al. Citation2014, Alquézar et al. Citation2017, Morawiec et al. Citation2018, Chenevier-Gobeaux et al. Citation2019).
Therefore, we aggregated individual data from 5 study cohorts on 10,325 patients to a) overcome the shortcoming of small sample size and low event rates among low risk patients with suspected ACS, b) corroborate previous findings on the added value of cTn combined with Copeptin for immediate rule-out of NSTEMI across the spectrum of assay sensitivities, c) to demonstrate that combination of hs-cTn and Copeptin improve rule-out and prediction of 30-day mortality, if statistical analysis is confined to comparison of rule-out instead of the entire diagnostic process, and d) to compare DMS with the rule-out arm of the ESC 0 hour pathway that allows rule-out with an initial hs-cTn concentration below the limit of detection, called the single marker strategy (SMS). DMS and SMS were tested in the overall cohort and in the respective label populations as recommended in the 2015 ESC guidelines (Roffi et al. Citation2016).
Clinical significance
There is a debate whether a dual biomarker strategy (DMS) combining Copeptin and cardiac troponin for instant rule-out of NSTEMI is effective and safe when fast hs-cTn based protocols are being increasingly used.
So far, studies on DMS with hs-cTn were small, and findings were interpreted incorrectly due to inappropriate statistical methods. In addition, there are no data on the comparative performance of DMS compared to an immediate rule-out of NSTEMI based on a single very low hs-cTn.
Findings on 10,329 patients show a comparative performance of DMS versus a serial cTn testing strategy, regardless the sensitivity of the cTn assay, and irrespective of time to presentation or other pre-specified subgroups.
The DMS is as effective and safe for instant rule-out of NSTEMI as a very low initial hs-cTn but DMS can be applied to 2.4-fold more patients with suspected ACS.
DMS is a potential alternative to the ESC 0/3 hour algorithm, irrespective the cTn assay used, and is superior to the ESC 0-hour rule-out arm of the ESC 0/1 hour algorithm.
Methods
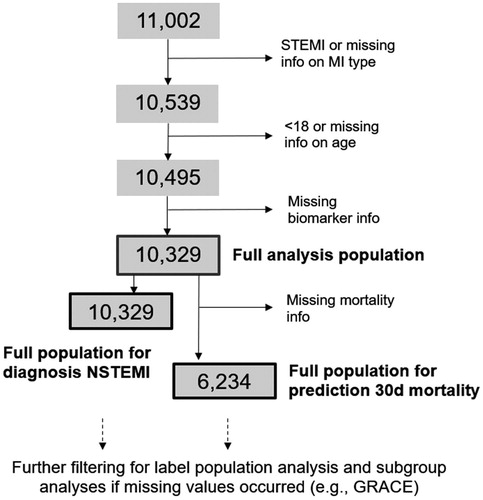
We pooled individual patient data from 5 trials. Findings from the Chopin trial (Reichlin et al. Citation2009), the BIC-8 trial (Möckel et al. Citation2015), the Pro-Core registry (Giannitsis et al. Citation2019), as well as an unpublished study from the Wilhelminenhospital (Vienna, Austria) and the pooled data from multiple diagnostic evaluations at different study sites in Germany, Switzerland, Austria and France (recruitment 2009–2010), including Heidelberg. The latter has been published earlier (Giannitsis et al. Citation2011). Data from the diagnostic evaluations were collected using the same standardized format and hence treated as one multi-center trial. Follow-up for mortality or complications was not part of the protocol of the diagnostic evaluations. Patients were eligible for the pooled analysis if they were aged ≥18 years, presented with symptoms suggestive of an ACS to the ED of a study site. Patients presenting with ST-segment elevation or a final diagnosis of ST-segment elevation myocardial infarction (STEMI), patients without information on age and those with missing values for Copeptin or (hs)-cTn were excluded (, patient flow). A description of the individual studies that were aggregated for this pooled analysis has been published earlier (Giannitsis et al. Citation2020).
Patients underwent clinical assessment per local practice. As a minimal requirement, routine assessment included medical history, physical examination, standard blood testing including measurements of local (hs)-cTn, Copeptin, creatinine, 12-lead ECG, and clinical information to calculate the GRACE score (Granger et al. Citation2003). Other clinical scores were not tested prospectively, prohibiting any conclusion on their clinical usefulness. Levels of (hs)-cTn were measured at presentation and serially thereafter for as long as clinically indicated. Treatment of patients was left at the discretion of the attending physician of the respective study site and was not influenced by Copeptin results in the observational studies. However, Copeptin was used to guide early discharge in the randomized BIC-8 interventional trial (Möckel et al. Citation2015) and in the prospective Pro-Core registry (Giannitsis et al. Citation2019). In this pooled analysis, we evaluated the diagnostic and prognostic performance and effectiveness of two ESC guideline (Roffi et al. Citation2016) recommended rule-out options, either Copeptin below a fixed pre-specified cut-off together with hs-cTn value below the 99th percentile cut-off at presentation, or an immediate rule-out based on a single hs-cTn level below the limit of detection (LoD). Adjudications of the final diagnoses were performed locally integrating all available information and based on local troponin assays which were used at the respective cut-off values recommended by the manufacturers.
All single-center observational studies contributing patients for this pooled analysis had obtained approvals from their local ethical committees. In addition, central ethics approvals and study registrations were obtained for the multicenter observational and interventional trials.
The study complies with the Declaration of Helsinki and all patients had provided written informed consent.
Definition of endpoints
The primary endpoint was diagnostic safety for rule-out of NSTEMI reflected by negative predictive values (NPV) and sensitivities. Sensitivity was the probability with which a true NSTEMI was correctly detected by a diagnostic biomarker strategy and NPV was the probability with which a negative test result leading to a rule-out decision was correct. For DMS, NSTEMI was ruled-out when Copeptin and initial troponin were below their respective cut-offs, i.e. Copeptin concentrations below 10 or 14 pmol/L (depending on the assay type used) together with a local cardiac troponin concentration below the ULN, i.e. the 99th percentile value of a healthy reference population - as recommended by the manufacturer in the package inserts. NSTEMI was defined according to the criteria of the second and third Universal MI definition (Thygesen et al. Citation2007, Thygesen et al. Citation2012), and was diagnosed locally by study centres without central adjudication. A further differentiation between non-cardiac chest pain and unstable angina was not in the scope of the present analysis.
The secondary endpoint consisted of NPV and sensitivity for prediction of all-cause death at 30 days.
An additional secondary endpoint was assessment of the effectiveness of the particular strategy, defined as the proportion of patients qualifying for the respective DMS or SMS strategies for rule-out (i.e. proportion of patients with values below the relevant cut-off(s)).
The endpoints were tested in the overall cohort and in the label populations. The label population for the DMS consisted of patients with low- or intermediate risk based on the GRACE score, i.e. ≤140 points (Möckel et al. Citation2015, Giannitsis et al. Citation2019). For SMS, early presenters, i.e. those with a last episode of pain less than 3 h before the blood draw, were excluded. In addition, safety and effectiveness were determined in subgroups of special interest. These subgroups included age dichotomized at 65 years, presence or absence of diabetes mellitus, severe kidney disease defined as calculated creatinine clearance (eGFR) <60 ml/min, a history of CAD, male or female sex, time to presentation ranging from very early presenters (3 h) to very late presenters (>12 h) after onset of symptoms, and GRACE score classes. For the unpublished study cohorts, sufficient information was available to calculate all GRACE scores retrospectively (Giannitsis et al. Citation2020). All eGFR values missing in the original datasets were calculated post hoc if age, creatinine and sex were known, using the CKD-EPI equation (Levey et al. Citation2009).
Copeptin and cardiac troponin
Copeptin concentrations were measured in plasma samples collected at baseline either by a conventional assay (B·R·A·H·M·S GmbH, Hennigsdorf, Germany) or using the automated fluoro-immunoassay B·R·A·H·M·S Copeptin proAVP KRYPTOR for the quantitative measurement of C-terminal pro-arginine-vasopressin (CT-proAVP, Copeptin) in human serum and plasma on the B·R·A·H·M·S KRYPTOR compact PLUS platform. The test has a detection limit of 0.69 pmol/L and a functional assay sensitivity (detected by inter-assay precision of 20% CV) of 1.08 pmol/L.
The recommended cut-off for the decision between a positive and a normal test used to be 14 pmol/L with the earlier generation of the Copeptin assay and is currently 10 pmol/L with the latest Copeptin assay generation corresponding to the 95th percentile of a healthy reference population. This cut-off was used in the randomized controlled BIC-8 trial (Möckel et al. Citation2015) and the ProCore registry (Giannitsis et al. Citation2019) and is the recommended cut-off for the rule-out algorithms for MI since 2015.
Cardiac troponin was measured at the discretion of the participating study centres and the local cut-off values were used together with the uniform Copeptin cut-off, i.e. either 14 pmol/L or 10 pmol/L.
Hs-cTnT concentrations were measured on either Cobas e411 or Elecsys 2010 (Roche Diagnostics). Per package insert, the limit of blank (LoB) and LoD are 3 ng/L and 5 ng/L, respectively. The 99th percentile of a healthy reference population has been determined as 14 ng/L, with a coefficient of variation (CV) of less than 10% at 13 ng/L (Saenger et al. Citation2011). Using the 99th percentile cut-off, a value of 14 ng/L or above was considered elevated, irrespective of age or sex (Thygesen et al. Citation2007, Citation2012, Roffi et al. Citation2016). Hs-cTnI was measured using the Abbott Arcitect STAT assay. This assay has a limit of detection of 1–2 ng/L, and an upper reference limit (99th centile) of 34 ng/L in men and 16 ng/L in women. It has a coefficient of variation of 23% at the limit of detection (1·2 ng/L) and less than 10% at 6 ng/L (Neumann et al. Citation2016).
For comparison of the DMS with a single low hs-cTn cut-off, a decision cut-off at the LoD of each respective hs-cTn was used. For the Roche hs-cTnT and the Abbott hs-cTnI assays, these cut-offs were 5 ng/L and 2 ng/L, respectively.
Statistical analysis
Pooled analyses were carried out on individual patient level. Aggregation of several studies was deemed appropriate since all studies had recruited patients with suspected NSTE-ACS presenting to an ED. All centres were located in Europe and in the United States.
The pre-specified diagnostic thresholds for dichotomous classification were examined for individual (hs)-cTn and combinations of (hs)-cTn and Copeptin. Diagnostic and prognostic performance was quantified as the NPV and sensitivity for NSTEMI and all-cause death at 30 days. Effectiveness was quantified as the percentage of the overall cohort assigned to the rule-out zone by the respective strategy. A pre-specified analysis was performed in the label population excluding all patients not eligible for DMS due to a GRACE Score > 140 points, and all patients presenting within 3 h after onset of symptoms for SMS. Effectiveness was only calculated for DMS and SMS (i.e. ESC 0-hour algorithm) in the label populations. Additional analyses were executed in important patient subsets including early presenters vs. late presenters, females, older patients, patients with a history of CAD, patients with diabetes, and severe renal kidney disease (eGFR < 60 ml/min).
NPV, sensitivity and effectiveness were calculated from 2 × 2 contingency tables comparing biomarker-based classification vs. true NSTEMI (30-day mortality) status and are reported with 95% Wilson score intervals. These intervals have more accurate coverage compared to standard Wald intervals for proportions close to 0 or 1 (Brown et al. Citation2001), as was the case in this study. In contrast to some previous studies, we deliberately do not report results from null hypothesis significance testing for differences between biomarker strategies via Chi-squared or McNemar’s test (although they fully support our conclusions), as the null hypothesis of no difference is invalid in the present case. For example, DMS will per definition always have a sensitivity at least as high as initial cardiac troponin alone, because all patients with NSTEMI detected by the latter are also detected by the former. P-values from mentioned tests would hence be misleading. Instead, we encourage the reader to directly interpret the estimated effect sizes and their associated uncertainty.
NPV and sensitivity and their confidence intervals of biomarker strategies were also estimated separately for important subgroups of the full analysis population. To determine if there was evidence that improvements in diagnostic accuracy differed among subgroups, we used interaction testing to compare multiple 2 × 2 tables according to the method proposed by Michael (Michael Citation2007). This procedure tests the joint null hypothesis that a difference in proportions is equal for all subgroups via a test statistic that approximates a Chi-squared distribution for large samples. Hence, a high p-value of interaction suggests that potential improvements of sensitivity or NPV between DMS and either initial cardiac troponin in a serial pathway or SMS were not statistically different among subgroups (e.g. non-hs-cTn assay vs. hs-cTn assay, early vs. late presenters, presence or absence of GFR <60 ml/min, etc). Note that nevertheless we interpret these p-values with some caution due to rather unequal sample sizes across subgroups, small event rates in some subgroups and the neglect of potential within-patient correlation in the comparison of different biomarker strategies on the same population. Point estimates and confidence intervals, which we show, provide an arguably more informative representation of the subgroup-specific results. All analyses were conducted in R 3.5.1 (R Core Team 2018).
Results
The pooled data included 11,002 patients of whom 463 patients were excluded due to presentation with persistent ST-segment elevations (STEMI), age <18 years (n = 5) or missing information on age (n = 39), or missing biomarker information for either Copeptin (n = 91) or initial (hs)-cTn (n = 75) (, patient flow-diagram). Accordingly, a total of 10,329 patients with suspected NSTE-ACS qualified for final analysis of the diagnostic performance. Studies that evaluated outcomes (mortality) post discharge had almost complete 30-day follow-up with missing follow-up information in 0.0% to 1.6% (Giannitsis et al. Citation2020). Conversely, in the diagnostic studies (Giannitsis et al. Citation2020), a follow-up was not in the scope of the trials. Accordingly, information on 30-day all-cause mortality was not available in 4,095 patients, and analysis of DMS for prediction of 30-day mortality was available in 6,234 patients. The baseline characteristics and main outcomes of the individual trials within the pooled study cohort have been published earlier (Giannitsis et al. Citation2020).
There was a wide range of troponin assay types including conventional sensitive, contemporary sensitive and high-sensitive cTn assays. For the BIC-8 trial (Möckel et al. Citation2015), re-measured hs-cTn concentrations were available for all but 7 patients in the full analysis set (881 of 888). For the CHOPIN trial (Maisel et al. Citation2013), centralized re-measured cTnI values were used instead of local measurements. The distribution of (hs)-cTn assay types is displayed in Supplemental Figure 1 and Table S1. Note that if both, cTn and hs-cTn values from the first blood draw were available, the hs-cTn result was considered in the analysis.
Hs-cTn assays, namely the Roche hs-cTnT (n = 4,089) or the Abbott Architect hs-cTnI (n = 508), were used in 44.5% of all patients. The conventional Copeptin on the KRYPTOR was used in 58.1% (n = 6,005) and the ultrasensitive Copeptin on the KRYPTOR compact PLUS platform in 41.9% (n = 4,324).
In the analysis regarding DMS vs. hs-cTn in an intended serial algorithm (i.e. considering only the initial troponin value), hs-cTn was also used in the DMS arm in all cases (4597 of 10,329 patients).
Overall rate of NSTEMI was 9.4% (976 of 10,329 patients), ranging from 1.2% to 15.4% across individual cohorts (Giannitsis et al. Citation2020). At 30 days, all-cause mortality was 0.3% (33 of 6,234 patients), with a range from 0.2% to 0.7% across cohorts. An analysis of the performance of rule-out of NSTEMI was carried out in all patients (n = 10,329), whereas the prognostic performance on 30-day all-cause mortality was evaluated in patients with available follow-up only (n = 6,234).
For comparisons of SMS vs. DMS regarding rule-out of NSTEMI or 30-day all-cause death, numbers of eligible patients were lower in the in-label SMS (2,901 and 1,556, respectively) and the in-label DMS (3,873 and 2,066) cohorts due to exclusion of (i) patients with conventional and contemporary cTn measurements and (ii) exclusion of patients who did not qualify for the labels (or have missing information).
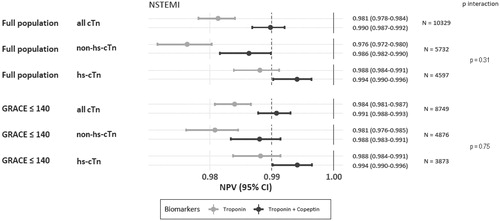
Findings on the diagnostic performance of DMS for rule-out of NSTEMI using any (hs)-cTn versus standard algorithm are summarized in and Supplemental Figure 2 and in the Supplemental materials (Supplemental Figures 2–6).
Figure 2. Rule-out of NSTEMI using DMS vs. non-hs-cTn or hs-cTn, displayed as NPVs with corresponding 95% confidence intervals, and p-values for interaction. NPVs were higher for DMS (dark dot for point estimate and lines for 95% confidence interval) versus cardiac troponin alone (light dots and lines), irrespective the troponin assay type (p interaction 0.31), both in the full and the label population (p interaction 0.75).
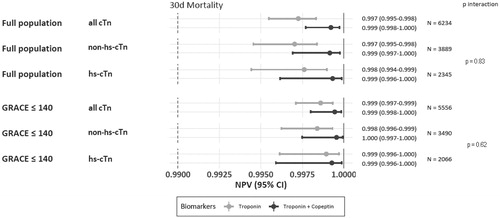
DMS vs. standard algorithm showed an improvement of NPVs and sensitivities for rule-out of NSTEMI, irrespective (interaction p = ns) of the troponin assay used ( and Supplemental Figure 2). NPV for 30-day mortality was very high exceeding 99.5%, irrespective of DMS or a strategy based on the initial (hs)-cTn alone, and regardless the sensitivity of the cTn assay used (). Analogous findings for sensitivities are displayed in Supplemental Figure 3.
Figure 3. Prediction of 30-day all-cause mortalities by DMS vs. hs-cTn or non-hs-cTn, displayed as NPVs with corresponding 95% confidence intervals, and p for interaction. NPVs were higher for DMS (dark dot for point estimate and lines for 95% confidence interval) versus cardiac troponin alone light dots and lines), irrespective the troponin assay type (p interaction 0.83), both in the full and the label population (p interaction 0.62).
Performance of DMS with high sensitivity troponin vs. initial (hs)-cTn in the serial algorithm according to the universal MI definition (UDMI)
Full study population
In the entire study population, irrespective of troponin assay, DMS was associated with higher NPVs and sensitivities (both p < 0.001) for rule-out of NSTEMI than the initial hs-cTn alone. The highest NPV (99.4% [95%CI: 99.02%-99.64%]; ) and sensitivity (94.9% [91.7%-97.8%], Supplemental Figure 2) were noted with DMS using hs-cTn. There was a gain in sensitivity but not in NPV when DMS was used against a conventional or contemporary cTn assay (p interaction = 0.03 and 0.31, respectively), although sensitivity and NPV also increased when DMS was used instead of hs-cTn.
Prediction of 30-day mortality was excellent with the initial hs-cTn alone or with DMS exceeding NPVs of 99.75%, and the predictive accuracy did not differ between cTn and hs-cTn assays (p interaction = 0.80). Using DMS with hs-cTn, an exceptionally high NPV for 30-day mortality was observed (99.9% [99.6%-100%]) with a lower confidence bound >99.5% (). Analogous findings for sensitivities regarding prediction of all-cause mortality are displayed in Supplemental Figure 3.
Pre-specified subgroups
Diagnostic performance was consistently better with DMS across subgroups.Improvement of NPV for rule-out of NSTEMI (Supplemental Figure 4(a,b)) or all-cause mortality (Supplemental Figure 5(a,b)) was consistent across subgroups of particular interest (all p interaction > 0.1), including age, sex, time since pain onset to initial presentation, history of CAD, diabetes, kidney function and GRACE score class, irrespective whether a hs-cTn or a non-hs assay was used. Yet, improvement in sensitivities for rule-out of NSTEMI was larger for early than late presenters when using DMS against cTn alone (Supplemental Figure 5(a,b)). For prediction of 30-day all-cause mortality, sensitivity improvement via DMS was consistent (p-interaction > 0.1) for all subgroups except for the GRACE score (p-interaction = 0.05).
Performance of DMS vs. ESC 0 h rule-out strategy in the respective label populations
Overall performance of both rule-out strategies was excellent with lower confidence limits of NPVs ≥99.0% (). NPVs of DMS and SMS in their respective label population were not different (99.4% [99.0%-99.6%] vs. 99.6% [99.0%-99.8%]).
Figure 4. Comparison of DMS vs. SMS regarding (a) NPV for rule-out of NSTEMI, (b) NPVs for 30-day all-cause mortality, and (c) effectiveness of the rule-out algorithm in the respective label population. NPVs for rule-out of NSTEMI and prediction of 30-day all-cause death were similar for DMS (dark dot for point estimate and dark lines for 95% confidence interval) versus hs-cTn alone (corresponding light dots and lines). The number of eligible patients qualifying for the respective strategy is 2.4-fold higher with DMS.
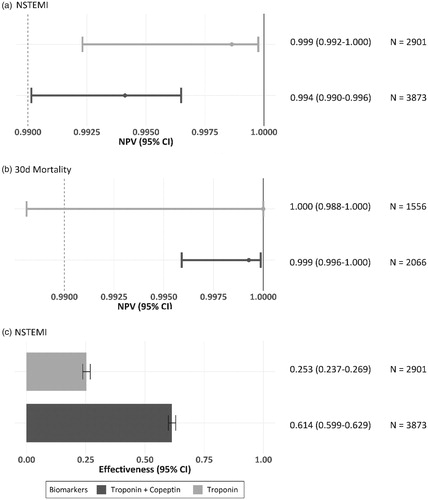
The safety of both instant rule-out strategies to predict 30-day all-cause death was excellent with NPVs >99.75% (), and similar between DMS and SMS (99.9%, [99.6%-100%] vs. 100% [98.8%-100%]).
The eligibility of patients for rule-out of NSTEMI () was 2.4-fold higher for DMS versus SMS (61.4% [59.9%-62.9%] vs. 25.3% [23.7%-26.9%]) in their respective label populations (3,873 vs. 2,901 patients).
Discussion
There are several important key findings regarding DMS versus the initial hs-cTn based on Universal MI definition criteria.
First, in the overall study population, DMS versus initial hs-cTn showed higher sensitivities and negative predictive values for rule-out of NSTEMI. DMS using hs-cTn was particularly effective yielding sensitivities of 96.4% [94.2%-97.8%] and NPV 99.4% [99.0%-99.6%] with lower confidence bounds above 99.0% for rule-out of NSTEMI. Second, DMS in combination with hs-cTn resulted in NPV for prediction of 30-day all-cause mortality close of 99.9% [99.6%-100%] with a lower confidence bound above 99.5% which is regarded as an acceptable rate for 30-day mortality (Than et al. Citation2013). The findings were consistent in the overall population and in the label population that excluded patients with a GRACE score >140 points (p interaction NS). Our findings corroborate previous findings on the excellent performance of DMS regarding instant rule-out of NSTEMI, obviating the need for a second blood draw, without a signal for increased mortality at 30 days. Thus, contrary to current belief, there is an improvement in diagnostic value of DMS, both in terms of sensitivity and NPV, even with the use of hs-cTn instead of cTn. Third, the diagnostic and prognostic performance of DMS compared to hs-cTn alone in terms of NPVs is similarly effective in all important subgroups including very early presenters, CKD (eGFR < 60 ml/min), a history of CAD, prevalent diabetes mellitus, female sex, or in older patients (>65 years).
The above findings corroborate the existing evidence on the usefulness of DMS to rule-out a NSTEMI with a serial cTn protocol using the 99th percentile as the diagnostic cut-off as the reference. Our data expand findings on hs-cTn as part of DMS showing improved rule-out and prediction of 30-day mortality, provided that statistical comparison is restricted to the rule-out indication for DMS and not for the rule-in process, as well.
The second part of our evaluation is novel and focussed on the comparison of DMS and a single marker hs-cTn based strategy.
At present, there are no data that compare DMS vs. rule-out of MI based on a single very low hs-cTn cut-off (SMS). We provide the following novel important findings. First, rule-out of NSTEMI in terms of NPV (99.4% vs. 99.9%, p = 0.21) is as effective using DMS or SMS. Second, 30-day all-cause mortality rates after rule-out are very low and similar with DMS and SMS (0.1% [0.0%-0.4%] vs. 0.0% [0.0%-1.2%]), as are corresponding NPVs (99.9% vs. 100%). Third, DMS demonstrates a consistent performance gain regarding rule-out of NSTEMI and prediction of 30-day mortality in all subgroups tested. In particular, rule-out of NSTEMI was extraordinarily effective in very early presenters with no interaction between times of symptom onset to admission. Thus, DMS has an advantage over SMS where very early presenters have to be excluded systematically. On the other hand, performance of DMS with hs-cTn is similarly effective in the overall study population including high risk patients with a GRACE score > 140 points as in the label population. Accordingly, DMS could allow to expand numbers of eligible patients who were previously excluded. Fourth, eligibility for a rule-out strategy with DMS is nearly 2.4-fold higher than with SMS (61.4% vs. 25.3%).
Thus, the present study reports several important findings that not only corroborate existing evidence on the safety and effectiveness of DMS but also add important and novel information on the comparative performance of DMS and SMS when a hs-cTn assay is used instead of a conventional or contemporary sensitive cTn assay.
Previous findings
While the diagnostic performance of DMS over an initial conventional or contemporary sensitive cTn is well established and is already recommended in the 2015 ESC guidelines (Roffi et al. Citation2016) as an alternative option for early rule-out, the safety of DMS in the era of hs-cTn assay has been questioned with the advent of hs-cTn and implementation of fast diagnostic protocols (Wildi et al. Citation2019). Rapid protocols enable a safe rule-out of MI with a single low hs-cTn concentration or an accurate diagnosis with serial testing after 60–120 min (Reichlin et al. Citation2012, Mokhtari et al. Citation2016, Mueller et al. Citation2016, Neumann et al. Citation2016, Than et al. Citation2018), but no immediate discharge, which has not been tested prospectively so far. Implementation of faster protocols were found to decrease stays in ED (Mueller et al. Citation2016, Twerenbold et al. Citation2016, Chew et al. Citation2019, Twerenbold et al. Citation2019, Stoyanov et al. Citation2020), increase discharge rates of low risk patients (Mueller et al. Citation2016, Twerenbold et al. Citation2016, Citation2019, Chew et al. Citation2019, Stoyanov et al. Citation2020), improve cost effectiveness compared to standard protocols, both for the ESC 0/1 hour (Ambavane et al. Citation2017) and the DMS (Reinhold et al. Citation2018).
Copeptin, although not specific to myocardial injury, responds to an immediate neural trigger with concentrations rising early and decreasing gradually over several hours in AMI (Slagman et al. Citation2015). This rapid-release kinetic may help to cover the troponin delayed release period known as ‘troponin-blind’ interval. Accordingly, DMS is believed to particularly improve diagnosis in very early presenters (Slagman et al. Citation2015). Stallone et al (Stallone et al. Citation2016) reported a lower NPV for Copeptin together with hs-cTn in patients presenting less than 2 h after onset of symptoms. Nevertheless, DMS further reduced numbers of misclassified NSTEMI compared to hs-cTn alone in early presenters (Chenevier-Gobeaux et al. Citation2019). Regarding other subgroups, very similar performance has been consistently reported for DMS vs. hs-cTn alone in females (Balmelli et al. Citation2013), older patients Bahrmann et al. Citation2013 (, Balmelli et al. Citation2013), patients with diabetes (Zellweger et al. Citation2015), or those with a history of CAD (Potocki et al. Citation2012).
Nevertheless, the role of DMS has been discussed controversially based on study findings that compared DMS against hs-cTn assays and fast protocols (Wildi et al. Citation2019). However, these studies were flawed by several limitations including the lack of prospective evaluation in the observational studies with few exceptions (Möckel et al. Citation2015, Giannitsis et al. Citation2019), small sample size and low event rates (Meune et al. Citation2011, Bahrmann et al. 2013, Sebbane et al. Citation2013, Alquézar et al. Citation2017, Morawiec et al. Citation2018, Chenevier-Gobeaux et al. Citation2019), false definition of dual marker testing (i.e. either component positive instead of both components negative (Meune et al. Citation2011, Eggers et al. Citation2012, Sebbane et al. Citation2013), use of the previous less sensitive Copeptin assay formulation, mostly used at a cut-off of 14–17.3 pmol/L (Meune et al. Citation2011, Eggers et al. Citation2012, Bahrmann et al. Citation2013, Sebbane et al. Citation2013, Lipinski et al. Citation2014, Raskovalova et al. Citation2014, Morawiec et al. Citation2018), or 12 pmol/L (Chenevier-Gobeaux et al. Citation2019) instead of the more sensitive cut-off at the 95th percentile at 9 or 10 pmol/L (Thelin et al. Citation2013, Möckel et al. Citation2015, Alquézar et al. Citation2017, Giannitsis et al. Citation2019, Wildi et al. Citation2019). Moreover, several studies did not exclude patients with persistent ST-segment elevations (Sebbane et al. Citation2013, Lipinski et al. Citation2014, Raskovalova et al. Citation2014). Along with the above shortcomings, there are two severe methodological errors that account for most inconsistencies. First, DMS tested in the randomized intervention trial (Möckel et al. Citation2015) and in the multicenter prospective registry (Giannitsis et al. Citation2019) explicitly excluded patients at high risk, defined as GRACE score > 140 points. In contrast, all other individual studies and meta-analyses evaluated DMS vs. hs-cTn retrospectively in the overall study cohorts without exclusion of patients at high risk. As a consequence, DMS was found to perform less effective than the initial hs-cTn value alone. Conversely, the two studies that explored the performance of DMS in selected patients with low-to-intermediate risk using either the GRACE score (Mueller-Hennessen et al. Citation2019) or the HEART Score (Morawiec et al. Citation2018, Mueller-Hennessen et al. Citation2019) revealed sensitivities and negative predictive values at or close to 100%. Second, receiver operating curve (ROC) analysis has been used systematically to compare the diagnostic performance of DMS against hs-cTn. In all studies, DMS provided no added benefit in terms of areas under the curve to hs-cTn alone. However, it is important to note that ROC tests the performance of continuous variables and provides an estimate on overall accuracy, i.e. rule-out and rule-in. However, the intended use of the DMS strategy is limited to rule-out of NSTEMI at pre-specified cut-offs for Copeptin and hs-cTn. In support, when looking at rule-out alone, the majority of individual studies and meta-analyses reported higher sensitivities and negative predictive values for DMS versus hs-cTn but only a few reported the significance level (Eggers et al. Citation2012, Thelin et al. Citation2013, Raskovalova et al. Citation2014, Wildi et al. Citation2015, Shin et al. Citation2018).
Conclusions
The combination of Copeptin and either cTn or hs-cTn represents a similarly effective rule-out strategy for NSTEMI compared to the initial cardiac troponin value, regardless whether a cTn or hs-cTn assay is used. Corroborating findings from BIC-8 (Möckel et al. Citation2015) and ProCore (Giannitsis et al. Citation2019) on the diagnostic and prognostic performance, a rule-out of MI and death within 30 days with lower confidence bounds >99.0% and >99.5% suggests that patients can be ruled-out effectively and probably sent home for outpatient work-up, thus facilitating the decongestion of crowded EDs. Compared to the alternative option of a single marker strategy based on a very low hs-cTn, the combination of Copeptin together with hs-cTn can be used as safely but in 2.4-fold more patients, including those patients presenting less than 3 h after onset of symptoms. From the global perspective, use of the dual marker strategy appears particularly interesting in highly prevalent scenarios where hs-cTn is not available permanently, or wherever fast protocols have not been fully adopted yet, or when a hs-cTn assay is still not available (Anand et al. Citation2019). The dual marker strategy has the advantage to rule-out an MI with the first blood draw, without need for repetitive blood draws or prolonged waiting times for laboratory results. On the other hand, the utilization of the dual marker strategy lags behind, mostly due to the inconvenience to measure Copeptin on a labour-intensive semi-automated laboratory platform. Implementation of Copeptin into a fully automated laboratory platform, or use of Copeptin on a point-of-care device cleared for a high sensitivity assay is anticipated to increase acceptance of the dual marker strategy globally. Despite the large dataset that could be gathered, natural limitations of our study are that rule-out strategies were compared retrospectively via correlational analysis and that statistical power may have been low to detect differences among some of the subgroups due to missing variables or low rates of NSTEMI or mortality.
Limitations
There are several shortcomings that have to be mentioned. First, event rates are low in the tested populations rendering the analysis susceptible to type II errors. Second, study cohorts show some heterogeneities regarding hospital settings, a broad use of cTn assays and assay sensitivities, and a variable range of MI prevalence. Statistical evaluation on prognostic performance was executed in only 6,234 of 10,329 patients (60%) of the entire pooled cohort because early studies only tested the diagnostic performance of DMS while later studies also looked at the safety of discharge following rule-out. However, rates of missing outcomes in studies where safety was assessed were only between 0 and 1.6%. While the effects in the overall cohort are robust, the interpretation of outcomes in subgroups has to be interpreted cautiously, as subgroups may reduce the statistical power. But the results in subgroups are consistent, corroborating the main findings and in addition may help to generate hypotheses for future studies. Another potential limitation is that adjudication of MI and events was left at the discretion of treating physicians in the respective trial or registry. Since the diagnosis of MI was made using the diagnostic criteria of the Universal MI definition, and because all-cause death was selected as the outcome measure without differentiation into cardiac or non-cardiac death, the numbers of over – or underdiagnosed cases are believed to be balanced.
Supplemental Material
Download PDF (993.9 KB)Acknowledgments
The authors express their gratitude to the statistician Jan Wiemer who supported statistical analyses.
Disclosure statement
EG has received honoraria for lectures from Roche Diagnostics, B·R·A·H·M·S Thermo Fisher, and Mitsubishi Chemical Europe. He holds institutional research grants from Roche Diagnostics and Daiichi Sanyko and is consultant for Roche Diagnostics and B·R·A·H·M·S Thermo Fisher. JOV and SG are employees of B·R·A·H·M·S Thermo Fisher. KH received honoraria for lectures from AstraZeneca, Bayer, Boehringer Ingelheim, B·R·A·H·M·S Thermo Fisher, Daiichi Sankyo, Pfizer, Sanofi and The Medicines Company and has received research funding form AstraZeneca and B·R·A·H·M·S Thermo Fisher. ChH report speaker fees and honoraria for consultancy from B·R·A·H·M·S Thermo Fisher Scientific. AS holds institutional research grants from B·R·A·H·M·S Thermo Fisher and Roche Diagnostics.
References
- Alquézar, A., et al., 2017. Combined high-sensitivity Copeptin and troponin T evaluation for the diagnosis of non-ST elevation acute coronary syndrome in the emergency department. Emergencias, 29, 237–244.
- Ambavane, A., et al., TRAPID-AMI investigators, 2017. TRAPID-AMI investigators.Economic evaluation of the one-hour rule-out and rule-in algorithm for acute myocardial infarction using the high-sensitivity cardiac troponin T assay in the emergency department. PloS one, 12 (11), e0187662.
- Anand, A., Shah, A.S.V., et al., 2019. Global adoption of high-sensitivity cardiac troponins and the universal definition of myocardial infarction. Clinical chemistry, 65 (3), 484–489.
- Bahrmann, P., et al., 2013. Additional diagnostic and prognostic value of Copeptin ultra-sensitive for diagnosis of non-ST-elevation myocardial infarction in older patients presenting to the emergency department. Clinical chemistry and laboratory medicine, 51, 1307–1319.
- Balmelli, C., et al., 2013. Comparison of the performances of cardiac troponins, including sensitive assays, and Copeptin in the diagnostic of acute myocardial infarction and long-term prognosis between women and men. American heart journal, 166 (1), 30–37.
- Body, R., et al., 2017. Troponin-only Manchester Acute Coronary Syndromes (T-MACS) decision aid: single biomarker re-derivation and external validation in three cohorts. Emergency medicine journal : EMJ, 34 (6), 349–356.
- Boeddinghaus, J., et al., 2017. Direct comparison of 4 very early rule-out strategies for acute myocardial infarction using high-sensitivity cardiac troponin I. Circulation, 135 (17), 1597–1611.
- Brown, L.D., Cai, T.T., and dasGupta, A., 2001. Interval estimation for a binomial proportion. Statistical science, 16 (2), 101–133.
- Chapman, A.R., et al., 2017. Comparison of the efficacy and safety of early rule-out pathways for acute myocardial infarction. Circulation, 135 (17), 1586–1596.
- Chenevier-Gobeaux, C., et al., 2019. Is high-sensitivity troponin, alone or in combination with Copeptin, sensitive enough for ruling out NSTEMI in very early presenters at admission? A post hoc analysis performed in emergency departments. BMJ open, 9 (6), e023994.
- Chew, D.P., et al., 2019. Randomized trial of a 1-hour troponin t protocol in suspected acute coronary syndromes: the Rapid Assessment of Possible Acute Coronary Syndrome in the Emergency Department With High-Sensitivity Troponin T Study (RAPID-TnT). Circulation, 140 (19), 1543–1556.
- Eggers, K.M., Venge, P., and Lindahl, B., 2012. High-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest pain. Clinica chimica acta, 413 (13-14), 1135–1140.
- Giannitsis, E., et al., 2011. Combined testing of high-sensitivity troponin T and Copeptin on presentation at prespecified cutoffs improves rapid rule-out of non-ST-segment elevation myocardial infarction. Clinical chemistry, 57 (10), 1452–1455.
- Giannitsis, E., et al., 2019. Multicentre cross-sectional observational registry to monitor the safety of early discharge after rule-out of acute myocardial infarction by Copeptin and troponin: the Pro-Core registry. BMJ open, 9 (7), e028311.
- Giannitsis, E., et al., 2020. Instant rule-out of suspected non-ST-segment elevation myocardial infarction using high-sensitivity cardiac troponin T with Copeptin versus a single low high-sensitivity cardiac troponin T: findings from a large pooled individual data analysis on 10,329 patients. Clinical research in cardiology : official journal of the German Cardiac Society.
- Granger, C.B., et al., Global Registry of Acute Coronary Events Investigators, 2003. Predictors of hospital mortality in the global registry of acute coronary events. Archives of internal medicine, 163 (19), 2345–2353.
- Keller, T., et al., 2010. Copeptin improves early diagnosis of acute myocardial infarction. Journal of the American College of Cardiology, 55 (19), 2096–2106.
- Levey, A.S., et al., CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration, 2009. A new equation to estimate glomerular filtration rate. Annals of internal medicine, 150 (9), 604–612.
- Lipinski, M.J., et al., 2014. A systematic review and collaborative meta-analysis to determine the incremental value of Copeptin for rapid rule-out of acute myocardial infarction. The American journal of cardiology, 113 (9), 1581–1591.
- Mahler, S.A., et al., 2015. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circulation: Cardiovascular quality and outcomes, 8 (2), 195–203.
- Maisel, A., et al., 2013. Copeptin helps in the early detection of patients with acute myocardial infarction: primary results of the CHOPIN trial (Copeptin Helps in the early detection Of Patients with acute myocardial INfarction. Journal of the American College of Cardiology, 62 (2), 150–160.
- Meune, C., et al., 2011. Combination of Copeptin and high-sensitivity cardiac troponin T assay in unstable angina and non-ST-segment elevation myocardial infarction: a pilot study. Archives of cardiovascular diseases, 104 (1), 4–10.
- Michael, G.A., 2007. A significance test of interaction in 2xK designs with proportions. Tutorials in quantitative methods for psychology, 3 (1), 1–7.
- Möckel, M., et al., 2015. Early discharge using single cardiac troponin and Copeptin testing in patients with suspected acute coronary syndrome (ACS): a randomized, controlled clinical process study. European heart journal, 36 (6), 369–376.
- Mokhtari, A., et al., 2016. A 1-h combination algorithm allows fast rule-out and rule-in of major adverse cardiac events. Journal of the American College of Cardiology, 67 (13), 1531–1540.
- Morawiec, B., et al., 2018. Combined use of high sensitive cardiac troponin, copeptin, and the modified HEART score for rapid evaluation of chest pain patients. Disease markers., 2018, 1–7.
- Mueller, C., et al., TRAPID-AMI Investigators, 2016. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Annals of emergency medicine, 68 (1), 76–87.
- Mueller-Hennessen, M., et al., 2019. Combined testing of Copeptin and high-sensitivity cardiac troponin T at presentation in comparison to other algorithms for rapid rule-out of acute myocardial infarction. International journal of cardiology, 276, 261–267.
- Neumann, J.T., et al., 2016. Diagnosis of myocardial infarction using a high-sensitivity troponin i 1-hour algorithm. JAMA cardiology, 1 (4), 397–404.
- Pickering, J.W., et al., 2017. Rapid rule-out of acute myocardial infarction with a single high sensitivity cardiac troponin T measurement below the limit of detection: a collaborative meta-analysis. Annals of internal medicine, 166 (10), 715–724.
- Potocki, M., et al., 2012. Diagnostic and prognostic impact of Copeptin and high-sensitivity cardiac troponin T in patients with pre-existing coronary artery disease and suspected acute myocardial infarction. Heart, 98 (7), 558–565.
- Raskovalova, T., et al., 2014. Diagnostic accuracy of combined cardiac troponin and Copeptin assessment for early rule-out of myocardial infarction: a systematic review and meta-analysis. European heart journal: acute cardiovascular care, 3 (1), 18–27.
- Reichlin, T., et al., 2009. Incremental value of Copeptin for rapid rule out of acute myocardial infarction. Journal of the American College of Cardiology, 54 (1), 60–68.
- Reichlin, T., et al., 2012. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Archives of internal medicine, 172 (16), 1211–1218.
- Reinhold, T., et al., 2018. Cost analysis of early discharge using combined Copeptin/cardiac troponin testing versus serial cardiac troponin testing in patients with suspected acute coronary syndrome. PloS one, 13 (8), e0202133.
- Roffi, M., et al., ESC Scientific Document Group, 2016. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). European heart journal, 37 (3), 267–315.
- Saenger, A.K., et al., 2011. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clinica chimica acta., 412 (9-10), 748–754.
- Sebbane, M., et al., 2013. Early rule out of acute myocardial infarction in ED patients: value of combined high-sensitivity cardiac troponin T and ultrasensitive Copeptin assays at admission. The american journal of emergency medicine, 31 (9), 1302–1308.
- Shin, H., et al., 2018. Diagnostic accuracy of adding Copeptin to cardiac troponin for non-ST-elevation myocardial infarction: a systematic review and meta-analysis. PLoS one, 13 (7), e0200379.
- Slagman, A., et al., 2015. Temporal release pattern of Copeptin and troponin T in patients with suspected acute coronary syndrome and spontaneous acute myocardial infarction. Clinical chemistry, 61 (10), 1273–1282.
- Stallone, F., et al., 2016. Incremental value of Copeptin in suspected acute myocardial infarction very early after symptom onset. European heart journal. Acute cardiovascular care, 5 (5), 407–415.
- Stoyanov, K.M., et al., 2020. RAPID-CPU: a prospective study on implementation of the ESC 0/1-hour algorithm and safety of discharge after rule-out of myocardial infarction. Eur Heart J Acute Cardiovasc Care, 9 (1), 39–51.
- Than, M., et al., 2013. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department?: a clinical survey. International journal of cardiology, 166 (3), 752–754.
- Than, M.P., et al., 2016. Effectiveness of EDACS versus ADAPT accelerated diagnostic pathways for chest pain: a pragmatic randomized controlled trial embedded within practice. Annals of emergency medicine, 68 (1), 93–102.
- Than, M.P., et al., ICare-ACS Implementation Group, 2018. ICare-ACS (Improving Care Processes for Patients With Suspected Acute Coronary Syndrome): a study of cross-system implementation of a national clinical pathway. Circulation, 137 (4), 354–363.
- Thelin, J., et al., 2013. The combination of high sensitivity troponin T and Copeptin facilitates early rule-out of ACS: a prospective observational study. BMC cardiovascular disorders, 13, 42.
- Thygesen, K., Alpert, J.S., and White, H.D, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction, 2007. Joint ESC/ACCF/AHA/WHF Task Force for the redefinition of myocardial infarction universal definition of myocardial infarction. Eur Heart journal, 28 (20), 2525–2538.
- Thygesen, K., et al., Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, 2012. ESC Committee for Practice Guidelines (CPG). Third universal definition of myocardial infarction. European heart journal, 33 (20), 2551–2567.
- Twerenbold, R., et al., 2016. Impact of high sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. European heart journal, 37 (44), 3324–3332.
- Twerenbold, R., et al., 2019. Outcome of applying the ESC 0/1-hour algorithm in patients with suspected myocardial infarction. Journal of the American College of Cardiology, 74 (4), 483–494.
- Wildi, K., et al., 2015. Incremental value of Copeptin to highly sensitive cardiac Troponin I for rapid rule-out of myocardial infarction. International journal of cardiology, 190, 170–176.
- Wildi, K., et al., APACE investigators, 2019. Comparison of fourteen rule-out strategies for acute myocardial infarction. International journal of cardiology, 283, 41–47.
- Zellweger, C., et al., 2015. Use of Copeptin and high-sensitive cardiac troponin T for diagnosis and prognosis in patients with diabetes mellitus and suspected acute myocardial infarction. International journal of cardiology, 190, 190–197.