Abstract
Background
While large GWAS analyses have not found convincing associations between MDM2 promoter SNP55 and gynaecological cancers, SNP55 is in linkage disequilibrium with two other functional SNPs in the same promoter, likely to obscure associations between single SNPs and cancer risk. Here, we assessed the impact of SNP55 on risk of endometrial and ovarian cancer, including sub-analyses stratified for other functional SNPs in the region.
Material and methods
Using a custom LightSNiP assay, we genotyped SNP55 in two large hospital-based cohorts of patients with ovarian (n = 1,332) and endometrial (n = 1,363) cancer and compared genotypes to healthy female controls (n = 1,858).
Results
Among individuals harbouring the SNP309TT genotype, the minor SNP55T-allele was associated with a reduced risk of endometrial (dominant model: OR = 0.63; CI = 0.45–0.88; p = 0.01). Regardless of the genotype in neighbouring SNPs, the SNP55T-allele was also associated with a reduced risk of endometrial cancer before 50 years of age (dominant model: OR = 0.56; CI = 0.34–0.90; p = 0.02). No association between SNP55 status and ovarian cancer risk was observed.
Conclusions
MDM2 SNP55T-allele may correlate with reduced risk for endometrial cancer in a SNP309T-, but not SNP309G, context.
Introduction
The Murine Double Minute 2 homolog (MDM2) is a well-established proto-oncogene and is known to negatively regulate p53 by targeting it for proteasomal degradation (Haupt et al. Citation1997, Honda et al. Citation1997). The MDM2 gene is regulated by two promoters directing expression of two transcripts with different first exons/5’UTRs (Barak et al. Citation1994, Zauberman et al. Citation1995). In human cancers, MDM2 expression is frequently upregulated through gene amplification, but also by enhanced transcription/translation in non-amplified tumours (Oliner et al. Citation1992, Momand et al. Citation1998).
Several polymorphisms in the MDM2 promoters have been found to influence MDM2 expression levels by modulating transcription factor binding affinities and also to be associated with cancer risk. (Bond et al. Citation2004, Knappskog et al. Citation2011, Citation2012a, Knappskog and Lønning Citation2011; Gansmo et al. Citation2017). The SNP309G-allele (SNP309T>G; rs2279744) of promoter P2 was previously found to increase MDM2 expression by elongating the binding site of the Sp1 transcription factor, leading to increased cancer risk (Bond et al. Citation2004). Subsequent case–control studies have generated conflicting results, reflecting potential differences in effect depending on tissue, gender and ethnicity (Hu et al. Citation2007, Wilkening et al. Citation2007, Bai et al. Citation2009, Economopoulos and Sergentanis Citation2010). Later, a second SNP (SNP285G>C; rs117039649) was identified 24 bp upstream of SNP309 (Paulin et al. Citation2008, Knappskog et al. Citation2011). Contrasting SNP309G, the SNP285C minor allele reduced Sp1 binding and MDM2 transcription (Knappskog et al. Citation2011, Citation2012b). Notably, the SNP285C-allele has been found in Caucasian and some neighbouring populations only, while it is absent in Eastern-Asian and Sub-Saharan African populations (Knappskog and Lønning Citation2011; Knappskog et al. Citation2014). In addition to SNP309 and SNP285, which both reside in the MDM2 promoter P2, a 40-bps insertion/deletion variant, del 1518 (rs3730485), located in the MDM2 promoter P1, has been found to affect promoter activity (Lalonde et al. Citation2012). Further, del1518 has been reported to have a strong linkage disequilibrium with the SNP309 locus (Hu et al. Citation2006, Gansmo et al. Citation2016, Gansmo et al. Citation2017). Despite the del variant was associated with decreased MDM2 expression in some cell lines (Lalonde et al. Citation2012), subsequent studies assessing the potential effects of the del1518 variant on cancer risk have been conflicting (Gansmo et al. Citation2016, Gallegos-Arreola et al. Citation2017, Gansmo et al. Citation2017, Hua et al. Citation2017, Moazeni-Roodi et al. Citation2019).
Recently, the MDM2 SNP55C > T (rs2870820) located to the MDM2 promoter P2 was identified and functionally characterized (Okamoto et al. Citation2015). SNP55 was thus found to be the third functional SNP located within the MDM2 promoter P2. The SNP55T-allele was reported to influence the binding affinity of both the Sp1 and NF-kB transcriptional factors (Okamoto et al. Citation2015). Recently, we reported the SNP55T-allele to be associated with elevated risk of left-sided colon cancer in women but not in men, while no impact was found on the risk of breast-, prostate-, or lung cancers (Helwa et al. Citation2016). Moreover, SNP55 was found to be in complete linkage disequilibrium with both SNP309 and SNP285, with the SNP55T-allele almost exclusively restricted to the SNP309T-285G-allele (i.e. high D’ but low r2) (Helwa et al. Citation2016).
Large GWAS analyses performed on gynaecological cancers have not reported SNP55 to be associated with risk (Phelan et al. Citation2017, O'Mara et al. Citation2018). However, these types of studies have typically assessed the risk associations related to single SNPs. As such, the role of SNP55 status and its potential interplay with the other SNPs, also affecting Sp1 binding sites in the same promoter, has remained unknown.
In the present study, MDM2 SNP55 was genotyped in large Norwegian hospital-based sample sets of ovarian- and endometrial cancer patients and the frequencies were compared with previously reported genotypes in population based healthy controls. The impact on cancer risk was estimated both for SNP55 per se and in the context of other SNPs affecting Sp1 binding in the MDM2 promoter.
Clinical significance
MDM2 SNP55T-allele may be associated with reduced risk for endometrial cancer in women younger than 50 years.
Among MDM2 SNP309TT genotype carries the SNP55T allele may be correlated with reduced risk for endometrial cancer.
Material and methods
Study population
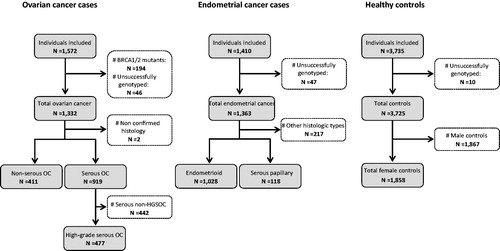
In this case–control study, the blood samples from cancer cases were obtained from hospital-based cohorts of Norwegian ovarian- (n = 1,332) and endometrial- (n = 1,363) cancer patients (). All patients have previously been genotyped for the MDM2 SNPs 285 (rs117039649) and 309 (rs2279744) (Knappskog et al. Citation2011, Citation2012b), and all ovarian cancer cases had been tested and found negative for BRCA1/2 mutations (patients with mutations were excluded). Other than the BRCA1/2 status, there were no selection criteria; consecutive patients were included, avoiding any selection bias. For comparison, the genotype data for all three SNPs (SNP55, SNP285 and SNP309) from blood samples of healthy female controls (n = 1,858) were extracted from our recently reported study (Helwa et al. Citation2016). These healthy individuals were drawn from the population-based Cohort of Norway (CONOR) study (Naess et al. Citation2008) and the matching to cancer cases (selection criteria) is described in detail previously (Lonning et al. Citation2018).
All experiments were executed according to the Norwegian guidelines for research on human material. Each sample donor involved in this study has provided written informed consent to use the samples for research purpose. The study was approved by the Regional Committee for Ethics in Medical Research (REK Midt-Norge).
GWAS data for ovarian cancer
GWAS data for ovarian cancer and MDM2 promoter known functional SNPs were mined form the available summary results look-up from the Ovarian Cancer Association Consortium (OCAC) at ocac.ccge.medschl.cam.ac.uk/data-projects/.
MDM2 SNP55 genotyping
Genotyping of MDM2 SNP55 (rs2870820) was performed using a custom LightSNiP assay (TIB MOLBIOL Syntheslabor GmbH, Berlin, Germany) on a LightCycler 480 II instrument (Roche, Basel, Switzerland) as previously described (Knappskog et al. Citation2014, Helwa et al. Citation2016). The high-resolution melting (HRM) curves were analysed using Melt Curve Genotyping module in the LightCycler 480 software version 1.5.
Statistical analysis
Potential associations between MDM2 SNP55 status and cancer risk were assessed by estimating odds ratios (ORs) and Fisher exact test. ORs are given with 95% confidence intervals (CIs). Since SNP55 is in complete linkage disequilibrium with SNP309 and SNP285 (Helwa et al. Citation2016), a pre-planned analysis strategy was followed, where ORs were also calculated within the subgroups of individuals that were homozygous or heterozygous for the SNP309 T-allele (i.e. the SNP309T/SNP285G-haplotpye). In addition, ORs were estimated within age groups (10 years intervals) and patients were stratified according to histopathological classification: Ovarian cancer patients were first classified as serous or non-serous, then analyses restricted to high-grade serous ovarian cancers (HGSOC) were performed. For endometrial cancer patients, sub-analyses were performed for the groups of endometrioid-, and the group of serous papillary cancers.
In addition to estimating odds ratios, the impact of SNP status on age at cancer onset was assessed. These differences were assessed using Kruskal–Wallis rank test for comparison of three groups and Mann–Whitney rank test for comparison of two groups.
All p values are given as two-sided and all statistical analyses were performed using SPSS software statistical package (version22).
Results
MDM2 SNP55 in GWAS data sets
Mining available GWAS reports for information on SNP55 (rs2870820), we found that this variants was not flagged as associated with risk of endometrial cancer in a recent large meta-analysis including a total of more than 12,000 cases and 108,000 controls (O'Mara et al. Citation2018). Among eight variants previously reported and nine found in the meta-analysis, none were located on band 12q15, where the MDM2 gene is located.
Similarly, SNP55 has not been flagged as associated with ovarian cancer risk (Phelan et al. Citation2017). Interestingly though, mining the available raw data from the Ovarian Cancer Association Consortium (OCAC), including more than 21,000 cases and 29,000 controls, we found the SNP55T to be weakly associated with reduced risk of ovarian cancer (OR = 0.96; CI = 0.94–0.99; p < 0.01). The risk reduction was slightly more pronounced when analyses were restricted to HGSOC (OR = 0.95; CI = 0.92–0.98; p < 0.01; Supplementary Table S1).
Neither for endometrial nor ovarian cancer, did the available information allow sub-analyses stratified for the other known functional SNPs in the proximity of SNP55.
Distribution of MDM2 SNP55 genotypes and cancer risk
The genotype distribution of SNP55 in female healthy controls, ovarian cancer, and endometrial cancer patients is summarized in . The distribution was found to be in Hardy–Weinberg equilibrium in all three groups.
Table 1. MDM2 SNP55 genotype distribution and risk estimates for ovarian and endometrial cancer.
To assess the impact of SNP55 status on ovarian and endometrial cancers risk, we estimated odds ratios (ORs) as compared to the healthy controls. Applying a dominant model for the minor allele (MDM2 SNP55TT + TC versus CC), no significant association was found between SNP55 status and risk of either ovarian (OR = 0.97; CI = 0.83–1.12; p = 0.65) or endometrial cancer (OR = 0.97; CI = 0.83–1.12; p = 0.65; ; ). Correspondingly, no association was observed applying a recessive model (MDM2 SNP55TT versus TC + CC; OR = 0.94; CI = 0.78–1.12; p = 0.49 and OR = 0.94; CI = 0.78–1.12; p = 0.52, respectively).
Figure 2. Impact of MDM2 SNP55 on ovarian and endometrial cancers risk. Forest plots illustrating the effect (odds ratio; OR) of SNP55 (dominant model) on risk of ovarian- and endometrial cancer in (A) overall cancer patients and (B) in patients harbouring the MDM2 SNP309TT genotype. Dot size indicate sample size (number of cases) and horizontal whiskers indicate 95% confidence interval for the OR estimate.
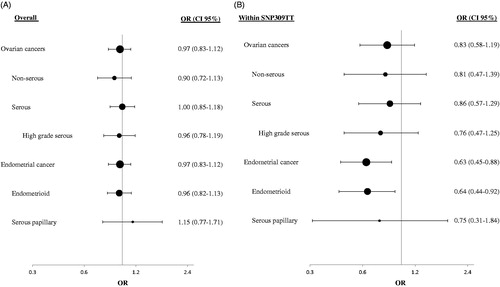
The ovarian cancer samples were stratified according to the main histological subtypes of serous versus non-serous and, within the group of serous cancers, further analysed for the sub-group of high-grade serous cancers (HGSOC). No significant association was found between MDM2 SNP55 and serous ovarian cancer (OR = 1.00; CI = 0.85–1.18; p = 1.00), HGSOC (OR = 0.96; CI = 0.78–1.19; p = 0.75), or non-serous cancers (OR = 0.90; CI = 0.72–1.13; p = 0.36).
Endometrial cancer cases were also stratified according to histological subtypes and we found no association to cancer risk in any of the two major subtypes, endometrioid-, or serous papillary cancers (OR = 0.96; CI = 0.82–1.13; p = 0.65, OR = 1.15; CI = 0.77–1.71; p = 0.55, respectively).
We further stratified all samples according to 10-year interval age groups and assessed ORs within each group (Supplementary Table S2). SNP55 status was not associated with risk of ovarian cancer in any of the age groups. Of note, individuals below 50 years of age and harbouring the minor T-allele were found to have decreased risk of endometrial cancer both in a dominant and a recessive model (OR = 0.56; CI = 0.34–0.90; p = 0.02 and OR = 0.43; CI = 0.18–1.0; p = 0.05, respectively).
Distribution of MDM2 SNP55 stratified according to SNP309 status
Given that MDM2 SNP55 and SNP309 are in complete linkage disequilibrium (Helwa et al. Citation2016), with SNP55T located on the SNP309 T-allele only, we performed further stratifications of our data and assessed the impact of SNP55 status among individuals carrying the SNP309 TT or TG genotypes (). Applying a dominant model, we found a reduced risk of endometrial cancer related to the SNP55T-allele among individuals carrying the 309TT genotype (OR = 0.63; CI = 0.45–0.88; p = 0.01; ). The same trend was found applying a recessive model (OR = 0.81; CI = 0.65–1.01; p = 0.07), and strong associations were observed when applying codominant or additive models (Supplementary Table S3). This observation was mirrored in the subgroup of endometrioid cancers, while the association was weaker for serous papillary cancers.
Table 2. MDM2 SNP55 genotype distribution among carriers of the SNP309TT and TG genotypes.
While no clear effect was observed for ovarian cancer in general, we observed a trend of towards SNP55T reducing the OR for HGSOC among SNP309TT-carriers (OR = 0.77; CI= 0.56–1.06; p = 0.11; ), however, this was restricted to the recessive model.
MDM2 promoter haplotypes and risk of ovarian and endometrial cancer
We further compared the four observed haplotypes across SNPs -55, -285 and -309 between cases and controls and estimated ORs (Supplementary Table S4). SNP55T/285G/309T was the most frequent haplotype. Setting this as reference, we found the individuals carrying the SNP55C/285G/309T to have a higher risk of endometrial cancer compared to SNP55T/285G/309T (OR = 1.13; CI = 0.99–1.29; p = 0.07), thereby corroborating the above findings of SNP55T to conferring a lower risk than SNP55C on a SNP309T background.
For ovarian cancer, no significant association between haplotypes and cancer risk was observed.
Influence of MDM2 SNP55 on age at cancer diagnosis
Since many germline risk factors are linked to younger age at disease onset, we compared the age at cancer diagnosis for patients carrying different SNP55 genotypes within our sample sets. In line with our findings of a reduced OR for endometrial cancer in individuals carrying the SNP55T-allele, we found patients harbouring this allele to have a numerically higher age at onset of disease, although this trend was not statistically significant (average age at onset for genotype CC; 65.5 years, CT; 66.3 and TT; 67.0; Supplementary Table 5). The same trend was observed for SNP55T within the subgroup of patients carrying the SNP309 genotype (Supplementary Table 5).
Discussion
SNP55 (rs2870820) was previously identified as a functional variant in the P2 promoter of the MDM2 proto-oncogene. In vitro studies have shown SNP55 to modulate the binding affinity of Sp1 and NF-kB to the P2 promoter and further, allele specific expression analysis also indicated a role for SNP55 with respect to MDM2 transcription (Okamoto et al. Citation2015). In their original paper characterizing SNP55, Okamoto and colleagues (Okamoto et al. Citation2015) also performed a limited case–control study to assess the potential influence on risk of endometrial cancer. Comparing SNP55 genotypes of 45 patients and a similar number of controls, they observed no differences. Subsequently, we found that the minor SNP55T-allele was associated with increased risk of left-sided colon cancer in women, while little effect was observed for prostate-, lung- or breast cancer (Helwa et al. Citation2016).
Over recent years, large scale GWASs have been performed to assess the relationship between SNPs and risk of endometrial and ovarian cancers (Phelan et al. Citation2017, O'Mara et al. Citation2018). While none of these have flagged SNP55 as a cancer risk modulating factor, it is important to note that the MDM2 promoter is a region in which there seems to be a complex interplay between several functional SNPs affecting binding sites of the same transcription factor (Sp1). Through several previous studies, it has been shown that the effects of single SNPs in this region are only detectable when assessed on the background of genotypes of other SNPs in the same region (Knappskog et al. Citation2011, Citation2012a, Citation2012b, Citation2014, Helwa et al. Citation2016). Therefore, in order to assess the real biological impact of SNP55, we here performed genotyping of this SNP in biobanks/data sets allowing for subgroup analyses, taking the genotypes of other SNPs in the proximity into account.
In the present study, we did not detect any major impact of SNP55 on either ovarian or endometrial cancer risk, when estimating ORs for the single SNP in the overall sample sets. Analysing different age intervals, a significantly decreased risk of endometrial cancer associated with the SNP55T allele was identified among individuals under the age of 50 years. This was in contrast to the lack of association seen among individuals above 50 years of age. Although the number of observations in the younger age group was low and the results should be interpreted with caution, these results may reflect differential impact of SNP55 on endometrial cancer before and after menopause. Notably, the SNP309G-allele has been associated with increased risk of colorectal cancer particularly in premenopausal women in a hormone-dependent manner (Bond et al. Citation2006a, Citation2006b), and SNP285 is located within an ER-half-site (Knappskog et al. Citation2012b). Taken together, this provides indications of a link between female hormonal status and the impact of MDM2 regulation on cancer risk.
The fact that we observe opposite effects of SNP55 in endometrial- versus colon cancer may seem puzzling. However, this is in line with recent observations for other MDM2 promoter SNPs, indicating that their functional role and potential impact on cancer susceptibility is highly tissue specific (Ortiz et al. Citation2018).
Several functional SNPs in the MDM2 promoter are in linkage disequilibrium (Helwa et al. Citation2016, Gansmo et al. Citation2017) and have been shown to interact with each other’s effect on cancer risk (Knappskog et al. Citation2011). Therefore, we considered stratified analyses according to neighbouring SNPs as the cleanest assessment of SNP55s role as a risk factor. Since the minor T-allele of SNP55 is present on SNP309T-alleles only, contrasting the SNP285C variant located on SNP309G, we assessed cancer risk within the subgroups of individuals harbouring the SNP309TT or TG genotypes. Accordingly, among the individuals harbouring SNP309TT, we found a decreased risk of endometrial cancer related to SNP55T. This result was confirmed across all applied genotype models. The reasons why SNP55 may be of importance among individuals with the SNP309TT-genotype but not among those carrying the TG-genotype remain unknown. Notably, however, a similar effect was previously observed for SNP285, where the C-allele was associated with reduced risk of breast cancer among individuals with SNP309GG-genotype but not among those carrying the SNP309TG-genotype (Knappskog et al. Citation2011).
The susceptibility to disease due to germline genetic variants is often associated with younger onset of disease comparable to sporadic disease (Berwick et al. Citation2007, Bonadona et al. Citation2011, de Voer et al. Citation2015). Thus, we compared the age of individuals carrying the different genotypes of SNP55. Although our analyses did not reach statistical significance, we observed a numerical increase in age at cancer diagnosis associated with the SNP55T allele in endometrial cancer patients. This mirrors our findings from the risk estimates and further indicate that the SNP55T-allele may be associated with a reduced risk of endometrial cancer.
Conclusions
We found that carrying the MDM2 SNP55T-allele may have a cancer-protective function for endometrial cancer among individuals harbouring the SNP309TT-genotype. In addition to providing risk information for this particular variant, the finding underlines the importance of assessing context/haplotypes in the MDM2 promoter. Further, the SNP55T allele is associated with reduced risk of endometrial cancer before the age of 50 years, potentially indicating a functional role in the premenopausal but not postmenopausal setting.
Supplemental Material
Download PDF (201.6 KB)Supplemental Material
Download PDF (214.5 KB)Supplemental Material
Download PDF (395.1 KB)Supplemental Material
Download PDF (32.1 KB)Supplemental Material
Download PDF (188.2 KB)Acknowledgements
The authors thank Beryl Leirvaag for technical assistance. The majority of this work was performed in the Mohn Cancer Research Laboratory. We also gratefully acknowledge the Ovarian Cancer Association Consortium (OCAC).
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Bai, J., et al., 2009. Cigarette smoking, MDM2 SNP309, gene-environment interactions, and lung cancer risk: a meta-analysis. Journal of toxicology and environmental health. Part A, 72 (11-12), 677–682.
- Barak, Y., et al., 1994. Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes & development, 8 (15), 1739–1749.
- Berwick, M., et al., 2007. Genetic heterogeneity among Fanconi anemia heterozygotes and risk of cancer. Cancer research, 67 (19), 9591–9596.
- Bonadona, V., et al., French Cancer Genetics Network, 2011. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA, 305 (22), 2304–2310.
- Bond, G.L., et al., 2004. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell, 119 (5), 591–602.
- Bond, G.L., et al., 2006a. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer research, 66 (10), 5104–5110.
- Bond, G.L., et al., 2006b. MDM2 SNP309 accelerates colorectal tumour formation in women. Journal of medical genetics, 43 (12), 950–952.
- De Voer, R.M., et al., 2015. Deleterious germline BLM mutations and the risk for early-onset colorectal cancer. Scientific reports, 5, 14060.
- Economopoulos, K.P. and Sergentanis, T.N., 2010. Differential effects of MDM2 SNP309 polymorphism on breast cancer risk along with race: a meta-analysis. Breast cancer research and treatment, 120 (1), 211–216.
- Gallegos-Arreola, M.P., et al., 2017. Association of the Del1518 promoter (rs3730485) polymorphism in the MDM2 gene with breast cancer in a Mexican Population. Ann clin lab sci, 47 (3), 291–297.
- Gansmo, L.B., et al., 2016. Associations between the MDM2 promoter P1 polymorphism del1518 (rs3730485) and incidence of cancer of the breast, lung, colon and prostate. Oncotarget, 7 (19), 28637–28646.
- Gansmo, L.B., et al., 2017. MDM2 promoter polymorphism del1518 (rs3730485) and its impact on endometrial and ovarian cancer risk. BMC cancer, 17 (1), 97
- Haupt, Y., et al., 1997. Mdm2 promotes the rapid degradation of p53. Nature, 387 (6630), 296–299.
- Helwa, R., et al., 2016. MDM2 promoter SNP55 (rs2870820) affects risk of colon cancer but not breast-, lung-, or prostate cancer. Scientific reports, 6, 33153.
- Honda, R., et al., 1997. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS letters, 420 (1), 25–27.
- Hu, Z., et al., 2006. Genetic variants in the MDM2 promoter and lung cancer risk in a Chinese population. International journal of cancer, 118 (5), 1275–1278.
- Hu, Z., et al., 2007. MDM2 promoter polymorphism SNP309 contributes to tumor susceptibility: evidence from 21 case-control studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 16 (12), 2717–2723.
- Hua, W., et al., 2017. MDM2 promoter del1518 polymorphism and cancer risk: evidence from 22,931 subjects. OncoTargets and therapy, 10, 3773–3780.
- Knappskog, S. and Lønning, P.E., 2011. MDM2 promoter SNP285 and SNP309; phylogeny and impact on cancer risk. Oncotarget, 2 (3), 251–258.
- Knappskog, S., et al., 2011. The MDM2 promoter SNP285C/309G haplotype diminishes Sp1 transcription factor binding and reduces risk for breast and ovarian cancer in Caucasians. Cancer cell, 19 (2), 273–282.
- Knappskog, S., et al., Norwegian Breast Cancer Group trial NBCG VI, 2012a. MDM2 promoter SNP344T > A (rs1196333) status does not affect cancer risk. PLoS one, 7 (4), e36263.
- Knappskog, S., et al., MoMaTEC study group, 2012b. SNP285C modulates oestrogen receptor/Sp1 binding to the MDM2 promoter and reduces the risk of endometrial but not prostatic cancer. European journal of cancer (Oxford, England : 1990), 48 (13), 1988–1996.
- Knappskog, S., et al., 2014. Population distribution and ancestry of the cancer protective MDM2 SNP285 (rs117039649). Oncotarget, 5 (18), 8223–8234.
- Lalonde, M.-E., et al., 2012. Identification of functional DNA variants in the constitutive promoter region of MDM2. Human genomics, 6 (1), 15
- Lonning, P.E., et al., 2018. White blood cell BRCA1 promoter methylation status and ovarian cancer risk. Annals of internal medicine, 168 (5), 326–334.
- Moazeni-Roodi, A., et al., 2019. The 40bp indel polymorphism of MDM2 increase the risk of cancer: an updated meta-analysis. Molecular biology research communications, 8 (1), 1–8.
- Momand, J., et al., 1998. The MDM2 gene amplification database. Nucleic acids research, 26 (15), 3453–3459.
- Naess, O., et al., 2008. Cohort profile: cohort of Norway (CONOR). International journal of epidemiology, 37 (3), 481–485.
- Okamoto, K., et al., 2015. SNP55, a new functional polymorphism of MDM2-P2 promoter, contributes to allele-specific expression of MDM2 in endometrial cancers. BMC medical genetics, 16, 67.
- Oliner, J.D., et al., 1992. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature, 358 (6381), 80–83.
- O'mara, T.A., et al., 2018. Identification of nine new susceptibility loci for endometrial cancer. Nature communications., 9 (1), 3166.
- Ortiz, G.J., et al., 2018. Contrasting effects of an Mdm2 functional polymorphism on tumor phenotypes. Oncogene, 37 (3), 332–340.
- Paulin, F.E., et al., 2008. MDM2 SNP309 is associated with high grade node positive breast tumours and is in linkage disequilibrium with a novel MDM2 intron 1 polymorphism. BMC cancer, 8, 281.
- Phelan, C.M., et al., OPAL study group, 2017. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nature genetics, 49 (5), 680–691.
- Wilkening, S., et al., 2007. MDM2 SNP309 and cancer risk: a combined analysis. Carcinogenesis, 28 (11), 2262–2267.
- Zauberman, A., et al., 1995. A functional p53-responsive intronic promoter is contained within the human mdm2 gene. Nucleic acids research, 23 (14), 2584–2592.