Abstract
Background
Identification of metastatic pancreatic cancer (mPC) patients with the worst prognosis could help to tailor therapy. We evaluated readily available biomarkers for the prediction of 90-day mortality in a nationwide cohort of mPC patients.
Methods
Patients with synchronous mPC were included from the Netherlands Cancer Registry (2015–2017). Baseline CA19-9, albumin, CRP, LDH, CRP/albumin ratio, and (modified) Glasgow Prognostic Score ((m)GPS composed of albumin and CRP) were evaluated. Multivariable logistic regression analyses were performed to identify predictors of 90-day mortality. Prognostic value per predictor was quantified by Nagelkerke’s partial R2.
Results
Overall, 4248 patients were included. Median overall survival was 2.2 months and 90-day mortality was 59.4% (n = 1629). All biomarkers predicted 90-day mortality in univariable analysis, and remained statistically significant after adjustment for clinically relevant factors and all other biomarkers (all p < 0.001). The prognostic value of the biomarkers combined was similar to WHO performance status. Patients who received chemotherapy had better outcomes than those who did not, regardless of biomarker levels.
Conclusions
In mPC patients, albumin, CA19-9, CRP, LDH, CRP/albumin ratio, and (m)GPS are prognostic for poor survival. Biomarkers did not predict response to chemotherapy. These readily available biomarkers can be used to better inform patients and to stratify in clinical trials.
Background
Patients with metastatic pancreatic cancer (mPC) face a disappointing outcome, with reported median overall survival (OS) rates of merely 2–3 months in population-based research (Vincent et al. Citation2011, van der Geest et al. Citation2017). FOLFIRINOX and Gemcitabine/nab-paclitaxel chemotherapy have shown to be the most effective treatment regimens in mPC (Conroy et al. Citation2011, Von Hoff et al. Citation2013, Ducreux et al. Citation2015). However, given the relatively high toxicity rates of chemotherapy and possible deterioration in quality of life (Conroy et al. Citation2011), it is crucial to select those patients who are most likely to benefit from systemic treatment. A significant proportion of patients with mPC has an extremely poor prognosis (e.g. survival <3 months) and might not benefit from further treatment (van der Geest et al. Citation2017). Identification of patients with such a poor prognosis could help to tailor treatment and supportive care options (e.g. to refrain from palliative chemotherapy, and/or consider endoscopic or surgical interventions).
Biomarkers could aid in the prediction of outcome and treatment success. Currently, many research efforts focus on the development of novel biomarkers. Unfortunately, many of such attempts fail (Ioannidis Citation2010, de Graaf et al. Citation2018). Thus far, Carbohydrate Antigen 19-9 (CA19-9) remains the only FDA-approved biomarker for pancreatic cancer (Ballehaninna and Chamberlain Citation2012). However, there is some evidence suggesting that other readily available biomarkers, such as C-reactive protein (CRP), albumin and lactate dehydrogenase (LDH), or combinations of those, may predict outcome in pancreatic cancer (McMillan Citation2013, Yu et al. Citation2017, Hang et al. Citation2018). These markers are nowadays not routinely used in clinical practice as current evidence is of limited quality and no potentially clinically relevant outcomes have been studied.
The aim of this study was to determine the prognostic value of readily available biomarkers for poor prognosis (defined as 90-day mortality) in a nationwide cohort of mPC patients. We also explored predictive value for benefit of palliative chemotherapy.
Clinical significance and impact
Many resources are spent in the search for novel prognostic biomarkers in metastatic pancreatic cancer.
However, readily available and non-expensive biomarkers could provide similar information.
We used a large (n = 4248) nationwide database (with many clinical variables available to adjust for) to evaluate readily available biomarkers (CA19-9, CRP, albumin, LDH, and combinations).
This is the largest study to show that all biomarkers predicted 90-day mortality after adjustment.
The prognostic value of the four biomarkers together is similar to that of WHO performance status, one of the most important clinical factors.
We recommend to include these biomarkers in future prognostic studies and clinical trials.
Materials and methods
Patient selection
This is a retrospective study for which data were derived from the Netherlands Cancer Registry (NCR). The NCR is a population-based registry, in which all newly diagnosed malignancies in the Netherlands (approximately 17 million inhabitants) using data extracted from patient’s medical records. Patients are identified using the automated pathological archive (PALGA) and the National Registry of Hospital Discharge Diagnosis. NCR data are registered by trained data administrators. These data administrators use the same standardized instructions, including those for biomarkers. Periodically, the NCR performs a quality check, after which data collection is optimized. Besides, as many research projects use NCR data, data quality is monitored continuously. Before using the biomarker data logic cheques were performed in order to search for outliers/illogical data entries; no signals of systematic errors were found. This study was reported according to the TRIPOD and REMARK guidelines where applicable (McShane et al. Citation2005, Collins et al. Citation2015, Sauerbrei et al. Citation2018).
All patients with synchronous mPC registered in the NCR between 2015 and 2017 were selected: ICD-O-3 topography C25 and morphology codes 8 010;8 012;8 020;8 140;8 141;8 260; 8 310;8 440;8 480;8 481;8 490;8 500;8 560, or a non-microscopic verified invasive neoplasm of the pancreas suspect for adenocarcinoma (excluding C25.4). A subgroup analysis was performed including only patients undergoing palliative chemotherapy.
Definitions
We considered CA19-9, albumin, CRP, and LDH. We studied each biomarker separately, the CRP/albumin ratio, and the (modified) Glasgow Prognostic Score (GPS and mGPS). In the GPS, 1 point each for CRP >10 mg/L or albumin <35 g/L is allocated (Forrest et al. Citation2004, McMillan Citation2013). For the mGPS, 1 point is assigned for CRP >10 mg/L, and 2 points for both CRP >10 mg/L and albumin <35 g/L (Supplementary Table A1) (McMillan Citation2013). Collected values were the highest value (for CA19-9, LDH and total bilirubin) or last value (for albumin and CRP) before start of treatment/decision to renounce treatment (for rationale see Supplementary Table A1). All biomarker tests were performed by the clinical laboratories of the individual hospitals as part of their standard clinical processes. Information on clinical outcome (survival) was therefore not available to the performers of the tests. Other extracted data were: age, sex, socioeconomic status, WHO performance score, comorbidity, tumour location, tumour diameter, clinical tumour (cT) stage, clinical lymph node (cN) stage, location/number of metastatic sites, pathology confirmation, treatment and OS. Socioeconomic status was assessed using social deprivation scores based on income, education and occupation per four-digit postal code area. Registered cT stage in 2 017 were based on the UICC TNM8 edition. For patients diagnosed in 2015–2016 cT was restaged according to the 8th edition using tumour size (Sobin et al. Citation2009). cN status was defined as lymph node negative (cN−), lymph node positive (cN+) or unknown (cNX). Number and sites of metastases were combined in one variable, based on the prognostic value of the number/location of sites as identified in previous work (one site lung/lymph nodes; one site other; two sites; three sites) (Mackay et al. Citation2019). Palliative chemotherapy was defined as administration of at least one dose of any cytotoxic regimen. Patients were treated according to standard clinical practice, usually with FOLFIRINOX or gemcitabine-based therapy. Primary outcome was 90-day mortality (any cause). Secondary outcome was OS. Vital status was checked with the civil municipal registry at 1 February 2019.
Statistical analysis
Missing data occurred frequently () and were deemed missing at random (possibly related to other parameters, but unrelated to outcome) (Schafer and Graham Citation2002). Missing data were estimated using 10-fold multiple imputation and Predictive Mean Matching (Mackinnon Citation2010). As sensitivity analyses complete case analyses were performed. Complete case analyses included complete data for the variables under study, except for the analyses comparing Nagelkerke’s partial R2 which were performed in the database with fully complete cases only to allow for adequate comparison.
Table 1. Characteristics and outcomes of 4248 patients diagnosed with metastatic pancreatic cancer (2015–2017) in the Netherlands.
The Box-Tidwell test was used to assess linearity between the continuous predictors and the Logit, which was violated in CRP. Hence, CRP was log-transformed (LN[CRP]). Median OS with 95% confidence interval (CI) was estimated using Kaplan–Meier analysis. First, the best clinical model using multivariable logistic regression analysis was constructed (backward selection; p > 0.10 for removal), including known relevant predictors and factors with some prognostic value (p < 0.10) in univariable analysis. Subsequently, biomarkers were added to assess their independent prognostic value. Multivariable analyses including (m)GPS or CRP/albumin ratio were not adjusted for individual albumin and CRP levels, as these variables are probably highly correlated to each other.
To illustrate the prognostic effect size for the continuous predictors and biomarkers, the odds ratio (OR) was calculated for comparison of the 75th versus the 25th percentile. This was achieved by scaling of the biomarker values (division of values by their interquartile range). The added prognostic value of each predictor and biomarker was explored by calculating Nagelkerke’s R2 for univariable analysis and after adjustment. The partial Nagelkerke’s R2 for each variable is the percentage of variability in the outcome which is explained by the specific prognostic factor(s) in the model (explained variance).
Predictive values (Ballman Citation2015) for the effect of palliative chemotherapy were explored. Biomarkers were categorized based on administration of palliative chemotherapy (yes/no) and biomarker levels (high vs. low using the median as cut-off value; or predefined scores for GPS and mGPS). To account for immortal-time bias in these analyses, 30-day mortality was excluded. Data were analysed using IBM SPSS Statistics for Windows version 24.0 (IBM corp., Armonk, NY). A p value <0.05 was considered statistically significant.
Results
Cohort description
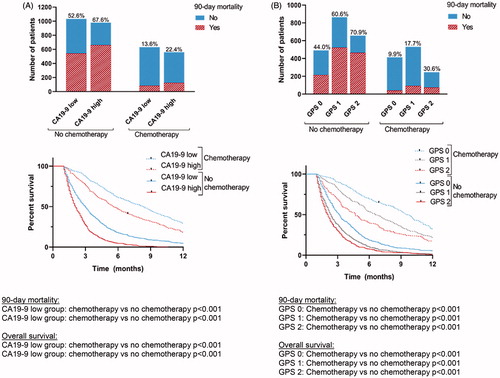
After exclusion of seven patients who were lost-to-follow-up within 90 days, 4 248 patients with mPC were analysed. Median age was 70 (interquartile range (IQR) 63–77) years and 53% was male (n = 2 239; ). The diagnosis of pancreatic cancer was pathologically confirmed in 3 054 patients (72%), and metastases were pathologically confirmed in 2 293 (54%). Palliative chemotherapy was administered to 1 218 patients (29%). Ninety-day mortality was 58% (n = 2 462) in the total cohort, and 20% (n = 238) in 1 218 patients receiving palliative chemotherapy. Median OS of the total cohort was 2.3 months (95%CI 2.2 to 2.4). Median biomarker levels are shown in . Of patients with a (m)GPS score of 2, 1 175/1 536 (77%) died within 90 days compared to 33% (317/965) and 34% (394/1 157) with a GPS or mGPS score of 0, respectively ().
Table 2. Ninety-day mortality for each (modified) Glasgow Prognostic Score category in metastatic pancreatic cancer.
Prognostic value of readily available biomarkers
In the total cohort, in univariable analysis, all biomarkers predicted 90-day mortality (all p < 0.001; ). After adjustment for age, WHO performance score, cardiovascular disease, primary tumour location, cN status and location/number of metastatic sites (best clinical model, Supplementary Table A2) all individual biomarkers were still prognostic (p < 0.001). After addition of also all other biomarkers (highly correlated biomarkers/scores were not added to the same model, see methods), all biomarkers predicted 90-day mortality. All biomarkers (individual and after adjustments) also predicted the secondary outcome OS (all p < 0.001; see Supplementary Table A3).
Table 3. Prognostic factors for 90-day mortality after diagnosis in patients with metastatic pancreatic cancer.
In the group of patients who received palliative chemotherapy (n = 1 218) all biomarkers predicted 90-day mortality after adjustment for WHO performance score, primary tumour location, cT stage, and location/number of metastatic sites (, for best clinical model see Supplementary Table A4). After including also all biomarkers, all biomarkers except for albumin, were strong prognostic factors.
The size of the prognostic value of each predictor in the total cohort is illustrated in . For example, patients with CA19-9 levels >9 000 ku/L rather than 160 kU/L had a two times higher odds of 90-day mortality (OR 1.95, 95%CI 1.59 to 2.39). Of all clinical factors, the Nagelkerke’s partial R2 was highest for WHO performance status (12% after adjustment for clinical factors, 7% after adjustment for also all biomarkers; ). LDH, albumin and LN[CRP] had a partial R2 similar to or higher than that of age and number/location of metastatic sites. In the full model, the four biomarkers together had a partial Nagelkerke’s R2 higher than that of WHO performance status (10% vs 7%, respectively). In other words, the prognostic value of CA19-9, LDH, LN[CRP] and albumin combined was higher than that of WHO. When comparing GPS, mGPS, CRP/albumin ratio, and LN[CRP] plus albumin as continuous parameters, (m)GPS and the two continuous parameters had the highest explained variance.
Figure 1. Prognostic factors for 90-day mortality after diagnosis in patients with metastatic pancreatic cancer. The odds ratios for the continuous variables (age and all biomarkers) are presented as an increase from the lower quartile (25th percentile) to the upper quartile (75th percentile). Other reference categories are the following: aWHO 0; bprimary tumour location head/body/overlapping/not otherwise specified; ccN − status; d1 metastatic site (lung/lymph nodes) eGPS 0; fmGPS 0.
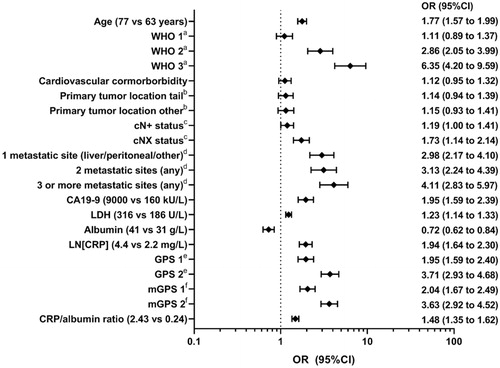
Figure 2. Prognostic value of predictors for 90-day mortality in metastatic pancreatic cancer, expressed as Nagelkerke’s partial R2 values. (A) Clinical predictors. (B) Biomarkers. The figure shows a) the prognostic value each individual biomarker (CA19-9, LDH, CRP, Albumin); unadjusted, adjusted for clinical factors, and after addition of also the biomarkers; b) the prognostic value of four combinations of CRP and albumin (GPS, mGPS, CRP/albumin ratio, and LN[CRP] and albumin as continuous variables), unadjusted, adjusted for clinical factors, and after addition of the remaining biomarkers (i.e. CA19-9 and LDH); c) all four biomarkers combined; unadjusted and adjusted for clinical factors (as there are no remaining biomarkers to add to the model, the bars with ascending stripes and descending stripes show the same results).
![Figure 2. Prognostic value of predictors for 90-day mortality in metastatic pancreatic cancer, expressed as Nagelkerke’s partial R2 values. (A) Clinical predictors. (B) Biomarkers. The figure shows a) the prognostic value each individual biomarker (CA19-9, LDH, CRP, Albumin); unadjusted, adjusted for clinical factors, and after addition of also the biomarkers; b) the prognostic value of four combinations of CRP and albumin (GPS, mGPS, CRP/albumin ratio, and LN[CRP] and albumin as continuous variables), unadjusted, adjusted for clinical factors, and after addition of the remaining biomarkers (i.e. CA19-9 and LDH); c) all four biomarkers combined; unadjusted and adjusted for clinical factors (as there are no remaining biomarkers to add to the model, the bars with ascending stripes and descending stripes show the same results).](/cms/asset/812db6bf-fb10-43e7-9f9b-7c9cca7fded1/ibmk_a_1893814_f0002_c.jpg)
Predictive value of readily available biomarkers
None of the biomarkers was predictive of treatment outcome; patients who underwent chemotherapy had lower rates of 90-day mortality and a more favourable OS compared to those who did not, regardless of biomarker levels. This was illustrated for GPS and CA19-9 in ; for the subgroup of patients with low CA19-9 both 90-day mortality and OS was better when receiving palliative chemotherapy compared to not receiving chemotherapy (p < 0.001 for both analyses). This was also found for the subgroup of patients with high CA19-9, GPS score 0, GSP 1 and GPS 2 (all p < 0.001 for both 90-day mortality and OS). Very similar results we found for the other biomarkers. Also, when including only patients with WHO performance status 0–1 and age <80 (as surrogate definition for the eligibility to receive chemotherapy), there was no predictive value of any biomarker under study.
Sensitivity analysis
Upon sensitivity analysis, comparing the outcomes before and after multiple imputation, similar results were found. The main differences were that in complete-case analysis albumin was not a statically significant prognostic factor after adjustment for all clinical factors/biomarkers in the total cohort, while it was after multiple imputation ( versus Supplementary Table A5). In the chemotherapy group, LDH, albumin and CRP/albumin ratio were not statically significant prognostic in part of the models. Although the (size of the) prognostic value was lower for LN[CRP], albumin, and (m)GPS in complete case analysis than with multiple imputation, the prognostic value of the four biomarkers together was still similar to that of WHO performance status (6.5% vs 7.6%, respectively; and versus A1 and A2). Imputation of all predictors except for the biomarker under study (hence creating separate datasets for each biomarker) showed results very similar to imputation of all variables in one database ( versus Supplementary Table A6).
Discussion
This population-based study showed that CA19-9, albumin, CRP, LDH, (m)GPS, and CRP/albumin ratio predict 90-day mortality independent of other clinical parameters including WHO performance status in 4 248 patients with mPC. The prognostic value of the four individual biomarkers combined was at least similar to that of WHO performance status. There was no predictive value in interaction with chemotherapy; regardless of biomarker levels, patients undergoing palliative chemotherapy had better outcomes than those who received best supportive care.
To the best of our knowledge, this is the largest study reporting on CA19-9, GPS or any of the other biomarkers under study in mPC. As no previous studies assessed the prognostic value of these biomarkers for 90-day mortality, outcomes are difficult to compare. However, the strong prognostic value of CA19-9 for OS is well-known (Haas et al. Citation2013, Goldstein et al. Citation2015). GPS is composed of the positive acute phase protein CRP, and albumin which is a negative acute phase protein but also reflects nutritional status. Since the first report on its prognostic value in non-small cell lung cancer, the GPS has shown strong prognostic value in various types of cancer (Forrest et al. Citation2004, McMillan Citation2013). However, in mPC, only a limited number of relatively small series [n = 81–174 (Inoue et al. Citation2015, Liu et al. Citation2017, Piciucchi et al. Citation2017); n = 439 (Imaoka et al. Citation2016)] studied GPS, most of them show prognostic value similar to our findings. LDH is released in the bloodstream in the case of cell damage and has been associated with poor prognosis in several cancer types, including mPC [n = 196–272 (Tas et al. Citation2014, Yu et al. Citation2017)].
In our analyses, we did not find predictive value of the biomarkers for the effectiveness of chemotherapy. As revealed by a recent review, to date there are no other established predictive biomarkers to guide treatment decisions in pancreatic cancer (van der Sijde et al. Citation2019). A recent exploratory study (n = 54) has suggested that repeated KRAS circulating tumour DNA measurements could be useful in early response prediction in advanced pancreatic cancer (Kruger et al. Citation2018). However, future, larger studies are needed to evaluate possible implementation of circulating tumour DNA in clinical practice (Creemers et al. Citation2017, Strijker et al. Citation2019).
In our study we adjusted for an elaborate set of clinical prognostic factors (‘the best clinical model’). This included 10 of the 12 mandatory prognostic variables as recommended by a recently published consensus statement (included: age, albumin, bilirubin, CA19-9, CRP, disease status, LDH, liver metastasis, number of metastatic sites and performance status; not available were neutrophil-to-lymphocyte ratio and pain at baseline), and three from the recommended set (sex, primary tumour location, pulmonary metastasis) (Ter Veer et al. Citation2018). Most earlier studies adjusted only for a limited set of clinical factors, often not including for instance WHO performance status (Haas et al. Citation2013).
The results of the present study must be interpreted in the light of some limitations. First, the missing data. We performed extensive sensitivity analyses with imputation, and did not find any important differences in the results. The small differences we found (some markers were not statistically significant after adjustment in complete case analysis), are attributable to the inefficiency of complete case analysis, which ignores information on patients even if a single predictor is missing from the set of required predictors for a statistical model. Indeed, shows very similar predictor and biomarker values among complete and completed cases. It seems unlikely that the currently studied relation between the biomarkers and outcomes would differ in the patients with missing data. Second, the exact dates of biomarker measurements were unknown. However, by NCR definitions, all biomarkers were highest (CA19-9, LDH and bilirubin) or last values (albumin and CRP) before start of cancer treatment/decision to renounce treatment. If current results are translated into clinical practice or new studies, the same definitions should be applied. Third, CA19-9 higher than 9 000 kU/L was registered as 9 000 kU/L, which could have led to attenuated prognostic effects in this study. The most important strength of this study is the large number of patients diagnosed with mPC over a recent period of only three years and adequate follow-up. This, combined with a population-based design and availability of many other prognostic factors allowing for adequate adjustment, makes the results generalizable to many different settings.
We recommend to include CA19-9, albumin, CRP and LDH measurements in future studies on mPC. These markers are readily available and non-expensive, but have a combined prognostic value at least similar to that of WHO performance status. When considering CRP and albumin, and its related scores, it seems that GPS, mGPS and the combination of LN[CRP] and albumin as continuous variables have the highest prognostic strength. We note that mGPS and GPS (which seem to perform equally) might be easier to interpret in clinical practice than the combination of (log-transformed) continuous variables. These biomarkers could be used to better inform patients about their prognosis and hence aid end-of-life decision-making, for instance to initiate palliative treatment. In addition, these biomarkers could be used to stratify in clinical trials in order to ensure well-balanced treatment-arms. In the future, the biomarkers could also be used to select patients eligible for specific treatment strategies, e.g. patients with a very poor prognosis of <90 days for best supportive care rather than palliative chemotherapy. A validated prediction model including for example WHO performance status, age, location/number of metastases, and biomarkers could be a next step. Moreover, the value of novel (often expensive) prognostic markers should be compared to these readily available biomarkers.
Ethics approval
The Medical Ethics Committee of the Amsterdam UMC, University of Amsterdam waived the need for ethical approval. The study was performed in accordance with the Declaration of Helsinki.
Presentations
This study was presented during the 13th Congress of the European-African Hepato-Pancreato-Biliary Association (E-AHPBA), Amsterdam 2019.
Author contributions
Study design: MS, EvV, LvdG, MFB, JWW, MGB, HvL. Data acquisition and statistical analysis: MS, EvV, LvdG, MFB, JWW, EWS, MGB, HvL. Manuscript preparation: MS, EvV, LvdG, MGB, HvL. Revising the manuscript: LvdG, ORB, MFB, NHM, MYH, JvH, JV, JdVG, JWW, EWS. Supervision: JWW, EWS, MGB, HvL.
Supplemental Material
Download PDF (612.7 KB)Supplemental Material
Download PDF (587.9 KB)Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the Netherlands Cancer Registry.
Disclosure statement
NHM has acted as a consultant for BMS, Eli Lilly, MSD, Servier, and Astra Zenenca. MFB has received research funding from Celgene and acted as a consultant for Servier. HWL has acted as a consultant for BMS, Celgene, Eli Lilly and Company, Nordic Pharma Group, Philips and Servier, has received research grants from, Amgen, Bayer Schering Pharma AG, BMS, Celgene, Eli Lilly and Company, GlaxoSmithKline Pharmaceuticals, Nordic Pharma Group, Philips, Roche Pharmaceuticals. JDV has received non-financial support from BTG, and Servier, and has served as a consultant for Shire and has received institutional research funding from Servier. The other authors report no conflict of interest.
Data availability statement
This is a retrospective study for which data were derived from the Netherlands Cancer Registry (NCR). All relevant data are available within the manuscript.
Additional information
Funding
References
- Ballehaninna, U.K., and Chamberlain, R.S., 2012. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. Journal of gastrointestinal oncology, 3 (2), 105–119.
- Ballman, K.V., 2015. Biomarker: predictive or prognostic? Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 33 (33), 3968–3971.
- Collins, G.S., et al., 2015. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ (clinical research ed.), 350, g7594.
- Conroy, T., et al., 2011. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New England journal of medicine, 364 (19), 1817–1825.
- Creemers, A., et al., 2017. Clinical value of ctDNA in upper-GI cancers: a systematic review and meta-analysis. Biochimica et biophysica acta. Reviews on cancer, 1868 (2), 394–403.
- de Graaf, G., et al., 2018. The early economic evaluation of novel biomarkers to accelerate their translation into clinical applications. Cost effectiveness and resource allocation : C/E, 16, 23.
- Ducreux, M., et al., ESMO Guidelines Committee, 2015. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the european society for medical oncology, 26(Suppl 5), v56–68.
- Forrest, L.M., et al., 2004. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. British journal of cancer, 90 (9), 1704–1706.
- Goldstein, D., et al., 2015. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. Journal of the National Cancer Institute, 107 (2), dju413.
- Haas, M., et al., 2013. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. Journal of cancer research and clinical oncology, 139 (4), 681–689.
- Hang, J., et al., 2018. Prediction of overall survival for metastatic pancreatic cancer: Development and validation of a prognostic nomogram with data from open clinical trial and real-world study. Cancer medicine, 7 (7), 2974–2984.
- Imaoka, H., et al., 2016. Evaluation of modified Glasgow prognostic score for pancreatic cancer: a retrospective cohort study. Pancreas, 45 (2), 211–217.
- Inoue, D., et al., 2015. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Japanese journal of clinical oncology, 45 (1), 61–66.
- Ioannidis, J.P., 2010. Expectations, validity, and reality in omics. Journal of clinical epidemiology, 63 (9), 945–949.
- Kruger, S., et al., 2018. Repeated mutKRAS ctDNA measurements represent a novel and promising tool for early response prediction and therapy monitoring in advanced pancreatic cancer. Annals of oncology : official journal of the European Society for Medical Oncology, 29 (12), 2348–2355.
- Liu, Z., et al., 2017. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Annals of surgical oncology, 24 (2), 561–568.
- Mackay, T.M., et al., 2019. Association between primary origin (head, body and tail) of metastasised pancreatic ductal adenocarcinoma and oncologic outcome: a population-based analysis. European journal of cancer, 106, 99–105.
- Mackinnon, A., 2010. The use and reporting of multiple imputation in medical research – a review. Journal of internal medicine, 268 (6), 586–593.
- McMillan, D.C., 2013. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer treatment reviews, 39 (5), 534–540.
- McShane, L.M., et al., Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics, 2005. REporting recommendations for tumour MARKer prognostic studies (REMARK). European journal of cancer (Oxford, England : 1990), 41 (12), 1690–1696.
- Piciucchi, M., et al., 2017. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. International journal of molecular sciences, 18 (4), 730.
- Sauerbrei, W., et al., 2018. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): an abridged explanation and elaboration. Journal of the National Cancer Institute, 110 (8), 803–811.
- Schafer, J.L., and Graham, J.W., 2002. Missing data: our view of the state of the art. Psychological methods, 7 (2), 147–177.
- Sobin, L. H., Gospodarowicz, M. K., and Wittekind, C., 2009. International Union Against Cancer (UICC) – the TNM classification of malignant tumours. 7th ed. Hoboken, NJ: Wiley-Blackwell.
- Strijker, M., et al., 2019. Circulating tumor DNA quantity is related to tumor volume and both predict survival in metastatic pancreatic ductal adenocarcinoma. International journal of cancer, 146 (5), 1445–1456.
- Tas, F., et al., 2014. Serum levels of LDH, CEA, and CA19-9 have prognostic roles on survival in patients with metastatic pancreatic cancer receiving gemcitabine-based chemotherapy. Cancer chemotherapy and pharmacology, 73 (6), 1163–1171.
- Ter Veer, E., et al., 2018. Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet oncology, 19 (3), e151–e160.
- van der Geest, L.G.M., et al., the Dutch Pancreatic Cancer Group, 2017. Nationwide trends in chemotherapy use and survival of elderly patients with metastatic pancreatic cancer. Cancer medicine, 6 (12), 2840–2849.
- van der Sijde, F., et al., 2019. Circulating biomarkers for prediction of objective response to chemotherapy in pancreatic cancer patients. Cancers (Basel), 11 (1), 93.
- Vincent, A., et al., 2011. Pancreatic cancer. The lancet (London, England), 378 (9791), 607–620.
- Von Hoff, D.D., et al., 2013. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New England journal of medicine, 369 (18), 1691–1703.
- Yu, S.L., et al., 2017. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Scientific reports, 7, 45194.