Abstract
Introduction
tRNA-derived fragments (tRFs) play an important role in immune responses. To clarify the role of tRFs in autoimmunity we studied circulating tRF-levels in patients with rheumatoid arthritis (RA) and psoriatic arthritis (PsA), and in a murine model for arthritis.
Material and methods
Circulating tRF-levels were quantified by miR-Q RT-qPCR. tRNA processing and modification enzyme expression was analysed by RT-qPCR and public transcriptomics data.
Results
Significant reduction (up to 3-fold on average) of tRF-levels derived from tRNA-Gly-GCC,CCC, tRNA-Glu-CTC and tRNA-Val-CAC,AAC was observed in RA patients, whereas tRNA-Glu-CTC and tRNA-Val-CAC,AAC tRFs were found at significantly higher levels (up to 3-fold on average) in PsA patients, compared to healthy controls. Also in arthritic IL1Ra-KO mice reduced levels of tRNA-Glu-CTC fragments were seen. The expression of NSUN2, a methyltransferase catalysing tRNA methylation, was increased in RA-peripheral blood mononuclear cells (PBMCs) compared to PsA, but this is not consistently supported by public transcriptomics data.
Discussion
The observed changes of specific tRF-levels may be involved in the immune responses in RA and PsA and may be applicable as new biomarkers.
Conclusion
Circulating tRF-levels are decreased in RA and increased in PsA and this may, at least in part, be mediated by methylation changes.
Introduction
Next-generation sequencing studies have revealed various types of circulating non-coding RNAs, including miRNA, tRNA, rRNA, Y RNA and long non-coding RNA. Although RNAs in general exert their function intracellularly, RNAs can also be released into the circulation, either passively upon tissue damage, or actively bound to lipid particles or encapsulated in extracellular microvesicles or exosomes. So far, miRNAs represent the best characterized class of circulating small non-coding RNAs (csncRNAs). They have a regulatory role in many biological processes, such as cell–cell communication and signalling pathways. Extracellular RNA is usually rapidly degraded by RNases, but the short length of csncRNAs and their association with other molecules protect them and increase their extracellular stability.
A prominent class of csncRNAs is tRNA-derived fragments (tRFs). Multiple tRF types have been found, including 5′-tRFs, i-tRFs (internal tRFs), 3′-tRFs, 5′-tRNA halves (5′-tRHs) and 3′-tRNA halves (3′-tRHs) (Magee and Rigoutsos Citation2020). They differ in the cleavage position(s) in the mature or precursor tRNAs and their length ranges from 18 to 36 nucleotides. For a number of specific tRNAs tRFs are much more abundant than for other tRNAs. tRFs are constitutive components of some cell types and are upregulated during specific developmental stages, proliferation, stress or infection (Shigematsu et al. Citation2014). They act as molecular decoys of RNA binding proteins, mediate neuroprotective responses, inhibit angiogenesis after ischemic injury and are involved in sex hormone-related pro-proliferative signalling (Yu et al. Citation2020). The association of tRFs with several human diseases, such as cancer and viral infections, has been demonstrated recently (Zhu et al. Citation2018). Moreover, it has been suggested that tRFs may alter immune responses due to their enrichment in mouse lymphoid organs (Dhahbi, Spindler, Atamna, Boffelli, et al. Citation2013).
Many studies have focused on the role of miRNA in the pathophysiology of rheumatoid arthritis (RA) (Moran-Moguel et al. Citation2018), while nothing is known about the contribution of tRFs to RA development and progression. Recently, we identified tRFs among the most abundant RNA species in RA patient sera (Dunaeva et al. Citation2018). In this study, we investigated whether the levels of circulating cell-free tRFs in serum samples of patients with RA and psoriatic arthritis (PsA) are altered compared to those in healthy subjects. In addition, tRF levels in an arthritis animal model, interleukin-1 receptor antagonist (IL-1Ra) deficient mice, which spontaneously develop polyarthritis, were determined. To obtain more insight in the molecular mechanisms leading to altered circulating tRF levels, the expression of several factors that might be involved in their generation was analysed.
Materials and methods
Study population
Serum samples were obtained from patients with RA (n = 15), patients with PsA (n = 15) and from healthy subjects (CTR; n = 15). Patient serum samples were collected at the Radboud University Medical Center in Nijmegen. CTR serum samples were obtained from the Sanquin Blood Bank in Nijmegen. Demographic and clinical characteristics of the study participants are summarized in Supplementary Table S1. For each patient/subject, several separate tubes of blood were collected to obtain paired samples. One tube was used to prepare the serum sample; the other tubes were used to prepare peripheral blood mononuclear cells (PBMCs) and plasma. Material from all donors was processed within 1 h after blood collection. Patient sera were used in accordance with the code of conduct of research with human material in The Netherlands. All subjects gave informed consent and the study protocol was approved by local medical ethics committee. See Supplementary Figure S1 for a patient flow diagram.
PBMC preparation and total RNA isolation
Human PBMCs were isolated using a density gradient technique (Lymphoprep). Whole blood samples were diluted with an equal volume of phosphate-buffered saline (PBS) and layered on top of Lymphoprep. The tubes were centrifuged at 800 g for 20 min at room temperature. The upper plasma layer was removed and PBMCs were collected and washed with PBS. Total RNA was isolated using the Trizol™ reagent according to the manual (Thermo Fisher Scientific, Waltham, MA). RNA was quantified by spectroscopy using a Nanodrop 2000 spectrophotometer.
Animal samples
Serum samples of IL-1Ra−/− mice, age between 13 and 15 weeks showing macroscopic signs of paw inflammations, were kindly supplied by Dr. Shahla Abdollahi-Roodzas. The IL-1Ra−/− mice were backcrossed onto the arthritis-susceptible strain BALB/c for at least eight generations. BALB/c mice from Charles River (Wilmington, MA) were used as wild-type (WT) controls. All mice were housed in filter-top cages under specific pathogen-free conditions, and a standard diet and water were provided ad libitum.
Small RNA identification, isolation and RT-qPCR
MINTbase version 2.0, a comprehensive database for tRNA-derived fragments (https://cm.jefferson.edu/MINTbase/), was used to identify tRFs (Pliatsika et al. Citation2018). Small RNA was isolated from serum samples using the miRCURY RNA isolation kit-Biofluids (Exiqon) according to the manufacturer’s procedure. The levels of specific small RNAs were analysed using the miR-Q RT-qPCR method (Sharbati-Tehrani et al. Citation2008). The RevertAid M-MuLV reverse transcriptase (Thermo Fisher Scientific) was employed to convert miRNA into cDNA, using primers with 3′-ends complementary to the terminal six nucleotides at the 3′-end of the small RNA of interest. The primer sequences are listed in Supplementary Table S2. The designed primers were applicable for both human and mouse tRFs. Subsequently, qPCR was performed by using GoTaq qPCR Master Mix (Promega, Madison, WI) in combination with specific forward primers containing at least 16 nucleotides corresponding to the 5′-end of the RNA of interest. All reactions were performed in duplicate, in parallel with ‘no-template’ controls. The csncRNA levels were normalized either based upon the serum volume used for RNA isolation, or the 5′ fragment of hY4 RNA as endogenous reference, because stable levels of this molecule were found in all samples. LinRegPCR software (version 11.0) was used to determine amplification efficiency for each of the csncRNA samples tested. The comparative threshold cycle (CT) method was applied to analyse qPCR data. The delta threshold cycle (ΔCT) values were used for ΔΔCT value calculation and the relative tRF levels via the 2− ΔΔCT methodology.
Plasma sample preparation for EV isolation and size exclusion chromatography (SEC)
Blood samples were taken in ethylenediaminetetraacetic acid tubes (BD, Plymouth, UK). Within 1 h, tubes were centrifuged for 10 min at 1690 g at 4 °C to obtain plasma. Thereafter, plasma was centrifuged at 10,000 g for 30 min at 4 °C to obtain platelet-free plasma. Platelet free plasma was filtered through a 0.22 μm syringe filter (Whatmann, GE Healthcare, Buckinghamshire, UK), aliquoted and stored at −80 °C. Plasma extracellular vesicles (EVs) were isolated using a standardized SEC protocol, previously described by Lobb et al. (Citation2015). In short, a sterile column was prepared using a 10 mL syringe stacked with Sepharose CL-2B (Pharmacia, Uppsala, Sweden). After washing the column with PBS containing 0.32% citrate (pH 7.4), 500 µL platelet-free plasma was loaded and the column was eluted with PBS containing 0.32% citrate. Fractions of 1 mL were collected and fraction five (containing EVs) was stored at 4 °C for until further use.
EV size distribution and concentration was estimated by the Brownian motion of particles under constant flow in a NanoSight NS300 equipped with a syringe pump. Concentrations were calculated using Nanoparticle Tracking Analysis 3.2 software (Nanosight Ltd, Amesbury, UK). Vesicles were diluted in PBS, till a suitable concentration for analysis was reached (20–60 particles per frame). Each sample was measured for 60 s, at a flow rate of 50, camera level 10 and detection threshold 5.
Public data analysis
The Gene Expression Omnibus (GEO) microarray datasets (GSE17755, GSE61281, GSE15573, GSE45291) generated for peripheral blood samples from healthy subjects and patients with RA and PsA were used to analyse the superoxide dismutase 2 (SOD2), angiogenin (ANG) and RNA cytosine C5-methyltransferase NSUN2 mRNA expression levels. Relevant details of these datasets are presented in Supplementary Table S3. Microarray data were analysed using GEO2R software (https://www.ncbi.nlm.nih.gov/geo/geo2r/).
CIBERSORT
The Cell type Identification By Estimating Relative Subsets Of Know RNA Transcripts (CIBERSORT) method is an analytical tool to provide an estimation of the abundance of cell types in a mixed cell population based on gene expression data (http://cibersort.stanford.edu/) (Newman et al. Citation2015). The microarray datasets GSE61281 and GSE15573 were used for the analysis. The LM22 matrix was used as a leukocyte gene reference signature (Newman et al. Citation2015). It contains 547 genes that distinguish 22 human hematopoietic cell phenotypes. The number of permutations was set to 1000.
Statistical analysis
All statistical analyses and calculations were performed by the GraphPad Prism version 5 software (La Jolla, CA). Graphics are presented as median with standard error of the mean. To examine the differences between two groups, Mann–Whitney two-sample tests were performed. Correlations of age or other clinical factors with tRF levels were performed using linear regression analysis. For multiple comparison, one-way ANOVA test with Bonferroni correction was applied. All p values were two-sided and p values <0.05 were considered statistically significant.
Sample size calculation
To calculate the sample size for our study, we applied the G*Power software (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower). The following input parameters were used: p value <0.05, power 80%, effect size 0.65 (estimated from our previous study). The G*Power estimation was 13 samples per group that would be sufficient to distinguish RA patients from healthy subjects.
Results
Circulating tRFs in patients with RA and PsA
We have previously analysed the profiles of small RNAs in sera of patients with early RA and healthy subjects by deep sequencing. The majority of the reads appeared to correspond to three classes of small RNAs: hY RNAs, tRNAs and miRNAs (Dunaeva et al. Citation2018). Circulating 5′-tRFs from tRNA-Gly, tRNA-Glu and tRNA-Val were most abundant in all samples. The initial analysis demonstrated that many tRF reads had identical sequences and differed only at the 3′ end. Specific tRF fragments of a certain length of the tRNA-Gly-GCC,CCC tRNA-Glu-CTC, and tRNA-Val-CAC,AAC types were enriched in both groups (Supplementary Table S4). To confirm that these reads are of tRNA origin, the sequences of tRFs were verified using ‘License Plates’, a nomenclature for the naming of short RNA molecules (https://cm.jefferson.edu/LicensePlates/).
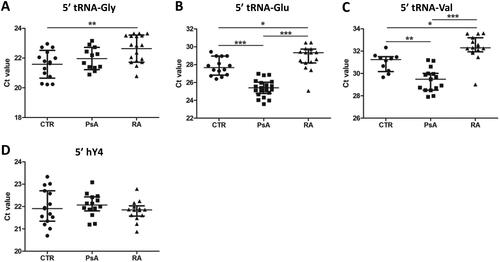
These results prompted us to compare the levels of the most abundant circulating tRF in sera of patients with RA or PsA with those in healthy subjects. Both RA and PsA are characterized by overt inflammation and bone erosion. tRF levels in serum samples were quantified by the miR-Q method. Reverse transcription primers were based on the most abundant isoforms with respect to the 3′ end. The products of miR-Q reactions were analysed by agarose gel electrophoresis and sequenced to confirm that the right tRFs were amplified (). Previously we demonstrated that the levels of circulating 5′ end fragments of hY4 RNA do not differ between patients with RA and healthy subjects (CTR). As a reference, these hY4 RNA fragments were analysed by the miR-Q method in parallel using sera of patients and healthy subjects. The levels of circulating tRFs derived from tRNA-Gly-GCC,CCC, tRNA-Glu-CTC and tRNA-Val-CAC,AAC were significantly lower in RA (higher Ct value) than those in CTR samples, whereas circulating tRFs derived from tRNA-Glu-CTC and tRNA-Val-CAC,AAC were found at significantly higher levels in sera of PsA patients in comparison with CTR and RA samples (). As expected, the levels of circulating 5′ fragments of hY4 RNA were similar in all groups. When the alternative ΔΔCq method was used to calculate relative tRF levels, using the 5′ fragment of hY4 RNA as a reference, similar results were obtained (Supplementary Figure S2).
Figure 1. Analysis of RT-qPCR products of circulating tRFs obtained by the miR-Q approach. (A) Products for tRNA-Gly-GCC, CCC 5′ end and full-length tRNA-Gly-GCC, CCC analysed by agarose gel electrophoresis. Lane 1: RT-qPCR product generated with primers for 5′ end of tRNA-Gly. Lane 2: minus cDNA control with primers for 5′ end. Lane 3: PCR markers; size in base pairs indicated on the left. Lane 4: RT-qPCR product generated with primers for full-length tRNA-Gly. Lane 5: minus cDNA control with primers specific for full-length tRNA. (B) Products for the 5′ ends of tRNA-Glu-CTC and tRNA-Val-CAC, AAC analysed by agarose gel electrophoresis. Like in a products obtained in the presence and absence of cDNA were analysed. The expected sizes for the miR-Q products (indicated by arrows) are 89 base pairs for tRNA-Gly and 94 base pairs for tRNA-Glu and tRNA-Val.
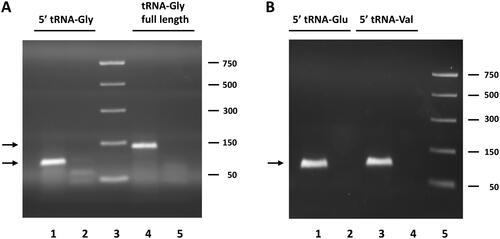
Figure 2. Circulating tRF levels in patient and control sera. (A–C) Results of miR-Q quantification of 5′-ends of tRNA-Gly-GCC,CCC (a), tRNA-Glu-CTC (B) and tRNA-Val-CAC,AAC (C) in sera from patients with PsA or RA and in sera of healthy subjects (CTR). (D) Results of similar analyses for the 5′ end of hY4 RNA. Mann–Whitney two-sample tests were performed to examine the differences between different groups (*p < 0.05; **p < 0.01; ***p < 0.001).
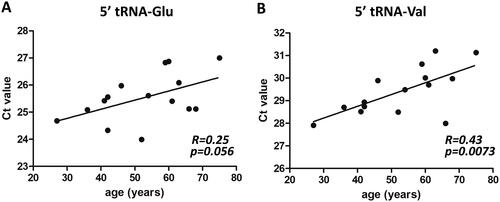
Because most of the RA patients were older than the CTR and PsA patients (Supplementary Table S1 and Supplementary Figure S3; p = 0.002 and 0.02, respectively), we assessed whether there was a correlation between age and the levels of circulating tRFs for the RA and PsA patients and the healthy individuals. A negative correlation between age and the levels of tRFs derived from tRNA-Glu-CTC, and tRNA-Val-CAC,AAC was observed only for patients with PsA (). PsA patients displayed an age-dependent decrease in their tRF levels. No correlation between circulating tRF levels and treatment of PsA patients and the disease activity score (DAS) of RA patients was found for all tRFs analysed (data not shown). For RA, the correlation with treatment could not be analysed, since all RA patients were treated with a DMARD, a biological, or both.
Circulating tRFs in IL1Ra KO mice
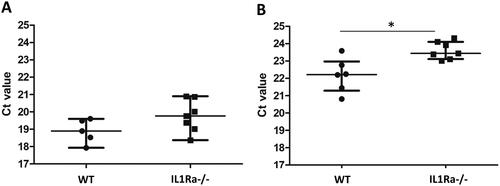
To investigate whether joint inflammation per se leads to alterations in circulating tRF levels, we analysed tRF levels in the IL1 receptor antagonist knockout (IL1Ra KO) mouse model for arthritis. These mice spontaneously develop arthritis in an IL-17 and T-cell dependent manner (Nakae et al. Citation2003). Like in RA patients, lower levels of tRNA-Glu-CTC fragments were seen in IL1Ra KO mice with arthritis in comparison with control mice, and a similar trend was observed for tRNA-Gly-GCC,CCC fragments, although this did not reach statistical significance (). The levels of circulating tRNA-Val fragments in the sera of these mice were very low and, therefore, could not be reliably assessed. These results not only demonstrate that the generation of tRFs and their release in the circulation are not restricted to humans, but also suggest that their levels are altered in mice under arthritic conditions.
Figure 4. Levels of extracellular tRFs in sera of IL1Ra knock-out mice. Small RNAs were isolated from sera of wild type (WT) and IL1Ra deficient mice (IL-1Ra−/−) and the levels of tRFs were determined by the miR-Q approach using tRNA-specific primers. Mann–Whitney two-sample tests were performed to examine the differences between different groups (*p < 0.05).
Extracellular vesicle concentrations
It has been previously shown that tRNAs and tRFs are enriched in EVs (Chiou et al. Citation2018). To investigate whether the altered levels of circulating tRFs were related differences in the quantity of EVs, we measured EVs in plasma of control subjects and patients with RA and PsA. No difference in plasma EV concentration was observed (Supplementary Figure S4). These results indicate that the altered tRF levels in the circulation were not due to changes in the production of EVs.
Blood cell composition
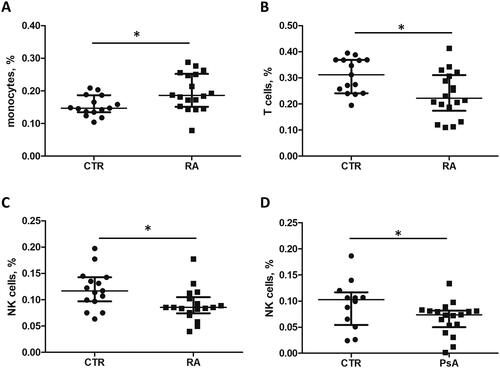
Winek and colleagues identified two main blood compartments based on their specific tRF profile: 1) monocytes, B-, T- and NK cells; and 2) neutrophils, serum, exosomes and erythrocytes (Winek et al. Citation2020). The specific profile of tRFs may also be related to tissue type and state and type of disease (Zhu et al. Citation2018). We hypothesized that the different levels of circulating extracellular tRFs may be caused by differences in blood cell composition. Systemic inflammation is known to affect circulating blood cell quantity and composition, as for example demonstrated by the change in relative levels of peripheral lymphocyte subsets during arthritis development (Lűbbers et al. 2016). To investigate potential differences in global blood cell composition between RA patients, PsA patients and CTR individuals, we applied CIBERSORT, a deconvolution tool based on cell type-specific gene expression signatures, using publicly available microarray data (GSE61281 and GSE15573) (Newman et al. Citation2015). The results revealed an increased number of circulating monocytes (p = 0.016) and a decreased number of natural killer (NK, p = 0.018) and T cells (p = 0.015) in RA patients in comparison with healthy controls (). A reduced number of NK cells was also observed for PsA patients (p = 0.037). These results are in line with a previous study that demonstrated a significantly lower number of circulating NK cells in RA and PsA patients in comparison with healthy controls, as measured by flow cytometry (Conigliaro et al. Citation2014). tRF-33-87R8WP9N1EWJDW (tRNA-Glu) and tRF-33-79MP9P9MH57SD3 (tRNA-Val) were found in compartment 1 (monocytes, B-, T- and NK cells) and serum (Winek et al. Citation2020). Therefore, it is possible that the changes of circulating extracellular tRFs are in part due to altered blood cell composition.
Figure 5. Relative fractions of monocytes, T cells and NK cell in whole blood from RA and PsA patients and healthy individuals. The microarray datasets GSE15573 and GSE61281 were used for the analysis of gene expression profiles and cell fractions. Monocyte, T cell and NK cell fractions were determined for each sample using CIBERSORT.
Superoxide dismutase expression in PBMCs
Many human diseases including RA are associated with the overproduction of reactive oxygen species (ROS), which contributes to the damage and degradation of a variety of macromolecules. In RA, ROS have been demonstrated to be directly involved in joint destruction (Datta et al. Citation2014). Synovial fluid and peripheral blood of RA patients show high levels of ROS and ROS-generated molecules that result from the oxidation and degradation of the major components of cartilage and bone, including collagen and hyaluronic acid (Ishibashi Citation2013). It has also been shown that oxidative stress is associated with the generation of tRFs (Thompson et al. Citation2008).
To investigate whether oxidative stress could explain the circulating tRF differences in RA and PsA patients, we analysed SOD2 enzyme expression. The human SOD family consists of three classes of metalloenzymes: cytoplasmic SOD1, mitochondrial SOD2, and extracellular SOD3. Their activity plays an essential role in cellular redox balance maintenance. SOD2 has been previously demonstrated to be associated with RA, although the function of SOD2 in RA is controversial. The expression of SOD2 mRNA levels in PBMCs from RA and PsA patients and from CTR subjects were analysed by RT-qPCR. The results showed similar SOD2 expression levels in RA and PsA patients and healthy controls (Supplementary Figure S5).
To validate the SOD2 mRNA expression data, SOD2 protein levels in PBMCs of CTR subjects, and RA and PsA patients were determined by western blotting. This revealed no significant difference in SOD2 protein levels between the CTR, RA and PsA groups (Supplementary Figure S6).
Publicly available datasets were used to assess the expression levels of SOD2 mRNAs in whole blood. In agreement with our data the analysis of datasets GSE61281 and GSE15573 showed similar levels of SOD2 mRNA in healthy individuals and PsA patients on the one hand and in healthy individuals and RA patients on the other hand (data not shown).
Although oxidative stress levels in blood may not properly reflect the situation in the inflamed joint, these data suggest that the altered levels of circulating tRFs were not due to elevated oxidative stress levels in PBMCs.
Angiogenin expression levels in PBMCs
Previous studies demonstrated that site-specific methylation of tRNAs protects from ANG-mediated cleavage and tRNAs lacking NSUN2-mediated methylation have a relatively high affinity for ANG. ANG is a ribonuclease involved in the cleavage of tRNA-Gly-GCC and tRNA-Glu-CTC (Pereira et al. Citation2021). Therefore, we also quantified ANG expression in PBMCs by RT-qPCR and observed similar levels in all tested groups (Supplementary Figure S7A). To obtain further support for similar ANG expression levels in PMBCs, we analysed publicly available microarray data (GSE61281, and GSE15573). Consistent with our experimental results, no difference in ANG expression in CTR, RA and PsA whole blood was observed (Supplementary Figure S7(B,C)). These results indicate that the altered tRF levels are unlikely due to altered ANG levels.
Association of NSUN2 expression with RA and PsA
tRNAs are the most extensively modified RNAs in all cells. Using the MODOMICs database, we identified the possible modifications of the tRNAs from which the tRFs were derived and the corresponding enzymes involved in the generation of these modifications (Supplementary Table S5). The modifications m2G (N2-methylguanosine), D (dihydrouridine), m5C (5-methylcytosine) and Ψ (pseudouridine) were found for all tRNAs.
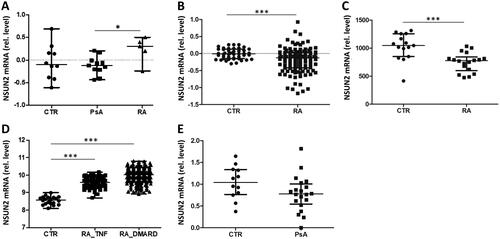
Previous studies demonstrated that loss of cytosine methylation (m5C) of tRNAs leads to accumulation of short tRFs (Blanco et al. Citation2014). NSUN2 and NSUN4 are the main cytosine methyltransferases for tRNAs in higher eukaryotes (Brzezicha et al. Citation2006). We decided to analyse NSUN2 expression, since the vast majority (77%) of actively transcribed tRNAs, including the tRNAs identified in our study, were NSUN2-specific substrates (Blanco et al. Citation2014). NSUN2 expression data in the human protein atlas (https://www.proteinatlas.org/) demonstrated low cell type specificity; all blood cells express more or less equal levels of this enzyme, except for neutrophils which showed very low expression levels. Quantification of NSUN2 gene expression in PBMCs by RT-qPCR revealed higher levels of NSUN2 mRNA in patients with RA compared to PsA patients and healthy individuals, although it did not reach statistical significance when compared to CTR (). Higher expression level of NSUN2 might indicate higher levels of tRNA methylation and as a consequence lower level of tRF compared with CTR and patients with PsA. To verify our results, we analysed four public gene expression datasets (GSE15573, GSE17755, GSE45291 and GSE61281). The analysis showed similar levels in patients with PsA and healthy controls (). A significant decrease in NSUN2 expression was observed for patients with RA (). In contrast, the analysis of GSE45291 revealed a significant increase of NSUN2 mRNA expression (). Of note, the latter dataset should be used with caution, since RA patients in this study showed an inadequate response to DMARD or anti-TNFα treatment. Moreover, all four datasets were generated using whole blood for RNA isolation, while we measured NSUN2 expression in PBMCs. Taken together, the data for NSUN2 expression is heterogeneous and only partially consistent with our experimental results.
Figure 6. Expression levels of NSUN2 in RA and PsA patients and in healthy individuals. (A) RT-qPCR analysis was used to analyse NSUN2 expression in PBMCs from CTR individuals and from patients with PsA and RA. (B–E) Microarray datasets GSE17755 (B), GSE15573 (C), GSE45291 (D) and GSE61281 (E) were used to quantify relative NSUN2 expression levels in whole blood (GSE17755, GSE45291, GSE61281) or PBMCs (GSE15573) of the same patient and control groups. RA_TNF and RA_DMARD indicate RA subgroups characterized by inadequate responses to anti-TNFα and DMARD treatment, respectively. Mann–Whitney two-sample tests were performed to examine the differences between different groups (*p < 0.05; **p < 0.01; ***p < 0.001).
Discussion
Both RA and PsA are chronic diseases that worsen over time. Their clinical symptoms and serological and radiographic features often overlap. Both conditions are associated with joint inflammation, tenderness, pain and swelling (Merola et al. Citation2018). Although in both RA and PsA joint inflammation is associated with the increased production of proinflammatory molecules, circulating inflammation markers such as C reactive protein (CRP) and serum amyloid A are significantly lower in the PsA population than in patients with RA, and this is also true for the erythrocyte sedimentation rate (Veale and Fearon Citation2015). Our study focused on differential levels of circulating tRFs in sera from patients with PsA and RA and healthy subjects. We showed that the levels of circulating extracellular tRFs from tRNA-Glu-CTC and tRNA-Val-CAC,AAC are decreased in patients with RA and increased in patients with PsA in comparison with healthy controls, and that also the levels of tRFs from tRNA-Gly-GCC,CCC are reduced in RA patients. Of interest, these parameters are altered independently of disease activity and treatment, indicating their usefulness as biomarkers to differentiate between disease conditions. Furthermore, this suggests a role in etiopathogenesis rather than being a consequence of increased systemic inflammation.
It should be noted that hydration levels of the individuals from whom blood samples were used may affect csncRNAs levels to some extent. However, it can be excluded that hydration levels affect our conclusions, because (i) the mouse model and human blood analysis gave similar results, (ii) alterations of hydration levels have been demonstrated to affect biomarker concentrations by not more than 10%, whereas more than 3-fold differences were observed for tRFs, and (iii) average values of multiple individuals in each group were compared.
In a previous study on the origin of the tRNA halves in sera several mouse organs were analysed and the presence of 5′ tRNA halves was observed in hematopoietic and lymphoid tissues including spleen, lymph nodes, foetal liver, leukocytes, bone marrow and thymus (Dhahbi, Spindler, Atamna, Yamakawa, et al. Citation2013). We applied a deconvolution approach and publicly available gene expression datasets to compare cell compositions of synovial tissues in patients with RA and PsA and healthy individuals and indeed observed differences in the relative abundance of specific blood cells between these groups. In particular reduced levels of NK cells were observed in both RA and PsA, which is consistent with previously reported data (Conigliaro et al. Citation2014). It is likely that serum tRFs are actually derived from circulating blood cells and, although this is not yet supported by experimental evidence, altered tRNA levels may be related to differences in cellular composition of the inflamed tissues.
It has been shown that EVs are enriched in tRFs. The levels of circulating tRFs may reflect the abundance of EVs, as well as their content and composition. In RA patients the levels of circulating EVs were reported to be increased and EV protein content was reported to be altered (Sellam et al. Citation2009, Skriner et al. Citation2006). Although we did not observe differences in EV levels, our data do not exclude the possibility that differences in EV content are related to the observed differences in circulating tRFs. Additional studies are needed to compare the content of the EVs from patients with RA and PsA and CTR.
In addition to EV-associated tRFs, circulating tRFs not associated with EVs have been reported as well. A key aspect for the accumulation of specific tRNA halves in the extracellular space appeared to be the relatively high stability of tRFs. tRNA-Gly and tRNA-Glu 5′ halves were able to form homo or heterodimers that increase their stability and elevate their steady state levels inside and possibly also outside living cells (Tosar et al. Citation2018). It would be interesting to investigate whether tRF dimerization is related to the differences in tRFs observed between the RA, PsA and healthy controls.
We analysed several other factors that may contribute to altered levels of circulating tRFs in RA and PsA patients. No differences were observed for SOD2 and ANG expression in PBMC, both at the mRNA (SOD2, ANG) and protein level (SOD2). It should be noted that we cannot completely exclude the possibility that differences exist between the mRNA and protein expression levels. Cleavage of tRNA molecules is mediated by ANG (Yamasaki et al. Citation2009). Analysis of our own and public gene expression datasets demonstrated that there are no differences in ANG mRNA expression levels between these groups. This is supported by the results of Liote and coworkers, who showed that RA and healthy control plasma contained similar ANG protein levels (Lioté et al. Citation2003). Taken together, these data indicate that the altered levels of circulating tRFs in RA and PsA in blood are not likely resulting from altered SOD2 and ANG expression levels. These results should be interpreted with care, because it is not known to what extent changes in the proportion of each cell type (discussed above) have effect on the expression of SOD2 and ANG in a mixed cell PBMC or whole blood population.
Since nucleotide modifications increase the stability and rigidity of tRNAs (Durdevic et al. Citation2013), an alternative explanation for the differential levels of tRFs in RA and PsA would be differences in tRNA modifications between the patient groups. More than 100 post-transcriptional nucleotide modifications have been found in tRNAs (Agris Citation2004). Indeed, interference with NSUN2-mediated m5C methylation was shown to increase the cleavage of tRNA, leading to a significant enrichment of 5′-tRFs during brain development in mice (Flores et al. Citation2017). Deficiency of TRMT10A, another methyltransferase, led to tRNA-Gln-UUG, CUG fragmentation with accumulation of 5′-tRFs (Cosentino et al. Citation2018). Increased levels of NSUN2 in PBMCs from RA patients, as observed in our RT-qPCR experiments, might lead to increased levels of tRNA methylation in blood cells and decreased levels of tRFs in RA patient sera. Also other tRNA modifications may play a role in tRNA stability. For example, it has been demonstrated in yeast that pairs of nonessential modifications outside of the anticodon region can be highly important for in vivo stability of tRNA (Alexandrov et al. Citation2006).
This study has some limitations. The number of samples used for this study is relatively small, although the calculation of sample size indicated that our sample size was large enough. We also used publicly available datasets generated from large numbers of patients to verify part of our data. In addition, we did not analyse the modification status of the relevant tRNAs. As already noted above, additional studies are required to clarify the involvement of nucleotide modifications and the enzymes responsible for their generation in the production of extracellular tRFs. Although the results obtained with the animal model support the observations with material from RA patients, additional arthritis models will have to be studied to clarify whether tRF changes are universally associated with arthritis.
In summary, we demonstrated that the levels of circulating extracellular tRFs differ between RA and PsA patients and healthy individuals, which may reflect different molecular processes in the pathophysiology of these diseases. Our results suggest that blood cell composition as well as nucleotide modification differences may affect circulating tRF levels, but more work is required to elucidate the role of these phenomena in more detail. In addition, more insight in the biochemical processes mediated by tRFs may shed more light on the potential molecular mechanisms involved in the pathophysiology of RA and PsA and this may also provide new opportunities to apply tRF level differences in the diagnosis of RA and PsA.
Clinical significance statement
Further research will be needed to assess the diagnostic value of the altered tRF levels in RA and PsA patient sera.
Supplemental Material
Download MS Word (734 KB)Acknowledgements
We thank Bartijn Pieters (10x Genomics, Leiden) for providing the samples of extracellular vesicles from patients. We thank Dr. Shahla Abdollahi-Roodzas (Bristol Myers Squibb, Cambridge, USA) for her help with mouse tissue sample collection.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, GJMP, upon reasonable request.
Additional information
Funding
References
- Agris PF. 2004. Decoding the genome: a modified view. Nucleic Acids Res. 32(1):223–238. doi: 10.1093/nar/gkh185
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell. 21(1):87–96. doi: 10.1016/j.molcel.2005.10.036
- Blanco S, Dietmann S, Flores JV, Hussain S, Kutter C, Humphreys P, Lukk M, Lombard P, Treps L, Popis M, et al. 2014. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 33(18):2020–2039. doi: 10.15252/embj.201489282
- Brzezicha B, Schmidt M, Makalowska I, Jarmolowski A, Pienkowska J, Szweykowska-Kulinska Z. 2006. Identification of human tRNA:m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNA Leu (CAA). Nucleic Acids Res. 34(20):6034–6043. doi: 10.1093/nar/gkl765
- Chiou NT, Kageyama R, Ansel KM. 2018. Selective export into extracellular vesicles and function of tRNA fragments during T cell activation. Cell Rep. 25(12):3356–3370.e4. doi: 10.1016/j.celrep.2018.11.073
- Conigliaro P, Triggianese P, Perricone C, Chimenti MS, Di Muzio G, Ballanti E, Guarino MD, Kroegler B, Gigliucci G, Grelli S, et al. 2014. Restoration of peripheral blood natural killer and B cell levels in patients affected by rheumatoid and psoriatic arthritis during etanercept treatment. Clin Exp Immunol. 177(1):234–243. doi: 10.1111/cei.12335
- Cosentino C, Toivonen S, Diaz Villamil E, Atta M, Ravanat JL, Demine S, Schiavo AA, Pachera N, Deglasse JP, Jonas JC, et al. 2018. Pancreatic β-cell tRNA hypomethylation and fragmentation link TRMT10A deficiency with diabetes. Nucleic Acids Res. 46(19):10302–10318. doi: 10.1093/nar/gky839
- Datta S, Kundu S, Ghosh P, De S, Ghosh A, Chatterjee M. 2014. Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin Rheumatol. 33(11):1557–1564. doi: 10.1007/s10067-014-2597-z
- Dhahbi JM, Spindler SR, Atamna H, Boffelli D, Mote P, Martin DI. 2013. 5’-YRNA fragments derived by processing of transcripts from specific YRNA genes and pseudogenes are abundant in human serum and plasma. Physiol Genomics. 45(21):990–998. doi: 10.1152/physiolgenomics.00129.2013
- Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Boffelli D, Mote P, Martin DIK. 2013. 5′ tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics. 14(1):298. doi: 10.1186/1471-2164-14-298
- Dunaeva M, Blom J, Thurlings R, Pruijn GJM. 2018. Circulating serum miR-223-3p and miR-16-5p as possible biomarkers of early rheumatoid arthritis. Clin Exp Immunol. 193(3):376–385. doi: 10.1111/cei.13156
- Durdevic Z, Mobin MB, Hanna K, Lyko F, Schaefer M. 2013. The RNA methyltransferase Dnmt2 is required for efficient Dicer-2-dependent siRNA pathway activity in Drosophila. Cell Rep. 4(5):931–937. doi: 10.1016/j.celrep.2013.07.046
- Flores JV, Cordero-Espinoza L, Oeztuerk-Winder F, Andersson-Rolf A, Selmi T, Blanco S, Tailor J, Dietmann S, Frye M. 2017. Cytosine-5 RNA methylation regulates neural stem cell differentiation and motility. Stem Cell Rep. 8(1):112–124. doi: 10.1016/j.stemcr.2016.11.014
- Ishibashi T. 2013. Molecular hydrogen: new antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr Pharm Des. 19(35):6375–6381. doi: 10.2174/13816128113199990507
- Lioté F, Champy R, Moenner M, Boval-Boizard B, Badet J. 2003. Elevated angiogenin levels in synovial fluid from patients with inflammatory arthritis and secretion of angiogenin by cultured synovial fibroblasts. Clin Exp Immunol. 132(1):163–168. doi: 10.1046/j.1365-2249.2003.02117.x
- Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Moller A. 2015. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 4:27031. doi: 10.3402/jev.v4.27031
- Lübbers J, van Beers-Tas MH, Vosslamber S, Turk SA, de Ridder S, Mantel E, Wesseling JG, Reijm M, van Hoogstraten IM, Bijlsma JW, et al. 2016. Changes in peripheral blood lymphocyte subsets during arthritis development in arthralgia patients. Arthritis Res Ther. 18(1):205. doi: 10.1186/s13075-016-1102-2
- Magee R, Rigoutsos I. 2020. On the expanding roles of tRNA fragments in modulating cell behavior. Nucleic Acids Res. 48(17):9433–9448. doi: 10.1093/nar/gkaa657
- Merola JF, Espinoza LR, Fleischmann R. 2018. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open. 4(2):e000656. doi: 10.1136/rmdopen-2018-000656
- Moran-Moguel MC, Petarra-del Rio S, Mayorquin-Galvan EE, Zavala-Cerna MG. 2018. Rheumatoid arthritis and miRNAs: a critical review through a functional view. J Immunol Res. 2018:2474529–16. doi: 10.1155/2018/2474529
- Nakae S, Nambu A, Sudo K, Iwakura Y. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173
- Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. 2015. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 12(5):453–457. doi: 10.1038/nmeth.3337
- Pereira M, Ribeiro DR, Pinheiro MM, Ferreira M, Kellner S, Soares AR. 2021. m5U54 tRNA hypomodification by lack of TRMT2A drives the generation of tRNA-derived small RNAs. Int J Mol Sci. 22(6):2941. doi: 10.3390/ijms22062941
- Pliatsika V, Loher P, Magee R, Telonis AG, Londin E, Shigematsu M, Kirino Y, Rigoutsos I. 2018. MINTbase v2.0: a comprehensive database for tRNA-derived fragments that includes nuclear and mitochondrial fragments from all the Cancer Genome Atlas projects. Nucleic Acids Res. 46(D1):D152–D159. doi: 10.1093/nar/gkx1075
- Sellam J, Proulle V, Jüngel A, Ittah M, Miceli Richard C, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM, et al. 2009. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 11(5):R156. doi: 10.1186/ar2833
- Sharbati-Tehrani S, Kutz-Lohroff B, Bergbauer R, Scholven J, Einspanier R. 2008. miR-Q: a novel quantitative RT-PCR approach for the expression profiling of small RNA molecules such as miRNAs in a complex sample. BMC Mol Biol. 9:34. doi: 10.1186/1471-2199-9-34
- Shigematsu M, Honda S, Kirino Y. 2014. Transfer RNA as a source of small functional RNA. J Mol Biol Mol Imaging. 1:8. https://austinpublishinggroup.com/molecular-biology/fulltext/jmbmi-v1-id1007.php
- Skriner K, Adolph K, Jungblut PR, Burmester R. 2006. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 54(12):3809–3814. doi: 10.1002/art.22276
- Thompson DM, Lu C, Green PJ, Parker R. 2008. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 14(10):2095–2103. doi: 10.1261/rna.1232808
- Tosar JP, Gámbaro F, Darré L, Pantano S, Westhof E, Cayota A. 2018. Dimerization confers increased stability to nucleases in 5’ halves from glycine and glutamic acid tRNAs. Nucleic Acids Res. 46(17):9081–9093. doi: 10.1093/nar/gky495
- Veale DJ, Fearon U. 2015. What makes psoriatic and rheumatoid arthritis so different? RMD Open. 1(1):e000025–e000025. doi: 10.1136/rmdopen-2014-000025
- Winek K, Lobentanzer S, Nadorp B, Dubnov S, Dames C, Jagdmann S, Moshitzky G, Hotter B, Meisel C, Greenberg DS, et al. 2020. Transfer RNA fragments replace microRNA regulators of the cholinergic poststroke immune blockade. Proc Natl Acad Sci USA. 117(51):32606–32616. doi: 10.1073/pnas.2013542117
- Yamasaki S, Ivanov P, Hu GF, Anderson P. 2009. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 185(1):35–42. doi: 10.1083/jcb.200811106
- Yu M, Lu B, Zhang J, Ding J, Liu P, Lu Y. 2020. tRNA-derived RNA fragments in cancer: current status and future perspectives. J Hematol Oncol. 13(1):121. doi: 10.1186/s13045-020-00955-6
- Zhu L, Liu X, Pu W, Peng Y. 2018. tRNA-derived small non-coding RNAs in human disease. Cancer Lett. 419:1–7. doi: 10.1016/j.canlet.2018.01.015