?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
Low back disorder (LBD) is a major cause of disability worldwide. Inflammation results in proliferation of cytokines or consequent degradation products (collectively known as inflammatory biomarkers) that activate pain pathways which can result in non-specific LBD. This systematic review and meta-analysis aim to evaluate the relationship between inflammatory biomarkers and clinical outcomes in patients with LBD.
Methods
The PRISMA guideline was followed for the systematic reivew. Three online databases were searched. Four RCTs and sixteen observational studies with 1142 LBD patients were analysed. The primary outcomes were back and leg pain scores, back-specific disability scores and expression of inflammatory biomarkers. Standardized mean difference (SMD) and their 95% confidence intervals (CI) were evaluated. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to summarize the strength of evidence.
Results
Four RCTs and sixteen observational studies were included in the analysis of 1142 patients with LBD. There was a statistically significant reduction in back pain score and IL-1 beta and increase in the expression of CTX-1 and IL-10 levels post treatment. There was a significant relationship between increase in the expression of MCP- and reduction in the expression of hsCRP with increase in back pain. Significant relationship was also observed between increase in the expression of MCP-1 and reduction in the expression of IL-6 with increase in leg pain. Increase in the expression of IL-8 and reduction in the expression of hsCRP was also associated with increased disability score.
Conclusion
Inflammatory biomarkers play a significant role in the pathogenesis of LBD. CTX-1, IL-10 and IL-1 beta may be responsible for the decrease in back pain scores post treatment. There is a relationship between MCP-1, IL-6, IL-8 and hsCRP with clinical and functional assessments for LBD. Further studies will improve understanding of the pathogenesis of LBD and aid in targeted management strategies.
Introduction
The World Health Organization defines low back pain (LBP) as ‘pain and discomfort below the costal margin above the inferior gluteal folds, with or without referred leg pain’. (Ehrlich Citation2003). Clinically, patients may present with a myriad of descriptions for their experience from sharp, dull, aching, stabbing, localised or diffuse pain with moderate to intense severity (Chou Citation2014). Globally in industrial countries approximately 84% of the population has experienced LBP during their life, resulting in significant economic burdens caused by loss of productive work days. Around 85% of these patients seeking physician intervention have no underlying cause of LBP (Wynne-Jones et al. Citation2014). Low back disorder (LBD) is LBP without specific spinal pathologies (i.e. vertebral fracture, malignancy, spinal infection, axial spondylarthritis, cauda equina syndrome). However, spondylolisthesis, spondylosis, disc herniation, disc degeneration, scoliosis, deformity (e.g. hemivertebrae) and radicular syndromes (e.g. radicular pain [leg pain or sciatica], radiculopathy, spinal stenosis) are included as potential low back disorders.
Globally, the prevalence of LBD is high, however, effective treatment options for these patients are limited. Thus, it is imperative to deepen our understanding of the aetiology and risk factors for LBD to reduce this burden. Many studies have shown that inflammatory granulation tissues can causes a markup of pro-inflammatory cytokines which result in the sensitization of nociceptors within the disc resulting in low back pain (Takahashi et al. Citation1996; Habtemariam et al. Citation1998; Burke et al. Citation2002). Rathod et al found that there was a significant elevation of high sensitive C-reactive protein (hsCRP) levels in patients with LBD compared to controls (Rathod et al. Citation2014). Takahashi et al. reported a higher expression of Interleukin 1 (IL-1), interleukin 6 (IL-6) and tumour necrosis factor alpha (TNFa) in patients undergoing surgery for lumbar disc herniation (Takahashi et al. Citation1996). Although Burke et al. demonstrated elevated levels of IL-6 and interleukin 8 (IL-8) in patients with LBD, there was no significant difference in the levels of TNFa and IL-1 (Burke et al. Citation2002). Queiroz et al. also found no significant difference in IL-6 levels between the LBD and healthy control group (Queiroz et al. Citation2015). Interestingly in some studies the author demonstrated a negative correlation between inflammatory biomarker and LBP, for example Capossela et al. demonstrated a negative correlation between level of monocyte chemoattractant protein 1 (MCP-1) and LBP (Capossela et al. Citation2018).
Observed associations between inflammatory biomarkers and LBD provide insight on the possible inflammatory causes of LBP that is occurring in these patients. This will allow for improved understanding into the pathophysiology and allow clinicians to provide more targeted treatment. Moreover, biomarkers could also be predictive of treatment outcome for low back disorder. Rathod et al. found that patients with lower hsCRP levels showed better recovery compared to patients with higher levels (Rathod et al. Citation2014). Krock et al. also demonstrated that a reduction in IL-8 levels in a mouse level resulted in decreased behavioural signs of LBP (Krock et al. Citation2019).
Despite the many proposed associations between inflammatory biomarkers, LBD and clinical outcomes of treatment, there is no validated tool for stratifying the role of specific biomarkers in the pathophysiology of LBD. As such, we conducted a systematic review and the first meta-analysis of the literature to understand the clinical significance of inflammatory biomarkers in patients with LBD. This study aims to identify the inflammatory biomarkers that have been identified in patients with LBD and evaluate the association of these markers with back pain, leg pain and disability score pre-treatment if any. It also aims to be the first to determine changes in clinical outcomes and analyse whether they are related to changes in inflammatory biomarker levels pre- and post-intervention.
Methods
Search strategy
Online databases PubMed, Embase and Cochrane Central Register of Controlled Trials (CENTRAL) were searched in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify all relevant studies published between January 1980 and February 2023. The search included the following terms: ‘inflammation’, ‘cytokines’, ‘low back pain’, ‘biological marker’, ‘cytokine’, ‘intervertebral disk displacement’, ‘herniated disk’ and specific inflammatory biomarkers with appropriate combinations of operators ‘AND’, ‘OR’, and ‘NOT’ as described in supplemental material 1. It was intentionally designed to be of high sensitivity to reduce the number of relevant studies that were excluded based on poor key word assignment or variations in terminology and spelling. Covidence was used for article screening and data extraction from the list of relevant studies. The listed reference reviews and potentially relevant studies were evaluated. The language of the included studies was restricted to English. The review protocol was registered on PROSPERO (International Prospective Register of Systematic Reviews number, CRD42021255302).
Inclusion criteria
Inclusion criteria followed the participants, interventions, comparators, outcomes and study design (PICOS) framework.
Adults (>18years) with LBD. LBD is defined as LBP without specific spinal pathologies and included spondylolisthesis, spondylosis, disc herniation, disc degeneration, scoliosis deformity and radicular syndromes. ‘Failed back surgery syndrome’ is included as this is not a specific disease. Inflammatory activity was measured in serum, cerebrospinal fluid, urine and saliva.
Studies which reported the level of inflammatory biomarkers in patients with LBD and/or healthy controls, before and/or after treatment
Studies which reported back pain with or without leg pain intensity and disability in patients with LBD and/or healthy controls, before and/or after treatment.
Randomized controlled trials (RCTs), cross sectional studies and observational cohort studies (with and without control group).
Exclusion criteria
Studies that did not include inflammatory biomarker level, pain intensity and disability scores.
Studies that included patients with specific spinal pathologies.
Animal studies, in vitro biomechanical studies and computational modelling studies
Case reports, reviews, and conference papers
Types of outcomes measures
Primary outcome was the changes of pain and/or the expression of inflammatory biomarkers pre- and post- intervention, as measured in Visual Analogue Scale (VAS) and Numeric Pain Rating Scale (NRS), and the association between LBP and/or leg pain and/or back-specific disability scores and the expression of inflammatory biomarkers.
Secondary outcome was back-specific disability questionnaires.
Selection of studies
Two reviewers (SS and XLC) independently reviewed all titles and abstracts that were identified in the search of databases. Full text review for the eligibility criteria were used to include relevant studies in this analysis. A third reviewer (ADD) was consulted when a consensus could not be made between the two reviewers.
Data extraction
Two reviewers (SS and XLC) independently read, analyzed and extracted data from the studies included. Relevant publication information (i.e. author, title, year, journal, country), study design (i.e. two-arm or multi-arm parallel trail or single-arm), number of participants, participant characteristics (e.g. age, sex, and body mass index (BMI)), interventions considered (type and duration, etc.) and patient outcomes associated with biomarker concentrations, location of different biomarker, and clinical outcomes (pain and disability) was extracted. Any disagreements on data extraction will be resolved by a third reviewer (ADD).
Risk of bias
Cochrane Collaboration Risk of Bias Tool was used to assess the risk of bias of RCTs that were included in this meta-analysis (Higgins et al. Citation2011). For each source of bias (selection, performance, detection, attrition, reporting and other), studies will be classified as having a ‘low’, ‘high’, or ‘unclear’ (if reporting was not sufficient to assess a particular domain) risk. For the overall risk of bias evaluation, the two reviewers (SS and XLC) independently determined the evidence, direction and magnitude of bias.
The Newcastle-Ottawa Scale (NOS) will be used to assess the methodological quality of the included cohort studies. The ‘star system’ of NOS ranges from 0 to 9, which is judged on three broad perspectives: a selection of the study, comparability, and the ascertainment of the outcome of interest. In this meta-analysis, a study with 3 or 4 starts in the selection domain and 1 or 2 stars in comparability and 2 or 3 stars in the outcome domain will be regarded as high-quality (Wells et al. Citation2000).
Controversial scores were resolved by the third reviewer (ADD)
Statistical analysis
A qualitative assessment of the clinical similarity of the different populations and treatments was performed by important variables such as baseline pain intensity, baseline disability, and the expression of inflammatory biomarkers. Between-study standard deviation (SD) was estimated and reported from random effects models, and the impacts of subgrouping or meta-regression on this was examined. Hedges’ d standardized mean difference (SMD) was used to standardize results of studies using different outcome measures to a uniform scale. SMD was calculated by subtracting the mean change of the pre-operative data from the mean change for the post-operative data, and the difference was divided by the pooled SD. The SD was calculated by dividing the standard deviations by the square root of the study population. Chi-squared (I2) statistic will be used to measure heterogeneity among the trials. I2 < 50% implies homogeneity and the analysis followed a fixed effects model according to the Mantel-Haenszel method. I2 > 50% indicates heterogeneity and, consequently, a random effect model was used according to the DerSimonian-Laird method. A subgroup analysis based on different pain forms and sensitivity analysis were conducted to assess the impact of heterogeneity. SMD and 95% confidence intervals (CI) were reported. A forest plot was used to calculate the effect size (ES). The statistical significance was set at 5% (α = 0.05).
Finally, to calculate the association of LBP and/or leg pain and/or back disability with measurements of inflammatory biomarkers, the inflammatory biomarkers were included as a predictor in a meta-regression. This meta-regression proportion of between-study variance was explained with a Knapp-Hartung modification. STAT software (release 15, StatCorp LLC, TX) was used for the statistical analyses.
Evaluating the quality of evidence
The quality of evidence informing this meta-analysis was assessed using Grading of Recommendations Assessment Development and Evaluation (GRADE) scale (supplemental material 2), which rated evidence quality as high, moderate, low, or very low (Guyatt et al. Citation2011). All discrepancies will be referred to an adjudicator.
Results
Study selection
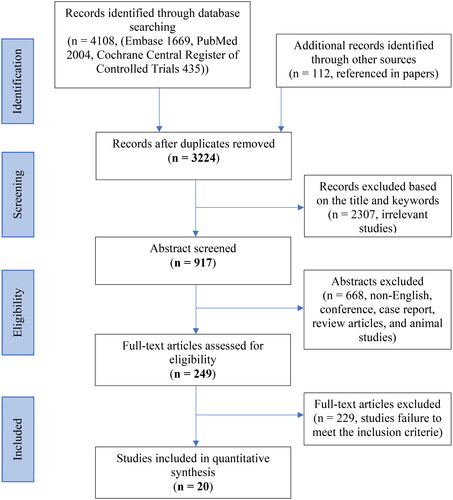
The search identified 3224 studies out of a possible 4108 studies after duplicates were removed. Following a review of the titles and abstracts 249 studies were identified for full text screening. On examination of the full text with the inclusion criteria, 20 papers were included in the final review. Of these 16 were observational studies (France and Urban Citation1991; Gebhardt et al. Citation2006; Rannou et al. Citation2007; Cuellar et al. Citation2010; Kraychete et al. Citation2010; Ohtori et al. Citation2011; Park and Lee Citation2011; Cheng et al. Citation2013; Elkan et al. Citation2016; Queiroz et al. Citation2017; Capossela et al. Citation2018; Teodorczyk-Injeyan et al. Citation2018; Hider et al. Citation2019; Surbakti and Nasution Citation2020; Al-Rawaf et al. Citation2021; Gjefsen et al. Citation2021) and 4 were RCTs (Lin et al. Citation2015; Degenhardt et al. Citation2017; Yuen et al. Citation2017; Koivisto et al. Citation2019). The PRIMSA flow diagram illustrating the literature search is shown in .
Study characteristics
A total of 1142 patients were analysed in the eligible studies, the mean age was 51, of which 53.2% were female (M/F: 489/653). All 20 included studies recorded clinical outcomes and inflammatory biomarkers pre-treatment. Of these, nine observational studies (France and Urban Citation1991; Gebhardt et al. Citation2006; Kraychete et al. Citation2010; Lin et al. Citation2015; Elkan et al. Citation2016; Degenhardt et al. Citation2017; Hider et al. Citation2019; Surbakti and Nasution Citation2020; Al-Rawaf et al. Citation2021) and two RCTs (Deshmane et al. Citation2009; Yuen et al. Citation2017) recorded pre-treatment disability outcomes and four observational studies (France and Urban Citation1991; Kraychete et al. Citation2010; Park and Lee Citation2011; Gjefsen et al. Citation2021), and one RCT (Lin et al. Citation2015) recorded pre-treatment leg pain outcomes. Three RCTs and four observational studies (France and Urban Citation1991; Gebhardt et al. Citation2006; Rannou et al. Citation2007; Deshmane et al. Citation2009; Yuen et al. Citation2017; Koivisto et al. Citation2019; Gjefsen et al. Citation2021) (total of 366 patients with a mean follow up duration of 17.2 weeks) recorded pre- and post-treatment back pain scores and inflammatory markers. Two observational studies (total of 208 patients with a mean follow up duration of 38.26 weeks) (France and Urban Citation1991; Gjefsen et al. Citation2021) recorded pre- and post-treatment leg pain scores. However, one study did not include standard deviations for post-treatment pain scores so was not included in the analysis (Gjefsen et al. Citation2021).
The specific biomarkers included in each study and the characteristics of all studies with and without treatment are provided in and , respectively.
Table 1. Demographic information, list of inflammatory markers and clinical outcomes of the cohort studies included for meta-analysis.
Table 2. Demographic information, list of inflammatory markers and clinical outcomes of the treatment studies included for meta-analysis.
Quality assessment
Four of the included RCTs had a low overall risk of bias. 1in 4 studies had an unclear risk of radon sequence generation bias, 1 in 4 had a high risk and unclear risk of allocation concealment bias, 2 in 4 and 1 in 4 studies had a high risk and unclear risk of performance bias, respectively, and 2 in 4 studies had a high risk of self-reported outcome detection bas. All studies had a low risk of objective measure selection bias, attribution bias and reporting bias (). All observational studies met the criteria of high quality as outlined in the risk of bias section ().
Figure 2. Assessment of the methodological quality of randomized controlled trials according to the Cochrane collaboration’s tool for assessing risk of bias.
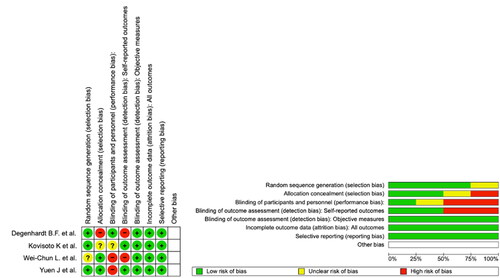
Table 3. Assessment Of the methodological quality of observational studies according to the Newcastle-Ottawa scale (NOS).
Outcomes for pain
Based on all the six included studies (France and Urban Citation1991; Gebhardt et al. Citation2006; Rannou et al. Citation2007; Deshmane et al. Citation2009; Yuen et al. Citation2017; Koivisto et al. Citation2019), there was an overall significant reduction in back pain scores post-treatment when compared to pre-treatment (SMD = 1.38 (95%CI= 1.00 to 1.76), =90.8%, P = < 0.001). The sensitivity analysis performed based on different pain forms showed patients that used VAS 100 mm and NRS had a significant reduction in pain (SMD = 1.17 (95%CI = 1.05 to 1.29) and SMD = 0.72 (95%CI = 0.01 to 1.43) respectively). Summary of the back pain results are provided in .
Figure 3. (A–E) Pre- and post-treatment back pain scores in visual analogue scale 10 cm (VAS 10 cm), visual analogue scale 100 mm (VAS 100 mm), numerical rating scale (NRS) and brief pain inventory short form were reported as the mean score and standard deviations (SD), and represented in , respectively. Forest plot of back pain score changes in different scales following treatments were reported as standardized mean difference (SMD) and 95% confidence intervals (CI). Sensitivity analysis based on the type of pain scale used was performed. Four methods were used to measure pain, VAS (10 cm), VAS (100 mm), NRS and the brief pain inventory short form.
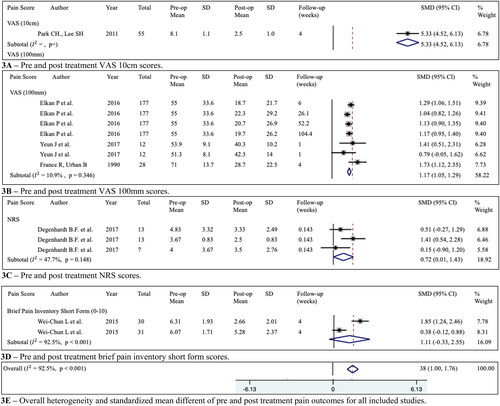
Outcomes for inflammatory biomarkers
In one study with one medication and one placebo arm (Lin et al. Citation2015), there was a significant increase in the expression of CTX-1 levels post-treatment (SMD = –0.54 (95%CI= −0.99 to −0.10), =0.0%, p = 0.969).
In one study with one manual treatment and one placebo arm (Yuen et al. Citation2017), there was a significant increase in the expression of IL-10 levels (SMD = –0.91 (95%CI= −1.28 to −0.53), =0.0%, p = 0.418) and decrease in the expression of IL-1 beta levels (SMD = 1.05 (95%CI= 0.56 to 1.54),
=40.0%, p = 0.197) post treatment.
Summary of the changes in inflammatory biomarkers are provided in .
Table 4. Inflammatory biomarker changes following different treatments were reported as standardized mean difference and 95% confidence intervals. Grading of recommendations assessment, development and evaluation (GRADE) level of quality assessment.
The association of inflammatory biomarkers and clinical scores
The relationship between pre-treatment inflammatory biomarker and clinical scores are provided in . There was a significant relationship between decreasing expression of hsCRP levels and increasing back pain score (r = –3.44 (95%CI = –5.16, −1.69), p = 0.003) (France and Urban Citation1991; Rannou et al. Citation2007; Lin et al. Citation2015; Teodorczyk-Injeyan et al. Citation2018; Koivisto et al. Citation2019; Surbakti and Nasution Citation2020; Al-Rawaf et al. Citation2021; Gjefsen et al. Citation2021). The same statistically significant relationship occurred between decreasing expression of hsCRP levels and disability score (r = –4.04, (95%CI = –4.54, −3.55), p < 0.001) (France and Urban Citation1991; Lin et al. Citation2015; Surbakti and Nasution Citation2020; Al-Rawaf et al. Citation2021). There was a significant relationship between increasing expression of MCP-1 levels and increasing back (r = 4.46, (95%CI= 2.72, 6.20), p = 0.004) and leg pain (r = 4.34 (95%CI = 1.30, 7.38), p = 0.025) (Kraychete et al. Citation2010; Cheng et al. Citation2013; Lin et al. Citation2015). There was a significant relationship between decreasing expression of IL-6 levels and increasing leg pain score (r = –1.20 (95%CI = –1.20, −0.41), p = 0.023) (Kraychete et al. Citation2010; Park and Lee Citation2011; Lin et al. Citation2015). There was a significant relationship between increasing expression of IL-8 levels and increasing disability score (r = 3.36 (95%CI = 2.71, 4.01), p < 0.001) (Lin et al. Citation2015; Hider et al. Citation2019).
Table 5. The table presents a detailed summary of the evidence, including statistical model (effect size and associated confidence intervals, regression data and the certainty of evidence).
Discussion
This is the first meta-analysis and meta-regression addressing the relationship between inflammatory biomarkers and low back disorder. The work identified 20 studies with a total of 1142 patients suffering from low back disorder. All studies reported level of inflammatory biomarker and back pain score pre-treatment, ten studies reported disability score pre-treatment, five studies reported pre-treatment leg pain score, eight studies reported post-treatment inflammatory markers, seven studies reported post-treatment pain scores, four studies reported disability score post treatment and two studies reported post-treatment leg pain score. In the studies that recorded change in inflammatory biomarkers, there was a moderate quality of evidence. This study demonstrated that despite the use of various methods to determine the severity of pain in LBP, there is a decrease in VAS 100 mm and NRS scores post-treatment (, ). The results also suggest that as MCP-1 levels increase there is a corresponding increase in back and leg pain. Similarly, as IL-8 increases so does disability score. The level of hsCRP was found to decrease when levels of back and leg pain increased. IL-6 levels also decreased as leg pain increased.
MCP-1 is a chemoattractant cytokine (chemokine) also known as chemokine ligand 2 (CCL2). It binds to G-protein-coupled receptors on cell surface of monocytes and results in the activation of an intracellular cascade which promotes chemotaxis of macrophages to the source of MCP-1 (Deshmane et al. Citation2009). Macrophages play an important role in upregulating IL-1 and TNFa which subsequently upregulates PGE2, an important culprit in nerve root injury resulting in the development of a symptomatic disk (O’donnell and O’donnell Citation1996). Similarly, monocyte expression has been found to be elevated in patients with LBD compared to asymptomatic controls (Weber et al. Citation2015). This study found that an increase in MCP-1 levels correlated to an increase in low back and leg pain severity. An exploratory study presented similar findings with NRS being positively correlated with changes in MCP-1 (Modi et al. Citation1990). MCP-1 has also been found to be independently associated with duration of pain, which higher levels of MCP-1 correlating to longer duration. However, some studies have found no association or even negative correlation between MCP-1 levels and LBD (Al-Rawaf et al. Citation2021; Gjefsen et al. Citation2021). The discrepancy related to the findings highlights the need for longitudinal studies with longer follow-up periods that target all aspects within the inflammatory pathway, this will generate a clearer understanding of inter-cell regulation patterns and how that leads to LBP, its resolution or flare up of pain.
IL-8 is a chemokine that is primarily produced by macrophages and induces chemotaxis of predominantly neutrophils and granulocytes and stimulates phagocytosis. Neutrophils induces neurogenic inflammation resulting in the release of substance P, causing pain (Ren and Dubner Citation2010). This study demonstrated that as IL-8 increases, a corresponding increase in disability score occurs. Literature that includes IL-8 and functionality in patients with LBD is limited. Dhahri R. et al. found IL-8 to be positively correlated with anxiety and functional scores, however, no correlation was found to pain intensity (VAS) (Dhahri et al. Citation2021). Studies have found correlation between IL-8 and LBP intensity (Pedersen et al. Citation2015; Krock et al. Citation2019), consideration should be noted for these studies owing to the positive correlation between pain intensity and disability (Grandidge et al. Citation2015). In future, biomarker studies should include disability and psychological evaluations in order to analyze the multifactorial comorbidities that result from low back pain.
In this study IL-6 levels have been shown to decrease as patients leg pain intensity increases. The role of IL-6 in radicular leg pain has been debated. Wang K. et al. found IL-6 levels to be 1.5 times higher in patients with severe sciatica compared to asymptomatic controls (Wang et al. Citation2016). Clinically, administering patients with subcutaneous tocilizumab (anti IL-6 receptor antibody has been shown to improve leg pain symptoms (Sainoh et al. Citation2022). However, other studies have found no correlation between IL-6 and leg pain (Andrade et al. Citation2013). Hider S et al., also found no difference in IL-6 levels in patients with clinically confirmed sciatica and those without, there was also no difference between patients with confirmed nerve root compression on MRI scan and those without (Hider et al. Citation2019). The uncertainty behind the role of IL-6 in leg pain and irregularities in results of current studies could be attributed to its dual mechanism. IL-6 acts as a pro-inflammatory cytokine when signalling in monocyte and macrophages and is dependent on the NFkB pathway, however, it also acts as an anti-inflammatory myokine when expressed intramuscularly (Brandt and Pedersen Citation2010).
In predominant number of pain conditions such as fibromyalgia, chronic headache, mixed musculoskeletal pain there has been a significant association found between elevated CRP levels and increased pain (Hagen et al. Citation2019; Zetterman et al. Citation2022; Hodges et al. Citation2023). In patients with disk degeneration, the immune response to nucleus pulposus material releases inflammatory cytokines leading to increased production of CRP (Bobechko and Hirsch Citation1965). CRP is a homopentameric acute phase inflammatory protein synthesized primarily by IL-6 dependent hepatocytes. Traditionally it has been utilized as an indirect indicator for systemic inflammation, however, recent studies suggest CRP plays an important role in the inflammatory process (Baumeister et al. Citation2016). Although the main role of CRP is the activation of C1q molecule in the complement pathway, it also binds to Fc receptors of IgG which leads to the release of proinflammatory cytokines indicated in low back pain (Du Clos Citation2000). Literature on hsCRP and its role in LBD is conflicted. Some authors have demonstrated an elevated hsCRP level in patients with LBP compared to health controls (Rannou et al. Citation2007; Al-Rawaf et al. Citation2021), others have also demonstrated a positive association between hsCRP and pain/disability intensity (Klyne et al. Citation2018). Contrarily, studies have also found no association between hsCRP and low back pain/disability (Park and Lee Citation2011; Elkan et al. Citation2016; Degenhardt et al. Citation2017; Surbakti and Nasution Citation2020).
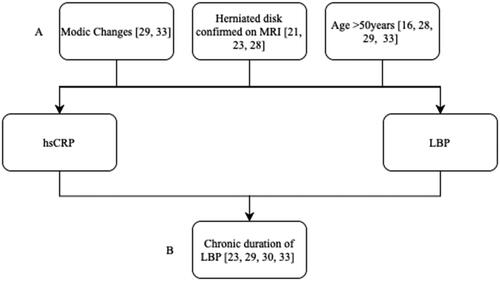
Interestingly in this study we observed a non-biological negative correlation between hsCRP, low back pain and disability score. This finding could be attributable to confounder and collider bias due to the inclusion and exclusion criteria of the studies analysed. Confounding refers to a distortion of the true association between exposure and outcome due to the introduction of additional factors (Skelly et al. Citation2012). Collider bias occurs when exposure and outcome influence a third variable (Holmberg and Andersen Citation2022). The common confounders and colliders included in the studies analysed are illustrated in . Modic changes are regarded as a pathognomic indicator for LBP and hsCRP has also been found to be elevated in patients with modic changes (Rannou et al. Citation2007; Koivisto et al. Citation2019). Similarly, a herniated disk results in an inflammatory response due to the foreign nature of the herniating material causing hsCRP to be elevated, it is also known to be associated with LBP (Yang et al. Citation2015). Increasing age is also associated with increasing serum levels of CRP and prevalence of LBP (Tang et al. Citation2018). Only recruiting chronic LBP patients in certain studies provided a colliding effect as both elevated hsCRP levels and increased severity of LBP is associated with increased duration of pain (Gebhardt et al. Citation2006; Rannou et al. Citation2007; Koivisto et al. Citation2019; Surbakti and Nasution Citation2020). Both confounding and colliding effects can mask the actual association, falsely demonstrate an association and in the studies case, reverse the sign of the hypothetical effect. Future analysis should focus on papers with similar inclusion and exclusion criteria to determine the true clinical significance of hsCRP in LBD.
Figure 4. Relationship between hsCRP, LBP and confounding (A) factors (modic changes, herniated disk confirmed on MRI and age) and collider (B) factors (chronic duration of LBP) that are included in the studies analysed.
The main driver of inflammatory response in the intervertebral disk is the imbalance between anti-inflammatory and pro-inflammatory cytokines (Ansar et al. Citation2016). In theory, the mean level of pro-inflammatory markers should decrease post treatment and correlate with a reduction in pain score, similarly, anti-inflammatory markers should increase with a corresponding reduction in pain score. There was no significant difference in demographic information between studies that analysed cytokine levels pre and post treatment.
In this study we demonstrated an increase in IL-10 levels post treatment. IL-10 is an anti-inflammatory cytokine which inhibits antigen presentation by dendritic cells, inhibits macrophage activation and infiltration, and inhibits production of immune-response genes necessary to produce pro-inflammatory cytokines (O’garra et al. Citation2008). Furthermore, low concentrations of IL-10 have been linked to chronic widespread pain (Uçeyler et al. Citation2006). A study by Li Y et al. found an imbalance between pro- and anti-inflammatory cytokines with an increase in IL-6 and decrease in IL-10 in patients with LBP compared to controls (Li et al. Citation2016). Similarly, a study conducted by Teodorczyk-Injeyan J et al. found the ratio of TNFa, IL-1b and IL-6 to IL-10 to be significantly elevated in patients with LBP (Teodorczyk-Injeyan et al. Citation2018). Studies on rats have shown IL-10 to alleviate radicular pain by inhibiting TNF-a expression (Jančálek et al., 2010). However, other studies have found no significant difference between IL-10 levels in patients with LBP and asymptomatic controls (Al-Rawaf et al. Citation2021).
IL-1b levels post treatment for LBD was demonstrated to decrease in this study. IL-1b induces upregulation of genes that encode matrix-degrading enzymes (matrix metalloproteases (MMPs) and A disintegrin-like and metalloprotease with thrombospondin type-1 motif (ADAMTS)), hence, its levels have been found to be increased in degenerated IVDs and patients with LBP (Risbud and Shapiro Citation2014). Increasing concentrations of IL-1b also upregulated production of IL-6 and COX-2 which are thought to be associated with pain hypersensitivity as a result of the inflammatory response (Jimbo et al. Citation2005). Studies have found IL-1b to be positively correlated with VAS pain scores and significantly increased in patients with neuropathic LBP (Luchting et al. Citation2016; Teodorczyk-Injeyan et al. Citation2018). Contrarily, studies have also shown no significant difference in IL-1b levels between patients with LBP and asymptomatic controls (Degenhardt et al. Citation2017; Al-Rawaf et al. Citation2021). An interesting study conducted by Biczo A et al. found different specific genetic variants of IL-1b to be associated with psychological status, LBP and improved outcome post-surgery (Biczo et al. Citation2022). The current debate on the role of IL-1b in LBD could be linked to the inadequate number of studies analyzing genotype subgroups, as a result future studies could analyze the genomic subgroup of inflammatory biomarkers.
Interestingly this study also demonstrated an increase in CTX-1 levels post-treatment. CTX-1 is a biomarker for bone resorption and is released during collagen degradation. It has primarily been used to analyse the risk of osteonecrosis in osteoporotic patients undergoing bisphosphonate treatment (Marx et al. Citation2007). A cross sectional population study with 7144 participants have found that higher bone mass density (BMD) in the lumbar spine is independently associated with LBP (Lee et al. Citation2013). Higher BMD has also been found to be related to rotational asymmetry and motion restriction at the affected vertebrae (Snider et al. Citation2011). However, other studies have found lower BMD to be related to LBP (Hoozemans et al. Citation2012). The causal link between increased level of bone resorption markers like CTX-1 and possible reduction of BMD leading to improved outcome for LBD treatment is unclear. More studies are required to determine the true role of bone metabolism biomarkers in the pathophysiology of LBP. A limitation of the studies analysed that included CTX-1 was that they did not specify if the blood was withdrawn in a fasting state as serum CTX-1 levels are reduced after food intake (Christgau Citation2000).
Our findings support the notion that an association between inflammatory biomarkers and low back disorder exists. Specifically, there was a positive association between IL-7 and MCP-1 with LBP, MCP-1 with leg pain and IL-8 with disability score. A negative association was observed between IL-6 and leg pain. A non-biological negative association was also observed between hsCRP and low back pain and leg pain. However, the lack of uniformity in patient populations between the different studies included and conflicting associations in literature make giving a definite conclusion to this meta-analysis difficult. The moderate quality of evidence of our results helps support the findings that CTX-1 and IL-10 increase after treatment and IL-1 beta decrease after treatment for LBD. Nevertheless, the numerous methods of determining pain, different treatments and follow up times provided a challenge. Although further high-quality longitudinal studies are needed to confirm these findings and evaluate the magnitude of these associations, our findings suggest a role of inflammation in the pathogenesis of LBD.
Limitations
Although this meta-analysis demonstrated many significant results, there are certain limitations which must be noted. The first is that there are only four RCTs included in this study which could have reduced the strength and robustness of the results. Secondly, the studies included did not have uniform inclusion and exclusion criteria’s resulting in a heterogenous population of patients suffering from different classifications of pain (acute, subacute, chronic etc.), diseases and ages, which are all known to affect the inflammatory environment. Additionally, treatment and follow-up protocol differed between studies which resulted in significant heterogeneity and increased bias due to unmeasured confounding and colliding effects. Thirdly, although the study found significant changes in biomarker level pre- vs post-treatment, the effect of these changes was assessed by very few studies, thus the results should be interpreted cautiously. There was also a lack of studies that included the analysis of biomarkers in different locations, this means discrepancies in localised and systemic levels of biomarkers were not distinguished. Fourthly, there was substantial heterogeneity between studies due to the wide variation in different pain scale and disability scale scores used. Finally, the primary literature is varied in its discussion of patient’s smoking status, exercise status and body mass index which are all known parameters that affect inflammation. This substantially increases heterogeneity of the results and effect the conclusion of the study. Guidelines for specific standardization of inclusion and exclusion criteria are needed to further analyse the significant association and changes in inflammatory markers in LBD to facilitate interpretation of this collective body of research.
Conclusion
Moderate grade of evidence was shown in this study for an increase in CTX-1 and IL-10 and decrease in IL-1 beta after treatment for LBD. There is a strong association between increase in MCP-1 with LBP, MCP-1 with leg pain, and IL-8 with disability score. There was also a negative association between hsCRP and LBP, and IL-6 and hsCRP with leg pain. However, the limitations of the results reduce the strength of evidence for the practical applications of this study’s findings. Standardizing inclusion and exclusion criteria, demographic status of the cohort, and pain and disability scale reported in literature will allow for further analysis.
Supplemental Material
Download MS Word (21.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
This work was supported by a University Postgraduate Award from the University of New South Wales to Stone Sima. Spine Labs is supported via unrestricted research grants to its institution by Baxter Inc and Nuvasive Inc. All authors declare that they have no conflict of interest related to this work.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
- Al-Rawaf HA, Gabr SA, Alghadir AH. 2021. Vitamin D deficiency and molecular changes in circulating microRNAs in older adults with lower back pain. Pain Res Manag. 2021:6662651–13. doi: 10.1155/2021/6662651
- Andrade P, Hoogland G, Garcia MA, Steinbusch HW, Daemen MA, Visser-Vandewalle V. 2013. Elevated IL-1β and IL-6 levels in lumbar herniated discs in patients with sciatic pain. Eur Spine J. 22(4):714–720. doi: 10.1007/s00586-012-2502-x
- Ansar W, Ghosh S, Ansar W, Ghosh S. 2016. Inflammation and inflammatory diseases, markers, and mediators: role of CRP in some inflammatory diseases. Biol C React Protein Health Dis. 24:67–107.
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. 2016. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 21(5):642–649. doi: 10.1038/mp.2015.67
- Biczo A, Bereczki F, Koch K, Varga PP, Lazary A, Genodisc Consortium. 2022. Genetic variants of interleukin 1B and 6 are associated with clinical outcome of surgically treated lumbar degenerative disc disease. BMC Musculoskelet Disord. 23(1):774. doi: 10.1186/s12891-022-05711-0
- Bobechko W, Hirsch C. 1965. Auto-immune response to nucleus pulposus in the rabbit. J Bone Joint Surg Br. 47:574–580.
- Brandt C, Pedersen BK. 2010. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010:520258–6. doi: 10.1155/2010/520258
- Burke J, Watson R, Mccormack D, Dowling F, Walsh M, Fitzpatrick J. 2002. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 84(2):196–201. doi: 10.1302/0301-620x.84b2.12511
- Capossela S, Pavlicek D, Bertolo A, Landmann G, Stoyanov JV. 2018. Unexpectedly decreased plasma cytokines in patients with chronic back pain. J Pain Res. 11:1191–1198. doi: 10.2147/JPR.S153872
- Cheng L, Fan W, Liu B, Wang X, Nie L. 2013. Th17 lymphocyte levels are higher in patients with ruptured than non-ruptured lumbar discs, and are correlated with pain intensity. Injury. 44(12):1805–1810. doi: 10.1016/j.injury.2013.04.010
- Chou R. 2014. In the clinic. Low back pain. Ann Intern Med. 160(11):ITC6–ITC1. doi: 10.7326/0003-4819-160-11-201406030-01006
- Christgau S. 2000. Circadian variation in serum CrossLaps concentration is reduced in fasting individuals. Clin Chem. 46(3):431–431.
- Cuellar JM, Golish SR, Reuter MW, Cuellar VG, Angst MS, Carragee EJ, Yeomans DC, Scuderi GJ. 2010. Cytokine evaluation in individuals with low back pain using discographic lavage. Spine J. 10(3):212–218. doi: 10.1016/j.spinee.2009.12.007
- Degenhardt BF, Johnson JC, Fossum C, Andicochea CT, Stuart MK. 2017. Changes in cytokines, sensory tests, and self-reported pain levels after manual treatment of low back pain. Clin Spine Surg. 30(6):E690–E701. doi: 10.1097/BSD.0000000000000231
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 29(6):313–326. doi: 10.1089/jir.2008.0027
- Dhahri R, Dghaies A, Slouma M, Metoui L, Gharsallah I, Dorgham I, Ayari R, Mallat Y, Amri K, Tezeghdenti A. 2021. POS1290 cytokine biomarkers in common low back pain. Ann Rheum Dis. 80:926.
- Du Clos TW. 2000. Function of C-reactive protein. Ann Med. 32(4):274–278. doi: 10.3109/07853890009011772
- Ehrlich GE. 2003. Back pain. J Rheumatol Suppl. 67:26–31.
- Elkan P, Sten-Linder M, Hedlund R, Willers U, Ponzer S, Gerdhem P. 2016. Markers of inflammation and fibrinolysis in relation to outcome after surgery for lumbar disc herniation. A prospective study on 177 patients. Eur Spine J. 25(1):186–191. doi: 10.1007/s00586-015-3998-7
- France RD, Urban BJ. 1991. Cerebrospinal fluid concentrations of beta-endorphin in chronic low back pain patients: influence of depression and treatment. Psychosomatics. 32(1):72–77. doi: 10.1016/S0033-3182(91)72114-2
- Gebhardt K, Brenner H, Stürmer T, Raum E, Richter W, Schiltenwolf M, Buchner M. 2006. The course of high-sensitive C-reactive protein in correlation with pain and clinical function in patients with acute lumbosciatic pain and chronic low back pain–a 6 months prospective longitudinal study. Eur J Pain. 10(8):711–719. doi: 10.1016/j.ejpain.2005.11.005
- Gjefsen E, Gervin K, Goll G, Bråten LCH, Wigemyr M, Aass HCD, Vigeland MD, Schistad E, Pedersen LM, Pripp AH, et al. 2021. Macrophage migration inhibitory factor: a potential biomarker for chronic low back pain in patients with Modic changes. RMD Open. 7(2):e001726. doi: 10.1136/rmdopen-2021-001726
- Grandidge L, Athanassacopolous M, Breakwell L, Chiverton N, Ivanov M, Michael R, Zaki H, Cole A. 2015. Oswestry disability index (ODI) and visual analogue score (VAS) in pre-operative patients with radicular leg pain. Spine J. 15(3):S53–S54. doi: 10.1016/j.spinee.2014.12.037
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. 2011. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 64(4):383–394., doi: 10.1016/j.jclinepi.2010.04.026
- Habtemariam A, Grönblad M, Virri J, Seitsalo S, Karaharju E. 1998. A comparative immunohistochemical study of inflammatory cells in acute-stage and chronic-stage disc herniations. Spine (Phila Pa 1976). 23(20):2159–2165; discussion 2166. doi: 10.1097/00007632-199810150-00003
- Hagen K, Stovner LJ, Nilsen KB, Kristoffersen ES, Winsvold BS. 2019. The impact of C-reactive protein levels on headache frequency in the HUNT study 2006–2008. BMC Neurol. 19(1):229. doi: 10.1186/s12883-019-1462-8
- Hider SL, Konstantinou K, Hay EM, Glossop J, Mattey DL. 2019. Inflammatory biomarkers do not distinguish between patients with sciatica and referred leg pain within a primary care population: results from a nested study within the ATLAS cohort. BMC Musculoskelet Disord. 20(1):202. doi: 10.1186/s12891-019-2604-2
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, Cochrane Statistical Methods Group. 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 343(2):d5928–d5928. doi: 10.1136/bmj.d5928
- Hodges S, Guler S, Sacca V, Vangel M, Orr S, Pace-Schott E, Wen Y, Ge T, Kong J. 2023. Associations among acute and chronic musculoskeletal pain, sleep duration, and C-reactive protein (CRP): a cross-sectional study of the UK biobank dataset. Sleep Med. 101:393–400. doi: 10.1016/j.sleep.2022.11.013
- Holmberg MJ, Andersen LW. 2022. Collider bias. JAMA. 327(13):1282–1283. doi: 10.1001/jama.2022.1820
- Hoozemans MJ, Koppes LL, Twisk JW, Van Dieën JH. 2012. Lumbar bone mass predicts low back pain in males. Spine (Phila Pa 1976). 37(18):1579–1585. doi: 10.1097/BRS.0b013e31825409d8
- Jimbo K, Park JS, Yokosuka K, Sato K, Nagata K. 2005. Positive feedback loop of interleukin-1β upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J Neurosurg Spine. 2(5):589–595. doi: 10.3171/spi.2005.2.5.0589
- Klyne DM, Barbe MF, Van Den Hoorn W, Hodges PW. 2018. ISSLS Prize in clinical science 2018: longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode—the good, the bad, and the ugly. Eur Spine J. 27(4):763–777. doi: 10.1007/s00586-018-5490-7
- Koivisto K, Karppinen J, Haapea M, Järvinen J, Kyllönen E, Tervonen O, Niinimäki J, Alini M, Lotz J, Dudli S, et al. 2019. The effect of zoledronic acid on serum biomarkers among patients with chronic low back pain and modic changes in lumbar magnetic resonance imaging. Diagnostics. 9(4):212. doi: 10.3390/diagnostics9040212
- Kraychete DC, Sakata RK, Issy AM, Bacellar O, Santos-Jesus R, Carvalho EM. 2010. Serum cytokine levels in patients with chronic low back pain due to herniated disc: analytical cross-sectional study. Sao Paulo Med J. 128(5):259–262. doi: 10.1590/s1516-31802010000500003
- Krock E, Millecamps M, Anderson KM, Srivastava A, Reihsen TE, Hari P, Sun YR, Jang SH, Wilcox GL, Belani KG, et al. 2019. Interleukin-8 as a therapeutic target for chronic low back pain: upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine. 43:487–500. doi: 10.1016/j.ebiom.2019.04.032
- Lee S, Nam CM, Yoon DH, Kim KN, Yi S, Shin DA, Ha Y. 2013. Association between low-back pain and lumbar spine bone density: a population-based cross-sectional study. J Neurosurg Spine. 19(3):307–313. doi: 10.3171/2013.5.SPINE12473
- Li Y, Liu J, Liu Z-Z, Duan D-P. 2016. Inflammation in low back pain may be detected from the peripheral blood: suggestions for biomarker. Biosci Rep. 36(4):e00361. doi: 10.1042/BSR20160187
- Lin W-C, Yeh CH, Chien L-C, Morone NE, Glick RM, Albers KM. 2015. The anti-inflammatory actions of auricular point acupressure for chronic low back pain. Evid Based Complement Alternat Med. 2015:1–9. doi: 10.1155/2015/103570
- Luchting B, Heyn J, Woehrle T, Rachinger-Adam B, Kreth S, Hinske LC, Azad SC. 2016. Differential expression of P2X7 receptor and IL-1β in nociceptive and neuropathic pain. J Neuroinflammation. 13(1):100. doi: 10.1186/s12974-016-0565-z
- Marx RE, Cillo JE, Jr, Ulloa JJ. 2007. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 65(12):2397–2410. doi: 10.1016/j.joms.2007.08.003
- Modi WS, Dean M, Seuanez HN, Mukaida N, Matsushima K, O’brien SJ. 1990. Monocyte-derived neutrophil chemotactic factor (MDNCF/IL-8) resides in a gene cluster along with several other members of the platelet factor 4 gene superfamily. Hum Genet. 84(2):185–187. doi: 10.1007/BF00208938
- O’donnell JL, O’donnell AL. 1996. Prostaglandin E2 content in herniated lumbar disc disease. Spine (Phila Pa 1976). 21(14):1653–1655. doi: 10.1097/00007632-199607150-00007
- O’garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. 2008. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 223(1):114–131. doi: 10.1111/j.1600-065X.2008.00635.x
- Ohtori S, Suzuki M, Koshi T, Takaso M, Yamashita M, Inoue G, Yamauchi K, Orita S, Eguchi Y, Kuniyoshi K, et al. 2011. Proinflammatory cytokines in the cerebrospinal fluid of patients with lumbar radiculopathy. Eur Spine J. 20(6):942–946. doi: 10.1007/s00586-010-1595-3
- Park CH, Lee SH. 2011. Prognostic usefulness of high sensitivity C-reactive protein for transforaminal epidural steroid injection in patients with radicular pain. Pain Med. 12(2):219–223. doi: 10.1111/j.1526-4637.2010.01039.x
- Pedersen LM, Schistad E, Jacobsen LM, Røe C, Gjerstad J. 2015. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and-8 (IL-8) in patients with lumbar radicular pain due to disc herniation: a 12-month prospective study. Brain Behav Immun. 46:132–136. doi: 10.1016/j.bbi.2015.01.008
- Queiroz BZ, Pereira DS, De Britto Rosa NM, Lopes RA, Andrade AGP, Felício DC, Jardim RMFVS, Leopoldino AaO, Silva JP, Pereira LSM. 2017. Inflammatory mediators and pain in the first year after acute episode of low-back pain in elderly women: longitudinal data from back complaints in the elders—Brazil. Am J Phys Med Rehabil. 96(8):535–540. doi: 10.1097/PHM.0000000000000661
- Queiroz BZ, Pereira DS, Rosa N. M d B, Lopes RA, Felício DC, Pereira DG, Dias JMD, Dias RC, Pereira LSM. 2015. Functional performance and plasma cytokine levels in elderly women with and without low back pain. J Back Musculoskelet Rehabil. 28(2):343–349. doi: 10.3233/BMR-140526
- Rannou F, Ouanes W, Boutron I, Lovisi B, Fayad F, Macé Y, Borderie D, Guerini H, Poiraudeau S, Revel M. 2007. High-sensitivity C-reactive protein in chronic low back pain with vertebral end-plate Modic signal changes. Arthritis Rheum. 57(7):1311–1315. doi: 10.1002/art.22985
- Rathod TN, Chandanwale A, Ladkat KM, Chavan S, Chavan A, Bhosale PB. 2014. High sensitive C-reactive protein-effective tool in determining postoperative recovery in lumbar disc disease. Indian J Orthop. 48(4):354–359. doi: 10.4103/0019-5413.136216
- Ren K, Dubner R. 2010. Interactions between the immune and nervous systems in pain. Nat Med. 16(11):1267–1276. doi: 10.1038/nm.2234
- Risbud MV, Shapiro IM. 2014. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 10(1):44–56. doi: 10.1038/nrrheum.2013.160
- Sainoh T, Orita S, Miyagi M, Suzuki-Narita M, Sakuma Y, Oikawa Y, Kubota G, Sato J, Shiga Y, Fujimoto K, et al. 2022. Improvements in intractable lumbar and lower-extremity symptoms after systemic administration of tocilizumab, an anti-interleukin-6 receptor antibody. Asian Spine J. 16(1):99–106. doi: 10.31616/asj.2020.0283
- Skelly AC, Dettori JR, Brodt ED. 2012. Assessing bias: the importance of considering confounding. Evid Based Spine Care J. 3(1):9–12. doi: 10.1055/s-0031-1298595
- Snider KT, Johnson JC, Degenhardt BF, Snider EJ. 2011. Low back pain, somatic dysfunction, and segmental bone mineral density T-score variation in the lumbar spine. J Osteopathic Med. 111:89–96.
- Surbakti KP, Nasution I. 2020. Association between serum levels of high sensitivity C-reactive protein, interleukin-1 and interleukin-6 with pain intensity in patients with low back pain without sciatica. Open Access Maced J Med Sci. 8(B):6–10. doi: 10.3889/oamjms.2020.4114
- Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. 1996. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976). 21(2):218–224. doi: 10.1097/00007632-199601150-00011
- Tang Y, Liang P, Chen J, Fu S, Liu B, Feng M, Lin B, Lee B, Xu A, Lan HY. 2018. The baseline levels and risk factors for high-sensitive C-reactive protein in Chinese healthy population. Immun Ageing. 15(1):1–8. doi: 10.1186/s12979-018-0126-7
- Teodorczyk-Injeyan JA, Mcgregor M, Triano JJ, Injeyan SH. 2018. Elevated production of nociceptive CC chemokines and sE-selectin in patients with low back pain and the effects of spinal manipulation: a nonrandomized clinical trial. Clin J Pain. 34(1):68–75. doi: 10.1097/AJP.0000000000000507
- Uçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. 2006. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 54(8):2656–2664. doi: 10.1002/art.22026
- Wang K, Bao J-P, Yang S, Hong X, Liu L, Xie X-H, Wu X-T. 2016. A cohort study comparing the serum levels of pro-or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. Eur Spine J. 25(5):1428–1434. doi: 10.1007/s00586-015-4349-4
- Weber K, Satoh S, Alipui DO, Virojanapa J, Levine M, Sison C, Quraishi S, Bloom O, Chahine NO. 2015. Exploratory study for identifying systemic biomarkers that correlate with pain response in patients with intervertebral disc disorders. Immunol Res. 63(1-3):170–180. doi: 10.1007/s12026-015-8709-2
- Wells GA, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. University of Ottawa. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- Wynne-Jones G, Cowen J, Jordan JL, Uthman O, Main CJ, Glozier N, Van Der Windt D. 2014. Absence from work and return to work in people with back pain: a systematic review and meta-analysis. Occup Environ Med. 71(6):448–456. doi: 10.1136/oemed-2013-101571
- Yang H, Liu H, Li Z, Zhang K, Wang J, Wang H, Zheng Z. 2015. Low back pain associated with lumbar disc herniation: role of moderately degenerative disc and annulus fibrous tears. Int J Clin Exp Med. 8:1634.
- Yuen JW, Tsang WW, Sonny H, Loo WT, Chan S-T, Wong DL, Chung HH, Tam JK, Choi TK, Chiang VC. 2017. The effects of Gua sha on symptoms and inflammatory biomarkers associated with chronic low back pain: a randomized active-controlled crossover pilot study in elderly. Complement Ther Med. 32:25–32. doi: 10.1016/j.ctim.2017.03.010
- Zetterman T, Markkula R, Kalso E. 2022. Elevated highly sensitive C-reactive protein in fibromyalgia associates with symptom severity. Rheumatol Adv Pract. 6(2):rkac053. doi: 10.1093/rap/rkac053