Abstract
Background
Smoking cessation reduces the risk of developing smoking-related diseases. Although smoking prevalence has declined, many continue smoking cigarettes. Switching completely to smoke-free alternatives like the Tobacco Heating System (THS) 2.2—a heated tobacco product for which there is evidence demonstrating significantly reduced formation and exposure to harmful chemicals compared to cigarettes—has the potential to reduce the harm caused by continuing to smoke cigarettes.
Methods
We conducted a 6-month clinical study (NCT02396381) with a 6-month extension (NCT02649556), initially randomizing 984 adult smokers to continue smoking or switch to THS (non-mentholated), of which 672 continued into the extension study. Endpoints were evaluated at baseline and at 3, 6, and 12 months. We longitudinally assessed biomarkers of potential harm (BoPHs) known to be reversible upon smoking cessation as indicators of pathways involved in the pathogenesis of cardiovascular or respiratory diseases and carcinogenicity. The need to cough and safety profile were also assessed. Impact on eight key BoPHs was used as a proxy to evaluate harm reduction potential.
Results
At 12 months, comparison of BoPH levels between the predominant THS use and cigarette smoking groups showed a positive effect in favor of switching, partially or in full, to THS.
Conclusion
These results provide additional evidence of the harm reduction potential of THS for smokers who would otherwise continue smoking, but they need to be verified in long-term confirmatory studies.
Clinical trial registration
Clinicaltrials.gov Identifier: NCT0264955. Date of registration: January 7, 2016 https://clinicaltrials.gov/ct2/show/NCT02649556
Introduction
Smoking cessation is the best choice for reducing the risk of developing cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), and lung cancer, and other smoking-related diseases. Quitting the use of any tobacco or nicotine-containing product must remain the primary medical intervention for any smoker, as recommended by standard of care. Although tobacco control measures implemented in recent decades (WHO 2003, Levy et al. Citation2004) helped reduce the prevalence of smoking, less than 10% of smokers successfully quit every year in the United States (U.S.) (Centers for Disease Control and Prevention Citation2020b). For those who decide to continue to smoke, a pragmatic approach is needed to reduce the risk of smoking-related diseases.
In principle, tobacco harm reduction can be achieved when cigarettes are replaced by substantially less harmful nicotine delivery systems (Rodu Citation2011, Royal College of Physicians Citation2016). According to the U.S. Institute of Medicine (IOM; now known as the National Academy of Medicine, which is part of the National Academy of Sciences), ‘a product is harm-reducing if it lowers total tobacco-related mortality and morbidity even though use of that product may involve continued exposure to tobacco-related toxicants’ (IOM 2001). Some public health authorities acknowledge that less harmful alternatives to cigarettes have the potential to reduce population harm (Public Health England Citation2016, Clarke et al. Citation2019, Centers for Disease Control and Prevention Citation2020a, McNeill et al. Citation2022). However, the concept of Tobacco Harm Reduction remains controversial from a public health perspective (Franck et al. Citation2016, Beaglehole et al. Citation2019, Hatsukami and Carroll Citation2020). This is partly because of the complexity of assessing the impact of new products owing to: (1) the large variety of alternatives available, (2) their recent market introduction, (3) the absence of a framework for assessing the long-term risk reduction potential and remaining risks associated with their use in the intended population (smokers) without epidemiological evidence, (4) the potential dual or poly-use of products including cigarettes, (5) their impact on non-intended users (e.g. non-smokers or youth) and (6) concerns related to the agendas of the major tobacco companies that produce these alternative products.
The Tobacco Heating System (THS; brand name IQOS®) is a heated tobacco product (HTP) developed by Philip Morris International (PMI). In 2020, the United States Food and Drug Administration (FDA) authorized IQOS to be marketed as a Modified Risk Tobacco Product with exposure modification orders (FDA Citation2020). IQOS was the first tobacco product to receive an exposure modification order, which permitted the marketing of IQOS ‘as containing a reduced level of or presenting a reduced exposure to a substance or as being free of a substance when the issuance of the order is expected to benefit the health of the population’ (FDA Citation2020).
Multiple studies have evaluated the reduced risk potential of THS, including studies conducted by scientists independent from PMI. The decrease in harmful and potentially harmful constituent (HPHC) emissions (including carcinogens, cardiovascular, reproductive, developmental, and respiratory toxicants (U.S. Department of Health and Human Services and FDA (Food and Drug Administration)) Citation2012)) in THS aerosol relative to cigarette smoke is well documented (Schaller et al. Citation2016, Bekki et al. Citation2017, Forster et al. Citation2017, Mallock et al. Citation2018, Drovandi et al. Citation2019, Li et al. Citation2019, Mallock et al. Citation2019, Goujon et al. Citation2020) and reviewed in Sussman et al. (Citation2023). Consistent with the chemistry findings, smokers switching from cigarettes to ad libitum THS use are less exposed to HPHCs (Bekki et al. Citation2017, Caponnetto et al. Citation2018, Farsalinos et al. Citation2018, Mallock et al. Citation2018, Simonavicius et al. Citation2018, Stephens Citation2018, Li et al. Citation2019). To understand if reduction of exposure was associated with early beneficial changes in biological and physiological functions underlying the development of clinical symptoms and disease, we evaluated changes in a set of biomarkers of potential harm (BoPHs) involved in the pathomechanisms of smoking-related diseases (listed in ). This panel was used as a proxy to further evaluate the potential disease risk modification (harm reduction) in smokers who switched to THS as suggested by the (IOM 2001). Participants categorized as ‘Other use’ (defined in Supplementary Table 3) were excluded from the biomarker analysis.
Table 1. Biomarkers of potential harma. (the eight core BoPH are highlighted.).
In smokers who predominantly switched to THS for 6 months (defined as ≥70% THS allowing for 30% cigarette use), changes in a core set of eight BoPHs (HDL-C; WBC; FEV1%pred; COHb; Total NNAL; sICAM-1; 11-DTX-B2; 8-epi-PGF2α) indicative of pathomechanisms (i.e. lipid metabolism, endothelial dysfunction, platelet activation, lung function, oxygen transport, carcinogenicity, inflammation, and oxidative stress) with evidence of a robust relationship with CVD, COPD, or carcinogenicity, were evaluated relative to participants randomized to continue smoking their own cigarettes (Philip Morris Products S.A. Citation2015, Lüdicke et al. Citation2019). Considering that clinical and epidemiological data on the short- to long-term health effects of smoking cessation and the association with reduced risk of disease is extensively documented (U.S. Department of Health and Human Services Citation2020), we compared the short-term changes associated with switching to THS to the reduction or increase in BoPHs characterized for smoking cessation use against continuing smoking. Our results were interpreted as favorable for THS across all BoPH (reduction of harm), as the direction of changes in the predominant THS group (predTHS) was comparable to that reported for smoking cessation within a similar timeframe against cigarettes (Lüdicke et al. Citation2019). These findings were in line with the fundamental principles of toxicology for which a significant reduction in exposure to HPHCs and toxicants should lead to a reduction in adverse health effects.
The present study was an extension of the initial 6-month study and aimed to evaluate the changes in a set BoPHs over 12 months in adult smokers who switched to THS in comparison to those who continued smoking. This extension study was exploratory in nature; therefore, no pre-specified hypothesis was tested. A pre-specified hypothesis was tested at 6 months in the main study, with the assumption that the switching effect would already be significant at 6 months.
The initial study was extended to 12 months to: (1) determine if the impact of predominant THS use was maintained over 12 months because switching to THS could have triggered a change in lifestyle that was not present at 6 months but was at 12 months (e.g. ordinary physical activity, change in diet, etc.) and (2) understand if people successfully continued to use THS over a longer period since it is well known that smokers who quit smoking can relapse at any time in a 12-month study.
Materials and methods
Study design
The overall study consisted of an initial 6-month randomized, controlled, two-arm ‘exposure response’ confirmatory study conducted in an ambulatory setting in the U.S. (clinicaltrials.gov: NCT02396381, March 23, 2015 (Lüdicke et al. Citation2019)), followed by an exploratory 6-month extension study (clinicaltrials.gov: NCT02649556, January 7, 2016). The Consolidated Standards of Reporting Trials (CONSORT) Guideline (Schulz et al. Citation2010) was used to report the present study. The initial study enrolled a total of 984 adult healthy smokers who were not willing to quit smoking (DiClemente et al. Citation1991, Velicer et al. Citation1995) and randomized them (1:1 ratio) into two groups:
to smoke their own cigarettes ad libitum (496 participants, cigarette arm), or
to use THS (non-mentholated variant) ad libitum (488 participants, THS arm).
The method used to calculate the sample size for the initial 6-month confirmatory study was reported in a separate publication (Lüdicke et al. Citation2019). Briefly, sample size was calculated to ensure an overall study power of at least 90% while maintaining at least 80% power to detect the expected effect of THS use as compared with continued cigarette smoking for each co-primary endpoint. Randomization was performed during the initial study through an interactive voice and web response system. Stratified randomization ensured that each sex was represented by at least 40% of the participants. A quota ensured that the White race did not represent more than 75% of the randomized participants.
Of the 984 randomized participants in the initial study, 803 (81.6%) completed month 6. Participants from the 19 sites who completed the initial study (381 in the THS arm and 422 in the cigarette arm) were asked to participate for an additional 6 months according to their initial randomization arm. The exploratory extension study was descriptive in nature and no sample size was calculated. The Midlands Independent Review Board (Overland Park, KS, USA) approved the study, and all participants provided written informed consent prior to screening. Recruitment to the extension study started in September 2015, and the last participant completed the study in March 2017.
The two studies covered a 12-month period, including a baseline visit when all participants smoked cigarettes; a run-in period of 8 ± 2 days during which all the participants used THS for familiarization; a visit for randomization; subsequent visits at 3, 6, and 12 months; and monthly visits for safety checks. A 28-day safety follow-up period occurred after the 12-month visit or after discontinuation. All data were collected in electronic case report forms.
Products
The product tested—THS 2.2—comprises a holder that heats a tobacco stick and a charger that is used to recharge the holder (Smith et al. Citation2016). The sponsor provided THS to the participants because it was not marketed in the U.S. at the time of the study. The tobacco sticks were provided on demand during or between site visits. The composition of the aerosol generated when heating the sticks, including nicotine content, was reported in a separate publication and compared to cigarette smoke (Schaller et al. Citation2016). All HPHCs, except nicotine, were shown to be much lower in THS aerosol than in cigarette smoke. Cigarettes, used as a comparator in this study, were purchased by participants.
Participants
The initial study: The initial 6-month study aimed to demonstrate that switching from cigarettes to THS over 6 months would result in favorable changes in BoPHs, with all endpoints shifting in the same direction as they would upon smoking cessation. The study enrolled adults with at least 10 years of smoking history who had smoked at least 10 non-menthol cigarettes per day over the year prior to screening. They were at least 30 years old and not willing to quit in the next 6 months (Stages of Change questionnaire (Prochaska and DiClemente Citation1983), administered by site staff). As per eligibility criteria (Supplementary Table 1), participants with COPD stage I according to the (GOLD guideline (GOLD Citation2013) could be enrolled based on the spirometry at the screening visit. The study excluded participants with stage II to IV COPD (GOLD Citation2020), pregnant or breastfeeding women, and participants with body mass index (BMI) <18.5 or ≥35 kg/m2. Baseline spirometry reflected that some participants may have been GOLD Stage II. This may have been due to variability in spirometry measurement.
The extension study: This study was extended for an additional 6 months for those who met the extension study criteria (Supplementary Table 1). All participants from the initial study were eligible to enroll in the extension study, unless they had made a quit attempt in the previous study, had medical conditions that would jeopardize their participation according to the investigator’s judgment, or were breastfeeding or pregnant.
The participants were instructed to continue using their allocated product exclusively without restrictions. Any participant who wanted to quit using tobacco-containing products during any stage of the study was encouraged to do so and referred to appropriate services. Such participants were not discontinued; they were encouraged to attend scheduled visits for assessment.
Compensation was provided, as per Institutional Review Board approval and according to a predefined payment schedule, irrespective of actual product use.
Procedures
A detailed schedule of assessments is provided in Supplementary Table 2. As in the initial study, all participants continued to record their daily use of tobacco or nicotine-containing products, received information on the risks of smoking and advice on smoking cessation during the study and were briefed that THS should not be considered a lower risk than cigarettes.
For assessment of urinary BoPHs and biomarkers of exposure (BoExps), participants started urine collection at home on the morning preceding their visit and stopped the collection 24 hours later.
Blood was collected from the participants using standardized laboratory procedures to measure BoPH levels in serum and plasma.
Spirometry was carried out following the guidelines of the American Thoracic Society and European Respiratory Society (Miller et al. Citation2005), with predicted values standardized to the National Health and Nutrition Examination Survey III-predicted set (Hankinson et al. Citation1999). All post-bronchodilator (BD) spirometry testing was performed 15–30-min post-administration of ∼400 μg of salbutamol (equivalent to 4 puffs, assuming 100 μg/puff).
Body height, body mass, waist circumference, and blood pressure were recorded.
Biomarkers of potential harm and biomarkers of exposure
The eight core BoPHs measured in this study (HDL-C; WBC; FEV1%pred; COHb; Total NNAL; sICAM-1; 11-DTX-B2; 8-epi-PGF2α) were the same as the core set tested in the primary objective in the initial 6-month study, for which results have been published (Lüdicke et al. Citation2019). Respectively, they are indicators of inflammation, lipid metabolism, lung function, endothelial function, oxidative stress, oxygen transport, platelet activity, and carcinogenicity, therefore representing some of the main pathomechanisms underlying CVD, COPD, and cancer as outlined in the U.S. Surgeon General’s report on smoking cessation (U.S. Department of Health and Human Services Citation2020). The commonly assessed BoPH representative of the pathways that contribute to the development of tobacco-related diseases are listed in , including biomarkers known to be impacted within 1 year of smoking cessation. In addition, BoExps to the following HPHCs were measured: total N-nitrosonornicotine, acrylonitrile, nicotine, and carbon monoxide. The formula used to obtain nicotine equivalent (NEQ) values is provided in the Supplementary Material (Supplementary Method 1).
All urinary BoPH and BoExp levels were adjusted to urinary creatinine concentration and analyzed by Covance Central Laboratories Services, Inc. (Indianapolis, IN, USA) or Celerion, Inc. (Lincoln, NE, USA). These laboratories used methods validated in accordance with the College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA) standards or the Bioanalytical Method Validation guidance for Industry, from the U.S. FDA (FDACitation2018).
Cough questionnaire
The participants were asked if they had experienced a regular need to cough within the previous 24 h; they indicated the intensity of the cough. If the answer was ‘yes’, participants were asked to complete a visual analog scale (VAS) and to self-rate their need to cough on a 100-mm scale. The VAS has been used in both clinical and academic studies as a subjective evaluation and longitudinal assessment of cough and has been validated in subjects with chronic cough (Lee et al. Citation2013, Spinou and Birring Citation2014).
Safety
The safety population consisted of all enrolled participants with at least one safety assessment. Safety was monitored under the responsibility of the Investigator supervising each site (Supplementary Table 2). The following tests were performed: medical interview to check health status, physical examination, vital sign measurements, electrocardiogram, and safety laboratory (hematology, blood biochemistry, and urine analysis). Adverse events (AEs) were recorded and assessed for seriousness and relatedness to product or study.
Population analysis sets and statistical analyses
The analysis of this study was exploratory. In contrast to the initial 6-month study, which was a confirmatory study, there was no pre-specified hypothesis tested and no control of the overall type I error.
The full analysis set included all randomized participants who enrolled into the extension study, with recorded product use, baseline values, and at least one post-randomization value for one of the eight core BoPHs.
To reflect the realistic use of THS, participants were classified into four groups (as exposed) according to their product use pattern. The groups were defined a priori for the safety population and the full analysis set based on self-reported product use (cigarettes and tobacco sticks) over the 12 months post-randomization (Supplementary Table 3). PredTHS use was defined as ≥70% of daily tobacco stick use (THS use) on average (on at least 50% of the days over 12 months). Dual use was defined as 1% to <70% THS use. Cigarette smoking (cig) was defined as <1% THS use. All participants who did not meet these criteria were included in the ‘other’ use group.
Each BoPH was analyzed ()—between the predTHS and cig groups and the dual and cig groups—by using a mixed-effects model for repeated measurements, adjusting for sex, White race, time, value at baseline, interaction with time, and product use group. Other relevant baseline covariates, as described in Supplementary Table 4, were tested by means of analysis of variance and Pearson chi-square test for continuous and categorical variables, respectively, for being significantly differentially distributed at Baseline at a 10% type I error level. Retained baseline covariates added to the BoPH analyses are described in Supplementary Table 4. Site was included as a random effect.
The least squares (LS) means and estimate of the difference, along with 95% confidence intervals (CI), were estimated for high-density lipoprotein cholesterol (HDL-C), white blood cell (WBC) count, and forced expiratory volume in 1 s % predicted (FEV1%pred). For soluble intercellular adhesion molecule-1 (sICAM-1), 11-dehydrothromboxane B2 (11-DTX-B2), 8-epi-prostaglandin F2α (8-epi-PGF2α), carboxyhemoglobin (COHb), and total 4-(methylnitrosamino)-1-(3 pyridyl)-1 butanol) (NNAL), values were analyzed on the log scale, and the results were back-transformed in the original scale as a ratio as well as % reduction relative to cigarette smoking (i.e. predTHS:cig ratio or dual:cig ratio).
Odds ratios for need to cough (Yes/No) between predTHS and cig users were derived from a mixed effects logistic regression model. The model accounted for visit, baseline value and interaction with visit, sex, White race, age, smoking intensity, product use group, and interaction with time, as fixed-effect factors and site as a random effect.
Considering that self-reported product use may not reflect actual behavior, a post hoc analysis of predTHS was performed to explore the magnitude of effects according to the intensity of cigarette smoking concomitant to THS use (Connor Gorber et al. Citation2009). For each timepoint, this analysis determined the levels of the eight BoPHs according to each 2-cyanoethylmercapturic acid (2CyEMA) quartile. 2CyEMA is a BoExp to acrylonitrile, which is an HPHC generated in cigarette smoke from tobacco combustion at >400 °C (Rodgman and Perfetti Citation2013) that exhibits a linear relationship with the number of cigarettes smoked (Minet et al. Citation2011). The half-life of 2CyEMA has been estimated at up to 8–9 hours (Jakubowski et al. Citation1987, Ashley et al. Citation2020). In previous studies, acrylonitrile levels were reduced by over 99% in THS aerosol relative to cigarette smoke (Schaller et al. Citation2016), and urinary 2CyEMA levels in smokers switching to THS exclusively for 5 days were decreased by approximately 80% relative to smokers who continued smoking (Haziza et al. Citation2016, Lüdicke et al. Citation2018b). In that context, the present study used 2CyEMA as an objective chemical marker to evaluate the degree of cigarette smoking in the predTHS group.
All analyses were performed with Statistical Analysis Software (SAS) version 9.2 (SAS Inc., Cary, NC, USA).
Results
Population analysis set and demographics
summarizes the CONSORT participant flow through the initial 6-month ‘Exposure Response Study’ and the 6-month extension study.
Figure 1. Flow of participants through the ‘exposure response study’ and study extension. GCP: good clinical practice; THS: tobacco Heating System.
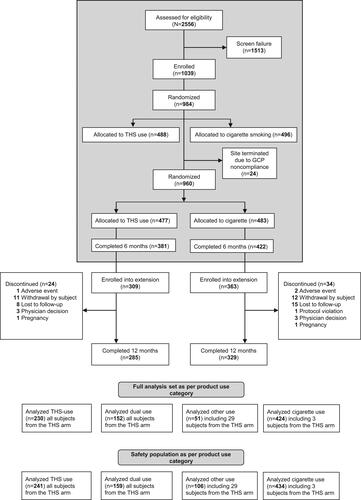
Participants who completed the initial study were asked to participate in the extension study. In the THS and Cigarette arms, 81% and 86% of participants, respectively, from the initial study extended their participation and of those, 285 and 329 participants, respectively, completed the study.
The safety population consisted of 940 participants classified according to product use: predTHS (N = 241), dual use (N = 159), cigarette (N = 434), and other (N = 106) groups.
The full analysis set consisted of 857 participants classified according to product use: predTHS (N = 230), dual use (N = 152), cigarette (N = 424), and other (N = 51) groups. Of the 230 participants in the predTHS category, 136 (59%) were classified as being exclusive users (≥95% use of THS).
The baseline characteristics of the participants () in the full analysis set were balanced across the four groups including, age, sex, race, smoking history, BMI, pack-year smoking history, and daily cigarette consumption. Cigarette consumption ranged between 18 and 20 cigarettes per day and smoking history was of 25–27 years on average. According to GOLD guidelines (GOLD Citation2013), pulmonary function was normal in 92.6%, 93.4%, 91.4%, and 92.2% of the predTHS users, cigarette smokers, dual users, and other users, respectively. Across the groups, 62 participants were classified as having COPD stage I and II according to the GOLD guidelines (GOLD Citation2020).
Table 2. Summary of demographic data and smoking-related characteristics at baseline; full analysis by product use group.
Product use
Over 12 months, the predTHS users reported a mean daily use of 16.5 (95% CI: 15.4; 17.7) THS tobacco sticks and 1.6 (95% CI: 1.4; 1.9) cigarettes per day (Supplementary Table 5); the dual users reported a mean use of 8.1 (95% CI: 7.2; 9.1) THS tobacco sticks and 9.2 (95% CI: 8.3; 10.2) cigarettes per day, and the cigarette smokers reported a mean use of 16.5 (95% CI: 15.9; 17.2) cigarettes per day. Use of other tobacco products (pipe, cigar, cigarillos, chewable/smokeless tobacco, etc.) was marginal (≤0.1 product per day).
Baseline NEQ levels were similar across the predTHS, dual, and cig groups, with overlapping CIs ranging from 9.1 to 11.8 mg/g creatinine; these levels were maintained throughout the 12 months (8.47 to 9.73 mg/g creatinine).
The average product consumption and exposure to nicotine were stable over the study.
BoPHs
Compared to the cig group, the predTHS group showed a change in trajectory in the eight core BoPH and in most of the supportive BoPH ( and ). The changes from baseline were overall in line with the results of the inferential analyses. The BoPH results are summarized below and provided in full (baseline, 3, 6, and 12 months) in . All comparisons below are described against the cig group.
Figure 2. Mean relative differences or reductions (and 95% confidence intervals) between the predTHS and cig groups after 3, 6, and 12 months of product use. A, lipid metabolism; B, endothelial and platelet function; C, lung function; D, inflammation and oxidative stress; E, oxygen transport; and F, carcinogenicity.
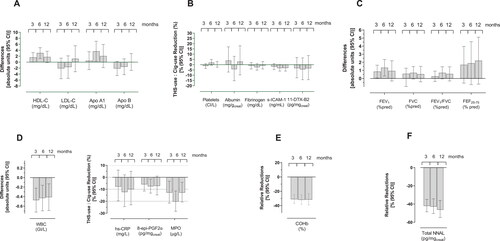
Table 3. Levels of BoPHs across the study groups and inferential analyses between the predTHS and dual groups and the cigarette (cig) group at 3, 6, and 12 months.
Lipid metabolism
The levels of HDL-C were 1.6 (0.0; 3.2), 3.1 (1.3; 4.9), and 1.8 (−0.2; 3.7) mg/dL higher at 3, 6, and 12 months respectively in the predTHS group as compared to continuing smoking. Concomitantly, apolipoprotein (Apo) A1 levels were 0.5 (−2.5; 3.5), 3.7 (0.3; 7.0), and 2.1 (−1.8; 6.0) mg/dL, respectively. The low-density lipoprotein cholesterol (LDL-C) and Apo B levels were lower at 3 and 6 months in the predTHS group with no notable difference in Apo B levels at 12 months, while a slight increase was observed for LDL-C.
Endothelial function and platelet activation
The levels of sICAM-1 and 11-DTX-B2 in the predTHS group were slightly reduced across the study, starting from 3 months. The mean reductions as compared to continuing smoking ranged from −1.9% (−4.7; 1.1) to −3.2% (−6.1; −0.3) and from −3.3% (−12.5; 6.9) to −4.5% (−14.3; 6.5), respectively. No meaningful changes were observed in platelet or fibrinogen levels.
Lung function
FEV1%pred values were slightly higher in the predTHS group across the study, starting from 3 months. The mean differences ranged from 0.8% (0.0; 1.6) to 1.3% (0.3; 2.3). FVC %pred, FEV1/FVC ratio, and forced expiratory flow between 25% and 75% of FVC % predicted (FEF25–75%pred) were consistently higher in the predTHS group.
Inflammation and oxidative stress
The WBC count was lower in the predTHS group across the study, starting from 3 months. The mean differences ranged from −0.47 GI/L (−0.73; −0.22) to −0.41 GI/L (−0.69; −0.13). In line with this result, the myeloperoxidase (MPO) and high-sensitivity C-reactive protein (hs-CRP) levels were reduced by at least −12.8% (−21.5; −3.3) and −7.8% (−19.7; 6) in the predTHS group, respectively, starting from 3 months. The mean reductions in 8-epi-PGF2α levels ranged from −5.9% (−11.5; 0.0) to −7.5% (−13.2; −1.5) over the study, starting from 3 months.
Oxygen transport and carcinogenicity
The mean reductions in COHb and total NNAL levels in the predTHS group ranged, respectively, from −31.2% (−37.0; −24.8) to −32.0% (−38.3; −25.1) and −42.1% (−48.5; −34.9) to −46.3% (−54.8; −36.2) across the study.
Impact of dual use
In the comparison between the dual and cig groups, the BoPH trajectories in the dual group were similar to those in the predTHS group, although the magnitude of the effect was much lower ().
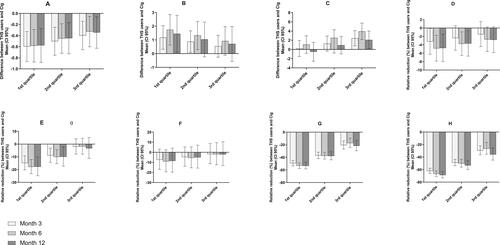
We used 2CyEMA as an objective marker of intensity for concomitant use of cigarettes. In a post hoc analysis, the magnitude of effect on BoPH of switching to THS versus continuing smoking was assessed according to the 2CyEMA levels. Looking at quartiles of 2CyEMA levels (presented in Supplementary Table 6), for all BoPH at month 12, except HDL-C, it was noted that with lower concomitant use of cigarettes, a stronger beneficial effect of switching to THS was observed ().
Figure 3. Mean relative difference or reduction (and 95% confidence intervals) between the predTHS and cig groups according to 2CyEMA quartile distribution for the set of eight BoPHs after 3, 6, and 12 months of product use. A, WBC (GI/L); B, FEV1%pred; C, HDL-C; D, sICAM-1; E, 8-epi-PGF2α; F, 11-DTX-B2; G, COHb; and H, total NNAL.
Exposure to HPHCs
In addition to total NNAL and COHb, the following HPHCs showed relative reductions in the predTHS group versus the cig group consistently across the study, starting from 3 months: total NNN level (N-nitrosonornicotine; LS mean predTHS use:cig ratios, 61.0 (50.7; 73.4) to 74.0% (58.7; 93.3)), 2CyEMA level (LS mean predTHS use:cig ratios, 48.5 (42.7; 55.2) to 52.4% (44.9; 61.2)%), and exhaled CO level (LS mean predTHS use-cig difference, −5.46 (−7.20; −3.72) to −7.90 ppm (−14.7; −1.10)). Dual users showed a markedly inferior reduction in BoExp levels (Supplementary Table 8). NEQ levels were comparable across the study to baseline irrespective of the product use group.
Cough
The proportion of participants who reported a regular need to cough was lower in the predTHS group than in the cig group (Supplementary Table 9). The mean odds ratio for the need to cough (predTHS use:cig) was consistent across the study, starting with 0.66 (95% CI, 0.43; 1.01) at month 3 and moving to 0.59 (0.37; 0.92) at month 6 and 0.60 (0.36; 1.01) at month 12.
Safety
There were no serious AEs (SAEs) related to THS use, while three SAEs reported by three randomized participants were considered related to continued cigarette smoking (transient ischemic attack, acute myocardial infarction, and vascular disorders). The most common AEs across all groups were upper respiratory tract infection, increased blood triglycerides, and hypertension. No randomized participant was discontinued because of AEs related to THS or cigarettes.
Among the predTHS group, 151 participants (62.7%) reported 371 AEs, in contrast to 278 participants (64.1%) in the cig group who reported 705 AEs; a total of 33 AEs in the two product use groups were classified as severe by the investigators.
The incidence of product use-related AEs was comparable between the predTHS group (8/241 participants; 3.3%) and cig group (13/434 participants; 3.0%). The incidence of product use-related AEs in the dual group was higher (11/159 participants; 6.9%).
There were very few clinically relevant findings in the clinical laboratory, vital signs, or ECG data, with comparable changes from baseline to month 6 and month 12 between the predTHS and cig groups.
Discussion
A panel of eight BoPHs was tested for the primary objective of the initial 6-month study to estimate the long-term risk reduction potential of switching to THS on the development of the main smoking-related diseases (CVD, COPD, and lung cancer) compared to continued smoking (Lüdicke et al. Citation2019). The initial study was extended by another 6 months to understand the pattern of change of these BoPH over a period of 12 months of THS use versus cigarette smoking among healthy participants.
In the absence of epidemiological data, we used a BoPH approach to evaluate the short-term benefit among healthy participants of switching from cigarette to THS, and to estimate the potential of THS to reduce the risk of developing smoking related diseases over the long-term for smokers who would otherwise continue smoking their combustible cigarettes. According to the IOM, a set of BoPH, to monitor the early beneficial changes in biological and physiological functions underlying the development of clinical symptoms and disease, could be used as a proxy to evaluate further disease risk modification in smokers without diagnosis of diseases compared to continued smoking (IOM 2001). This approach has also been suggested by others for assessing the cardiovascular risk associated with the use of smoke-free alternatives to cigarettes (Conklin et al. Citation2019).
As discussed in a previous publication (Lüdicke et al. Citation2019), these BoPHs were selected based on (1) a robust relationship with at least one known smoking-related disease, (2) clinical evidence linking cigarette smoking to negative changes, and (3) favorable changes (reduction or increase) within 12 months following smoking cessation. All eight BoPHs were discussed during an FDA-sponsored workshop on BoPHs to be assessed within the frame of tobacco harm reduction; however, none of them has been formally approved, and no threshold of changes has been established, even though a difference of 10% between smokers and non-smokers was deemed to demonstrate ‘sensitivity to differences by smoking status’ (Chang et al. Citation2019).
The primary analysis was performed in participants classified as predTHS users as the potential benefit from switching to THS is assumed only if cigarette consumption is reduced to an absolute minimum. However, it was expected that some smokers randomized to use THS would not be completely adherent as participants were not familiar with the product.
In the THS randomized arm, 55.6% of participants were classified as predTHS users, 36.7% as dual users (THS and cigarettes), 7.0% as other-users, and 0.7% as cigarette users. The reduction in exposure to HPHCs in the predTHS group compared to the cig group was lower compared to previous publications including an independent review (Drovandi et al. Citation2019, Akiyama and Sherwood Citation2021). This is likely related to the allowance of up to 30% concomitant use of cigarettes in the predTHS user category. Despite the substantial reduction from baseline in the daily cigarette consumption of the predTHS group, participants reported an average consumption of 1.6 cigarettes per day. In the dual use group (an average of 9.2 cigarettes per day and 8.4 tobacco sticks per day), the extent of exposure reduction was lower than in the predTHS group (self-reported product use is provided in Supplementary Table 5). While some studies suggest that smoking even one cigarette per day increases the risk of cardiovascular diseases (Hackshaw et al. Citation2018) and death from all causes (Bjartveit and Tverdal Citation2005), this increase remains smaller than when smoking more cigarettes, thereby still contributing to harm reduction. This was recently acknowledged by the FDA: ‘Published studies have shown that significantly reducing the number of cigarettes smoked per day is associated with lower risk of lung cancer and death, with greater reductions in cigarettes per day resulting in less risk’. Several countries have also adopted FDA’s position on the concept of reductions in daily cigarette use.
Our results among healthy participants in predTHS users describe the positive impact of THS use versus cigarette smoking, with most of the changes in the BoPHs (increases or decreases) in line with those reported in the literature upon 1 year of smoking cessation.
Lipid metabolism was improved, as reflected by higher levels of HDL-C and Apo A1 across the study. The absence of a clear or consistent decrease in LDL-C or Apo B levels was expected (Maeda et al. Citation2003, Ogawa et al. Citation2015, Chen et al. Citation2018).
Consistent with the lipid metabolism evidence, BoPHs for endothelial function and platelet activation (sICAM-1 and 11-DTX-B2), inflammation and oxidative stress (WBC and 8-epi-PGF2α), and oxygen transport impairment (COHb) were all consistently reduced in predTHS users in a similar manner as observed following smoking cessation (Palmer et al. Citation2002) (Rångemark et al. Citation1993, Chehne et al. Citation2002, Ergüder et al. Citation2006, Lee et al. Citation2014). Even though the reductions in COHb were smaller than in some of our previous studies (Haziza et al. Citation2016, Haziza et al. Citation2017, Lüdicke et al. Citation2018a), probably because participants were allowed up to 30% consumption of cigarettes, they remain substantial.
No intergroup differences were noted in platelet count, albumin, or fibrinogen levels. This is consistent with the findings in the literature, as changes in fibrinogen levels may take over 2–5 years to develop (Butkiewicz et al. Citation2006, Yasue et al. Citation2006), and findings on changes in platelet count are controversial (Morita et al. Citation2005, Caponnetto et al. Citation2011, Voulgari et al. Citation2011). It is important to note that in the present study among healthy participants, a consistent reduction in the level of hs-CRP was observed within 12 months of switching to THS, although this change is usually reported following 2–5 years of cessation (El-Deek et al. Citation2013).
Overall, the results from the predTHS group are characteristic of a lower atherogenic profile that could indicate a reduction in cardiovascular risk, particularly when considering that HDL-C levels (Gordon et al. Citation1989, Forey et al. Citation2013), WBC count, and 8-epi-PGF2α and sICAM-1 levels are reported to be predictive of decreased future adverse cardiovascular outcomes (Peck et al. Citation2018).
Predominant use of THS had a sustained impact on respiratory function, smaller declines in FEV1%pred, FVC %pred, FEV1/FVC ratio, and FEF25-75%pred in the predTHS group over 12 months. The likelihood for need to cough was substantially lower in predTHS users versus smokers. Together with the decrease in inflammation and oxidative stress, this could indicate a reduction in risk for respiratory diseases such as COPD and a favorable impact on quality of life. This inference is supported by the findings of other studies: (1) improvement in mucociliary clearance in smokers (Emma et al. Citation2020); (2) in a 1-year cohort, IQOS users showed improvement from baseline in total COPD assessment (40%), ability to walk longer, and spirometry outcomes (Sharman and Nurmagambetov Citation2020); and (3) a substantial decrease in annual exacerbations in a small sample of COPD patients who used IQOS for 3 years versus those who continued smoking (Polosa et al. Citation2021).
Similarly, there was a reduction in exposure to total NNAL in predTHS users—a carcinogen suggested as a risk marker for lung cancer (Hecht et al. Citation2016). The changes in the levels of total NNAL were the largest observed among all BoPHs, as this is directly linked to the composition of THS aerosol, where NNK is reduced by more than 95% (Schaller et al. Citation2016), and because NNK exposure can only come from tobacco consumption. This remains an important and clinically relevant finding, considering the described association between levels of TSNAs and lung cancer (Yuan et al. Citation2009).
Dual users also showed modifications in BoPHs, but to a much lower extent. This result is coherent with the lower reduction of exposure versus predominant THS use. The impact of cigarette smoking was verified in predTHS users when we evaluated the magnitude of changes in the eight key BoPHs by using 2CyEMA exposure to quantify cigarette smoking intensity. We observed that lower exposure to 2CyEMA was associated with a higher beneficial impact on the BoPHs, except in the case of HDL-C, which presented a different profile. At this stage, no plausible explanation can be found for the difference in the HDL-C profile.
Overall, the data clearly indicates an inverse dose response between the number of cigarettes smoked per day and the magnitude of the beneficial effects when switching to THS among healthy participants. Smoking cessation clearly remains the best option for any smoker (U.S. Department of Health and Human Services Citation2020). For those who do not quit smoking, complete switching from smoking to exclusive use of alternative products with the potential to reduce harm, such as THS, is critical to maximize harm reduction. In the real-world—where smokers choose to try a product and continue if they have a satisfactory experience—adherence is likely to be higher, and the beneficial impact on BoPHs could be more pronounced than was observed among healthy participants.
A strict comparison between the results observed in this extension study at 6 months and the first 6-month study (Lüdicke et al. Citation2019) cannot be done because the population differs slightly (not all subjects of the initial study were enrolled in the extension study). However, they are very comparable for all BoPHs. At 12 months, except for HDL and to some extent 11-DTX-B2 and FEV1%pred, which were less favorably changed than at 6 months, all other BoPH were still very comparable to the data observed in the 6-month ERS study.
The improvement in the levels of BoPH observed in healthy smokers who switch to THS are consistent, even though slightly smaller, with the findings described in a review of HTPs (Akiyama and Sherwood Citation2021) and two studies on HTPs (Gale et al. Citation2021, Sakaguchi et al. Citation2021). One Japanese observational, cross-sectional, study reported an increase of 13.9% in HDL-C levels and a decrease of 12.4% in sICAM-1, 17.4% in WBC, 32.6% in 11-DTX-B2, and 28.9% in 8-epi-PGF2α levels in exclusive Ploom TECH users versus continued smokers over an average of 1.2 years (Gale et al. Citation2022). Similarly, in the same study, a difference of 8.5% in FEV1%pred between Ploom TECH users and smokers in favor of HTP users was observed. In another 12-month study, where smokers were randomized to continue smoking or exclusive use of the Glo heated tobacco product, similar beneficial changes were reported, with changes from baseline in levels of BoPH also moving towards those of smoking cessation, in particular for sICAM-1 (−8%), 8-epi-PGF2α (−31%), 11-DTX-B2 (−19%), HDL-C (+6%) and WBC (−18%). FEV1 values remained unchanged in the HTP group, similarly to the smoking abstinence group, but unlike the cigarette group that decreased by 9% after 1 year, compared to baseline.
In our study, it is likely that up to 30% concomitant use of cigarettes by the predTHS users (as self-reported) in our study explains the lower magnitude of effects on BoPHs than those reported in these previous publications. As highlighted in a 2021 review (Akiyama and Sherwood Citation2021), there are some inconsistencies in the results obtained in the different studies looking at BoPH in HTP users. However, the populations and sample sizes differed quite significantly. In addition, our post hoc analysis conducted on predTHS users according to different 2CyEMA levels clearly indicated that the magnitude of effect on BoPH was inversely larger as a function of daily cigarette use intensity.
In terms of safety, the data did not indicate an increased frequency of AEs in THS users compared to the cigarette group. Because there is no causal association between an AE and the product used, AEs are not reliable indicators for harm or harm reduction for users of THS compared to cigarette smokers. Furthermore, the post-market surveillance data that are regularly submitted by PMI to regulatory authorities, including the FDA, show that the safety profile of THS is close to the safety profile of nicotine replacement therapy.
One of the strengths of our study was the large sample size, along with a multifaceted evaluation (e.g. multiple BoPHs, need to cough, safety profile, and product use patterns) over 12 months of THS switching. When compared with the effect of smoking cessation reported in the literature over the same timeframe, this approach is valuable to provide early insights into the long-term risk reduction potential associated with alternatives to cigarettes, such as THS, in the absence of epidemiological data. It is important to note that the present study was conducted among healthy participants where clinically relevant differences are undefined.
This study has some limitations. Self-reported product use may not reflect actual use behavior. Despite the balance in demographic characteristics across the four groups of product use, our ‘per-exposure’ analysis could have introduced an attrition bias from some unmeasured confounders. Also, allowing up to 30% concomitant use of cigarettes of the predTHS users clearly impacted the magnitude of the changes observed as some exposure to cigarette remained. If a smoker completely replaces cigarette smoking with exclusive THS use, it is expected that greater harm reduction benefits would be expected. This is suggested by our post hoc analysis on 2CyEMA in predominant THS users that clearly indicates larger effects in users with the lowest daily cigarette consumption. The extent of product patterns in this study does not necessarily reflect ‘real-life conditions’ as THS was provided for free to the participants because THS was not yet marketed in the U.S. at the time of the study.
Thresholds for clinically relevant changes in the selected BoPH do not exist. However, it is well recognized that smoking cessation has strong long-term-clinical benefits and the changes on BoPH are directionally in line with what is observed when smokers quit smoking. Finally, the study does not provide insights on hard clinical outcomes. A study to detect a difference in clinical outcomes would have necessitated a much longer-term follow-up and a larger sample size, particularly in smokers without a disease diagnosis.
In conclusion, reduction of exposure to HPHCs, favorable changes on multiple BoPH indicative of pathomechanistic pathways relevant for diseases associated with smoking, were in favor of THS use relative to cigarette smoking among healthy participants. In addition, a reduction in the self-reported need to cough was observed. When contextualized to the changes reported in the literature for smoking cessation, our findings suggest that THS has the potential to reduce smoking-related harms. These data bring additional scientific evidence of the potential of THS to reduce the risks of the main smoking-related diseases in smokers who would otherwise continue to smoke cigarettes. However, morbidity and mortality data from longer-term and epidemiological studies will be needed to provide ultimate evidence on the effect of long-term switching to THS, the level of harm reduction for smokers achievable, and remaining risks.
Ethical approval
The study was approved on August 25, 2015 (Approval Number: 08/25/2015), by the Midlands Independent Review Board, Overland Park, KS, USA. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
We obtained informed consent from all individual participants included in the study.
Author contributions
C.H., G.d.L.B., S.P., N.B., and S.M.A. were responsible for the accuracy of the scientific content and interpretation. M.D. and P.H. contributed to the creation of tables and figures and to ensure the quality control of the manuscript. The manuscript was written by C.H. and reviewed by all the authors prior to submission. P.H. provided writing and editorial support. All contributors were involved or knowledgeable in the design and the reporting of the clinical study described in this publication.
Supplemental Material
Download JPEG Image (97.4 KB)Supplemental Material
Download MS Word (98.2 KB)Acknowledgements
The authors deeply appreciate the contributions of all the investigators and other clinical and research staff involved in the present study.
Disclosure statement
All authors are employees of Philip Morris Products S.A. or worked for Philip Morris Products S.A. under contractual agreements.
Data availability statement
The data generated in this study are not publicly available but are available upon reasonable request from the corresponding author. The study protocol and study results are disclosed on ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02649556).
Additional information
Funding
References
- Akiyama Y, Sherwood N. 2021. Systematic review of biomarker findings from clinical studies of electronic cigarettes and heated tobacco products. Toxicol Rep. 8:282–294. doi:10.1016/j.toxrep.2021.01.014
- Andelid K, Bake B, Rak S, Lindén A, Rosengren A, Ekberg-Jansson A. 2007. Myeloperoxidase as a marker of increasing systemic inflammation in smokers without severe airway symptoms. Respir Med. 101(5):888–895. doi:10.1016/j.rmed.2006.09.023
- Ashley DL, De Jesús VR, Abulseoud OA, Huestis MA, Milan DF, Blount BC. 2020. Urinary acrylonitrile metabolite concentrations before and after smoked, vaporized, and oral cannabis in frequent and occasional cannabis users. Int J Environ Res Public Health. 17(18):6438. doi:10.3390/ijerph17186438
- Bake B, Oxhöj H, Sixt R, Wilhelmsen L. 1977. Ventilatory lung function following two years of tobacco abstinence. Scand J Respir Dis. 58(6):311–318.
- Beaglehole R, Bates C, Youdan B, Bonita R. 2019. Nicotine without smoke: fighting the tobacco epidemic with harm reduction. Lancet. 394(10200):718–720. doi:10.1016/S0140-6736(19)31884-7
- Bekki K, Inaba Y, Uchiyama S, Kunugita N. 2017. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J Uoeh. 39(3):201–207. doi:10.7888/juoeh.39.201
- Bjartveit K, Tverdal A. 2005. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 14(5):315–320. doi:10.1136/tc.2005.011932
- Brischetto CS, Connor WE, Connor SL, Matarazzo JD. 1983. Plasma lipid and lipoprotein profiles of cigarette smokers from randomly selected families: enhancement of hyperlipidemia and depression of high-density lipoprotein. Am J Cardiol. 52(7):675–680. doi:10.1016/0002-9149(83)90396-x
- Buist AS, Sexton GJ, Nagy JM, Ross BB. 1976. The effect of smoking cessation and modification on lung function. Am Rev Respir Dis. 114(1):115–122. doi:10.1164/arrd.1976.114.1.115
- Butkiewicz AM, Kemona-Chetnik I, Dymicka-Piekarska V, Matowicka-Karna J, Kemona H, Radziwon P. 2006. Does smoking affect thrombocytopoiesis and platelet activation in women and men? Adv Med Sci. 51:123–126.
- Caponnetto P, Maglia M, Prosperini G, Busà B, Polosa R. 2018. Carbon monoxide levels after inhalation from new generation heated tobacco products. Respir Res. 19(1):164. doi:10.1186/s12931-018-0867-z
- Caponnetto P, Russo C, Di Maria A, Morjaria JB, Barton S, Guarino F, Basile E, Proiti M, Bertino G, Cacciola RR, et al. 2011. Circulating endothelial-coagulative activation markers after smoking cessation: a 12-month observational study. Eur J Clin Invest. 41(6):616–626. doi:10.1111/j.1365-2362.2010.02449.x
- Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. 2009. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2012 Mar 1925(3):763–763. doi:10.1021/tx300048h
- Centers for Disease Control and Prevention. 2020a. Electronic cigarettes. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/index.htm. [Accessed September 10, 2020].
- Centers for Disease Control and Prevention. 2020b. Smoking cessation: fast facts. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
- Chang CM, Cheng YC, Cho TM, Mishina EV, Del Valle-Pinero AY, van Bemmel DM, Hatsukami DK. 2019. Biomarkers of potential harm: summary of an FDA-sponsored public workshop. Nicotine Tob Res. 21(1):3–13. doi:10.1093/ntr/ntx273
- Chehne F, Oguogho A, Lupattelli G, Palumbo B, Sinzinger H. 2002. Effect of giving up cigarette smoking and restarting in patients with clinically manifested atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 67(5):333–339. doi:10.1054/plef.2002.0438
- Chen L-F, Li S-C, Yang C-D, Zhang Y, Feng C, Wu W-L, Yan X-W. 2018. Cigarette smoking-induced low-density lipoprotein (LDL) dysfunction is partially reversible after smoking cessation. Int J Clin Exp Med. 11(11):11732.
- Clarke E, Thompson K, Weaver S, Thompson J, O’Connell G. 2019. Snus: a compelling harm reduction alternative to cigarettes. Harm Reduct J. 16(1):62. doi:10.1186/s12954-019-0335-1
- Conklin DJ, Schick S, Blaha MJ, Carll A, DeFilippis A, Ganz P, Hall ME, Hamburg N, O’Toole T, Reynolds L, et al. 2019. Cardiovascular injury induced by tobacco products: assessment of risk factors and biomarkers of harm. A Tobacco Centers of Regulatory Science compilation. Am J Physiol Heart Circ Physiol. 316(4):H801–h827. doi:10.1152/ajpheart.00591.2018
- Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. 2009. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 11(1):12–24. doi:10.1093/ntr/ntn010
- DiClemente CC, Prochaska JO, Fairhurst S, Velicer WF, Rossi JS, Velasquez M. 1991. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol. 59(2):295–304. doi:10.1037/0022-006x.59.2.295
- Drovandi A, Salem S, Barker D, Booth D, Kairuz T. 2019. Human biomarker exposure from cigarettes versus novel heat-not-burn devices: a systematic review and meta-analysis. Nicotine Tob Res. 22(7):1077–1085. doi:10.1093/ntr/ntz200
- El-Deek SE, Makhlouf HA, Saleem TH, Mandour MA, Mohamed NA. 2013. Surfactant protein D, soluble intercellular adhesion molecule-1 and high-sensitivity C-reactive protein as biomarkers of chronic obstructive pulmonary disease. Med Princ Pract. 22(5):469–474. doi:10.1159/000349934
- Emma R, Caponnetto P, Cibella F, Caruso M, Conte G, Benfatto F, Ferlito S, Gulino A, Polosa R. 2020. Short and long term repeatability of saccharin transit time in current, former, and never smokers. Front Physiol. 11:1109. doi:10.3389/fphys.2020.01109
- Ergüder IB, Ergüder T, Ozkan C, Bozkurt N, Soylu K, Devrim E, Durak I. 2006. Short-term effects of smoking cessation on blood antioxidant parameters and paraoxonase activity in healthy asymptomatic long-term cigarette smokers. Inhal Toxicol. 18(8):575–579. doi:10.1080/08958370600686325
- Farsalinos KE, Yannovits N, Sarri T, Voudris V, Poulas K, Leischow S. 2018. Carbonyl emissions from a novel heated tobacco product (IQOS): comparison with an e-cigarette and a tobacco cigarette. Addiction. 113(11):2099–2106. doi:10.1111/add.14365
- [FDA] Food and Drug Administration. 2018. Guidance for industry - Bioanalytical method validation.
- [FDA] Food and Drug Administration. 2020. FDA News Release, July 7 2020: FDA Authorizes Marketing of IQOS Tobacco Heating System with ‘Reduced Exposure’ Information.
- Forey BA, Fry JS, Lee PN, Thornton AJ, Coombs KJ. 2013. The effect of quitting smoking on HDL-cholesterol - A review based on within-subject changes. Biomark Res. 1(1):26. doi:10.1186/2050-7771-1-26
- Forster M, Fiebelkorn S, Yurteri C, Mariner D, Liu C, Wright C, McAdam K, Murphy J, Proctor C. 2017. Assessment of novel tobacco heating product THP1.0. Part 3: comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul Toxicol Pharmacol. 93:14–33. doi:10.1016/j.yrtph.2017.10.006
- Franck C, Filion KB, Kimmelman J, Grad R, Eisenberg MJ. 2016. Ethical considerations of e-cigarette use for tobacco harm reduction. Respir Res. 17(1):53. doi:10.1186/s12931-016-0370-3
- Gale N, McEwan M, Camacho OM, Hardie G, Proctor CJ, Murphy J. 2021. Changes in biomarkers after 180 days of tobacco heating product use: a randomised trial. Intern Emerg Med. 16(8):2201–2212. doi:10.1007/s11739-021-02798-6
- Gale N, McEwan M, Hardie G, Proctor CJ, Murphy J. 2022. Changes in biomarkers of exposure and biomarkers of potential harm after 360 days in smokers who either continue to smoke, switch to a tobacco heating product or quit smoking. Intern Emerg Med. 17(7):2017–2030. doi:10.1007/s11739-022-03062-1
- [GOLD] Global Initiative for Chronic Obstructive Lung Disease. 2020. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2020. https://goldcopd.org/gold-reports/
- [GOLD] Global Initiative for Chronic Obstructive Lung Disease. 2013. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary diseases. Updated 2013.
- Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Bangdiwala S, Tyroler HA. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79(1):8–15. doi:10.1161/01.cir.79.1.8
- Goujon C, Kleinhans S, Maeder S, Poget L, Schaller J. 2020. Robustness of HPHC reduction for THS 2.2 aerosol compared with 3R4F reference cigarette smoke under high intensity puffing conditions. Beiträge Zur Tabakforschung International. 29(2):66–83. doi:10.2478/cttr-2020-0008
- Hackshaw A, Morris JK, Boniface S, Tang J-L, Milenković D. 2018. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 360:j5855. doi:10.1136/bmj.j5855
- Hammett CJ, Prapavessis H, Baldi JC, Ameratunga R, Schoenbeck U, Varo N, French JK, White HD, Stewart RA. 2007. Variation in blood levels of inflammatory markers related and unrelated to smoking cessation in women. Prev Cardiol. 10(2):68–75. doi:10.1111/j.1520-037x.2007.05957.x
- Hankinson JL, Odencrantz JR, Fedan KB. 1999. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 159(1):179–187. doi:10.1164/ajrccm.159.1.9712108
- Hatsukami DK, Carroll DM. 2020. Tobacco harm reduction: past history, current controversies and a proposed approach for the future. Prev Med. 140:106099. doi:10.1016/j.ypmed.2020.106099
- Haustein KO, Krause J, Haustein H, Rasmussen T, Cort N. 2002. Effects of cigarette smoking or nicotine replacement on cardiovascular risk factors and parameters of haemorheology. J Intern Med. 252(2):130–139. doi:10.1046/j.1365-2796.2002.01014.x
- Haziza C, de La Bourdonnaye G, Skiada D, Ancerewicz J, Baker G, Picavet P, Lüdicke F. 2016. Evaluation of the Tobacco Heating System 2.2. Part 8: 5-day randomized reduced exposure clinical study in Poland. Regul Toxicol Pharmacol. 81(Suppl 2):S139–S150. doi:10.1016/j.yrtph.2016.11.003
- Haziza C, de La Bourdonnaye G, Skiada D, Ancerewicz J, Baker G, Picavet P, Lüdicke F. 2017. Biomarker of exposure level data set in smokers switching from conventional cigarettes to Tobacco Heating System 2.2, continuing smoking or abstaining from smoking for 5 days. Data Brief. 10:283–293. doi:10.1016/j.dib.2016.11.047
- Hecht SS, Stepanov I, Carmella SG. 2016. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc Chem Res. 49(1):106–114. doi:10.1021/acs.accounts.5b00472
- [IOM] Institute of Medicine. 2001. Clearing the smoke - Assessing the science base for tobacco harm reduction. Washington, DC: The National Academies Press. http://www.nap.edu/catalog/10029.html
- Jakubowski M, Linhart I, Pielas G, Kopecký J. 1987. 2-Cyanoethylmercapturic acid (CEMA) in the urine as a possible indicator of exposure to acrylonitrile. Br J Ind Med. 44(12):834–840. doi:10.1136/oem.44.12.834
- Jensen EJ, Pedersen B, Frederiksen R, Dahl R. 1998. Prospective study on the effect of smoking and nicotine substitution on leucocyte blood counts and relation between blood leucocytes and lung function. Thorax. 53(9):784–789. doi:10.1136/thx.53.9.784
- Kambam JR, Chen LH, Hyman SA. 1986. Effect of short-term smoking halt on carboxyhemoglobin levels and P50 values. Anesth Analg. 65(11):1186–1188.
- Lee KK, Matos S, Evans DH, White P, Pavord ID, Birring SS. 2013. A longitudinal assessment of acute cough. Am J Respir Crit Care Med. 187(9):991–997. doi:10.1164/rccm.201209-1686OC
- Lee PN, Forey BA, Fry JS, Thornton AJ, Coombs KJ. 2014. The effect of quitting smoking on white blood cell count - A review based on within-subject changes. http://www.pnlee.co.uk/documents/refs/lee2014D.pdf
- Lee PN, Fry JS. 2010. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med. 8(1):84. doi:10.1186/1741-7015-8-84
- Levy DT, Chaloupka F, Gitchell J. 2004. The effects of tobacco control policies on smoking rates: a tobacco control scorecard. J Public Health Manag Pract. 10(4):338–353. doi:10.1097/00124784-200407000-00011
- Li X, Luo Y, Jiang X, Zhang H, Zhu F, Hu S, Hou H, Hu Q, Pang Y. 2019. Chemical analysis and simulated pyrolysis of Tobacco Heating System 2.2 compared to conventional cigarettes. Nicotine Tob Res. 21(1):111–118. doi:10.1093/ntr/nty005
- Lüdicke F, Ansari SM, Lama N, Blanc N, Bosilkovska M, Donelli A, Picavet P, Baker G, Haziza C, Peitsch M, et al. 2019. Effects of switching to a heat-not-burn tobacco product on biologically relevant biomarkers to assess a candidate modified risk tobacco product: a randomized trial. Cancer Epidemiol Biomarkers Prev. 28(11):1934–1943. doi:10.1158/1055-9965.EPI-18-0915
- Lüdicke F, Picavet P, Baker G, Haziza C, Poux V, Lama N, Weitkunat R. 2018a. Effects of switching to the Menthol Tobacco Heating System 2.2, smoking abstinence, or continued cigarette smoking on clinically relevant risk markers: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (Part 2). Nicotine Tob Res. 20(2):173–182. doi:10.1093/ntr/ntx028
- Lüdicke F, Picavet P, Baker G, Haziza C, Poux V, Lama N, Weitkunat R. 2018b. Effects of switching to the Tobacco Heating System 2.2 menthol, smoking abstinence, or continued cigarette smoking on biomarkers of exposure: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (Part 1). Nicotine Tob Res. 20(2):161–172. doi:10.1093/ntr/ntw287
- Maeda K, Noguchi Y, Fukui T. 2003. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med. 37(4):283–290. doi:10.1016/s0091-7435(03)00110-5
- Mallock N, Böss L, Burk R, Danziger M, Welsch T, Hahn H, Trieu H-L, Hahn J, Pieper E, Henkler-Stephani F, et al. 2018. Levels of selected analytes in the emissions of “heat not burn” tobacco products that are relevant to assess human health risks. Arch Toxicol. 92(6):2145–2149. doi:10.1007/s00204-018-2215-y
- Mallock N, Pieper E, Hutzler C, Henkler-Stephani F, Luch A. 2019. Heated tobacco products: a review of current knowledge and initial assessments. Front Public Health. 7:287. doi:10.3389/fpubh.2019.00287
- McNeill A, Simonavičius E, Brose L, Taylor E, East K, Zuikova E, Calder R, Robson D. 2022. Nicotine vaping in England: an evidence update including health risks and perceptions, 2022.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. 2005. Standardisation of spirometry. Eur Respir J. 26(2):319–338. doi:10.1183/09031936.05.00034805
- Minet E, Cheung F, Errington G, Sterz K, Scherer G. 2011. Urinary excretion of the acrylonitrile metabolite 2-cyanoethylmercapturic acid is correlated with a variety of biomarkers of tobacco smoke exposure and consumption. Biomarkers. 16(1):89–96. doi:10.3109/1354750X.2010.533287
- Morita H, Ikeda H, Haramaki N, Eguchi H, Imaizumi T. 2005. Only two-week smoking cessation improves platelet aggregability and intraplatelet redox imbalance of long-term smokers. J Am Coll Cardiol. 45(4):589–594. doi:10.1016/j.jacc.2004.10.061
- Ogawa K, Tanaka T, Nagoshi T, Sekiyama H, Arase S, Minai K, Ogawa T, Yoshimura M. 2015. Increase in the oxidised low-density lipoprotein level by smoking and the possible inhibitory effect of statin therapy in patients with cardiovascular disease: a retrospective study. BMJ Open. 5(1):e005455. doi:10.1136/bmjopen-2014-005455
- Palmer RM, Stapleton JA, Sutherland G, Coward PY, Wilson RF, Scott DA. 2002. Effect of nicotine replacement and quitting smoking on circulating adhesion molecule profiles (sICAM-1, sCD44v5, sCD44v6). Eur J Clin Invest. 32(11):852–857. doi:10.1046/j.1365-2362.2002.01067.x
- Peck MJ, Sanders EB, Scherer G, Lüdicke F, Weitkunat R. 2018. Review of biomarkers to assess the effects of switching from cigarettes to modified risk tobacco products. Biomarkers. 23(3):213–244. doi:10.1080/1354750X.2017.1419284
- Philip Morris Products S.A. 2015. Evaluation of biological and functional changes in healthy Smokers after switching to THS 2.2 for 26 weeks [ZRHR-ERS-09-US]. Bethesda (MD): National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT02396381
- Pilz H, Oguogho A, Chehne F, Lupattelli G, Palumbo B, Sinzinger H. 2000. Quitting cigarette smoking results in a fast improvement of in vivo oxidation injury (determined via plasma, serum and urinary isoprostane). Thromb Res. 99(3):209–221. doi:10.1016/s0049-3848(00)00249-8
- Polosa R, Morjaria JB, Prosperini U, Busà B, Pennisi A, Gussoni G, Rust S, Maglia M, Caponnetto P. 2021. Health outcomes in COPD smokers using heated tobacco products: a 3-year follow-up. Intern Emerg Med. 16(3):687–696. doi:10.1007/s11739-021-02674-3
- Prochaska JO, DiClemente CC. 1983. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 51:3, 390–5.
- Public Health England. 2016. E-cigarettes: a developing public health consensus. London, England, United Kingdom.
- Rångemark C, Ciabattoni G, Wennmalm A. 1993. Excretion of thromboxane metabolites in healthy women after cessation of smoking. Arterioscler Thromb. 13(6):777–782. doi:10.1161/01.atv.13.6.777
- Rodgman A, Perfetti TA. 2013. The chemical components of tobacco and tobacco smoke. 2nd ed. CRC Press, Taylor & Francis Inc (United States). Public Health England.
- Rodu B. 2011. The scientific foundation for tobacco harm reduction, 2006-2011. Harm Reduct J. 8(1):19. doi:10.1186/1477-7517-8-19
- Roethig HJ, Zedler BK, Kinser RD, Feng S, Nelson BL, Liang Q. 2007. Short-term clinical exposure evaluation of a second-generation Electrically Heated Cigarette Smoking System. J Clin Pharmacol. 47(4):518–530. doi:10.1177/0091270006297686
- Royal College of Physicians. 2016. Nicotine without smoke: tobacco harm reduction. A report by the Tobacco Advisory Group of the Royal College of Physicians. London: RCP.
- Saareks V, Ylitalo P, Alanko J, Mucha I, Riutta A. 2001. Effects of smoking cessation and nicotine substitution on systemic eicosanoid production in man. Naunyn Schmiedebergs Arch Pharmacol. 363(5):556–561. doi:10.1007/s002100100398
- Sakaguchi C, Nagata Y, Kikuchi A, Takeshige Y, Minami N. 2021. Differences in levels of biomarkers of potential harm among users of a heat-not-burn tobacco product, cigarette smokers, and never-smokers in Japan: a post-marketing observational study. Nicotine Tob Res. 23(7):1143–1152. doi:10.1093/ntr/ntab014
- Schaller JP, Keller D, Poget L, Pratte P, Kaelin E, McHugh D, Cudazzo G, Smart D, Tricker AR, Gautier L, et al. 2016. Evaluation of the Tobacco Heating System 2.2. Part 2: chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul Toxicol Pharmacol. 81(Suppl 2):S27–S47. doi:10.1016/j.yrtph.2016.10.001
- Schulz KF, Altman DG, Moher D, CONSORT Group. 2010. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 8(1):18. doi:10.1186/1741-7015-8-18
- Scott DA, Stapleton JA, Wilson RF, Sutherland G, Palmer RM, Coward PY, Gustavsson G. 2000. Dramatic decline in circulating intercellular adhesion molecule-1 concentration on quitting tobacco smoking. Blood Cells Mol Dis. 26(3):255–258. doi:10.1006/bcmd.2000.0304
- Sharman A, Nurmagambetov T. 2020. Changes in respiratory function and physical capacity among smokers after switching to IQOS: one year follow-up. Glob J Respir Care. 6(1):21–27. doi:10.12974/2312-5470.2020.06.03
- Simonavicius E, McNeill A, Shahab L, Brose LS. 2018. Heat-not-burn tobacco products: a systematic literature review. Tob Control. 28(5):582–594. doi:10.1136/tobaccocontrol-2018-054419
- Sinha S, Luben RN, Welch A, Bingham S, Wareham NJ, Day NE, Khaw KT. 2005. Fibrinogen and cigarette smoking in men and women in the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population. Eur J Cardiovasc Prev Rehabil. 12(2):144–150. doi:10.1097/01.hjr.0000140719.09768.e2
- Smith MR, Clark B, Lüdicke F, Schaller J-P, Vanscheeuwijck P, Hoeng J, Peitsch MC. 2016. Evaluation of the Tobacco Heating System 2.2. Part 1: description of the system and the scientific assessment program. Regul Toxicol Pharmacol. 81(Suppl 2):S17–S26. doi:10.1016/j.yrtph.2016.07.006
- Spinou A, Birring SS. 2014. An update on measurement and monitoring of cough: what are the important study endpoints? J Thoracic Dis. 6(Suppl 7):S728–S34.
- Stephens WE. 2018. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob Control. 27(1):10–17. doi:10.1136/tobaccocontrol-2017-053808
- Stubbe I, Eskilsson J, Nilsson-Ehle P. 1982. High-density lipoprotein concentrations increase after stopping smoking. Br Med J (Clin Res Ed)). 284(6328):1511–1513. doi:10.1136/bmj.284.6328.1511
- Sussman RA, Sipala F, Emma R, Ronsisvalle S. 2023. Aerosol emissions from Heated Tobacco Products: a review focusing on carbonyls, methods, and experimental quality. Toxics. 11(12):947. doi:10.3390/toxics11120947
- Tan XJ, Jiao GP, Ren YJ, Gao XR, Ding Y, Wang XR, Xu H. 2008. Relationship between smoking and dyslipidemia in western Chinese elderly males. J Clin Lab Anal. 22(3):159–163. doi:10.1002/jcla.20235
- Tonstad S, Urdal P. 2002. Does short-term smoking cessation reduce plasma total homocysteine concentrations? Scand J Clin Lab Invest. 62(4):279–284. doi:10.1080/003655102760145834
- Tsai JS, Guo FR, Chen SC, Lue BH, Lee LT, Huang KC, Chen CY, Hung SH, Chuang LM, Chen CY. 2012. Changes of serum adiponectin and soluble intercellular adhesion molecule-1 concentrations after smoking cessation. Clin Chem Lab Med. 50(6):1063–1069. doi:10.1515/cclm-2011-0852
- U.S. Department of Health and Human Services. 2020. Smoking cessation: a report of the Surgeon General. Smoking cessation: a report of the Surgeon General. Washington (DC): US Department of Health and Human Services.
- U.S. Department of Health and Human Services & FDA (Food and Drug Administration). 2012. Docket No. FDA-2012-N-0143]: harmful and potentially harmful constituents in tobacco products and tobacco smoke; established list. Fed Regist. 77(64):20034–20037. [
- Velicer WF, Fava JL, Prochaska JO, Abrams DB, Emmons KM, Pierce J. 1995. Distribution of smokers by stage in three representative samples. Prev Med. 24(4):401–411. doi:10.1006/pmed.1995.1065
- Voulgari C, Katsilambros N, Tentolouris N. 2011. Smoking cessation predicts amelioration of microalbuminuria in newly diagnosed type 2 diabetes mellitus: a 1-year prospective study. Metabolism. 60(10):1456–1464. doi:10.1016/j.metabol.2011.02.014
- [WHO] World Health Organization. 2003. WHO framework convention on tobacco control.
- Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. 2005. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 26(5):835–845. doi:10.1183/09031936.05.00108904
- Yasue H, Hirai N, Mizuno Y, Harada E, Itoh T, Yoshimura M, Kugiyama K, Ogawa H. 2006. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ J. 70(1):8–13. doi:10.1253/circj.70.8
- Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, Han S, Wickham K, Gao YT, Yu MC, et al. 2009. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 69(7):2990–2995. doi:10.1158/0008-5472.CAN-08-4330
