Abstract
Background
Biomarkers play a role in identifying, managing, and predicting cancer outcomes. In lung cancer, they are used at various time points. Doubts remain regarding their accuracy for differential diagnosis and histological subtyping. A diagnostic test study was conducted. It included malignant lesions and controls with benign lesions. Before lung biopsy, all patients had the following biomarkers measured in serum (Pro-GRP,NSE,CYFRA21-1,SCC-Ag,CEA).
Methods
The predictive capacity of serum biomarkers was evaluated to discriminate between lung cancer and benign pathology. The accuracy was also assessed for distinguishing between SCLC and NSCLC and explored their ability to perform histological subtyping.
Results
93 patients were included, 60 with lung cancer, 33 with benign pathology. Pro-GRP and NSE were elevated in SCLC compared with NSCLC or nonmalignant disease. The most accurate for differentiating between malignant and benign pathology were CEA and CYFRA21-1. Pro-GRP had a poor predictive capacity for distinguishing NSCLC from SCLC. However, combined with CEA and CYFRA21-1, performance improved. For SCLC, the diagnostic capacity of Pro-GRP increased by combining with biomarkers, such as NSE/CYFRA21–1.
Conclusions
Biomarkers lacked the sensitivity and specificity for independent differential diagnosis or histological subtyping. However, the observed patterns in biomarker levels associated with specific histological subtypes suggest potential utility in a multi-biomarker approach or in conjunction with other diagnostic tools. This insight could guide future research to improve diagnostic accuracy and personalized treatment strategies in lung cancer.
CLINICAL SIGNIFICANCE
Biomarkers are crucial for identifying, managing, and predicting outcomes in lung cancer, though they lack accuracy in differentiating histological subtypes.
CEA and CYFRA21-1 were the most accurate biomarkers for distinguishing between malignant and benign pathology.
Pro-GRP and NSE levels were elevated in SCLC compared to NSCLC. Pro-GRP alone had poor predictive capacity for differentiating NSCLC from SCLC, but combining it with CEA and CYFRA21-1 improved diagnostic performance.
Patterns in biomarker levels suggest that a multi-biomarker approach, especially when combined with other diagnostic tools, could improve diagnostic accuracy.
Introduction
Lung cancer is the second most common cancer and the leading cause of cancer death worldwide (18.0% of total cancer deaths), with an estimated 2.2 million new cases and 1.8 million deaths reported in 2020 (Sung et al. Citation2021). The 5-year survival rate ranges from 4% to 17% depending on the stage of the disease (Nasim et al. Citation2019). Since lung cancer tends to be asymptomatic,85% of cases are diagnosed at advanced stages, and 57% of patients are diagnosed with metastatic disease (Nasim et al. Citation2019; Siegel et al. Citation2021). On the other hand, a better prognosis has been reported for localized stages, with a 5-year survival rate of the illness of 59% (Siegel et al. Citation2021). It usually develops after the fifth decade of life, being more common in men than women, and tobacco exposure is the most critical risk factor (Mao et al. Citation2016; Nasim et al. Citation2019).
Lung cancer can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), representing 15% and 85% of cases, respectively (Navada et al. Citation2006; Keogh et al. Citation2022). It is essential to distinguish between these two histological subtypes as they have different treatments and prognoses. NSCLC, at its early stages, is curable with surgery. However, SCLC is a rapidly growing, aggressive neoplasm that needs to be treated with chemotherapy and radiotherapy (Schiller Citation2001; Spira and Ettinger Citation2004; Barata and Costa Citation2007).
In the pursuit of early detection of lung cancer, screening efforts have focused on patients meeting the criteria of the National Institutes of Health (NIH)-sponsored National Lung Screening Trial (NLST) using low-dose computed tomography (CT). While this strategy resulted in a 20% reduction in lung cancer mortality after three rounds of screening, it is associated with a high rate of false positive results, increased radiation exposure, and healthcare costs (Chu et al. Citation2018; Seijo et al. Citation2019). Thus, researchers face the challenge of identifying a non-invasive, sensitive, and reliable biomarker for early disease detection (Fang et al. Citation2018).
Tumor-specific circulating proteins has been used for different purposes: as a screening tool in asymptomatic but high-risk patients, to aid in differential diagnosis for patients with suspicious symptoms or lung lesions in thoracic images, for histological classification and treatment strategy at the time of diagnosis, prognosis assessment, monitoring therapy response during oncologic management, and early detection of recurrence or progression (Lokshin et al. Citation2021). They can be measured in whole blood or serum samples obtained through minimally invasive approaches (Chu et al. Citation2018). Their attraction relies on the fact that they are believed to be released early in the disease, can be measured quickly, with non-invasive techniques, and are non-expensive (Schiller Citation2001; Navada et al. Citation2006; Mao et al. Citation2016; Keogh et al. Citation2022).
A limited number of individual serum biomarkers have been reported for lung cancer. However, none have provided enough sensitivity and specificity for its use in clinical practice (Bigbee et al. Citation2012). Some biomarkers, such as cytokeratin-19 fragment (CYFRA 21-1), carcinoembryonic antigen (CEA), and squamous cell carcinoma antigen (SCC-Ag), have been primarily investigated in NSCLC (Cedrés et al. Citation2011; Crosbie et al. Citation2013; Fang et al. Citation2018).
CYFRA 21-1 is the most sensitive tumor marker in NSCLC (Stieber et al. Citation1993; Molina et al. Citation1994; Niklinski et al. Citation1995), and its serum concentrations have been correlated with clinical–pathological characteristics such as tumor size, lymph node status and the stage of disease (Molina et al. Citation1994; Sertić Milić et al. Citation2015). CEA is a serum glycoprotein widely used as a biomarker for colorectal, breast and lung cancer (Dal Bello et al. Citation2019) and provides additive information on lung cancer histology (Liu et al. Citation2017). SCC-Ag is the only marker used in NSCLC with a clear relationship to histology. There is a greater than 95% probability that abnormal serum SCC-Ag levels indicate NSCLC (Nakahama et al. Citation1998; Molina et al. Citation2005).
Pro-Gastrin Releasing Peptide (Pro-GRP) has been identified as a promising biomarker for SCLC as higher levels have been detected even in patients with localized disease. At the same time, it is not considered appreciable in NSCLC or benign lung disease (Molina et al. Citation2005; Citation2010). Finally, Neuron Specific Enolase (NSE) is a cytosolic enzyme that is a better biomarker for SCLC given its neuroendocrine cellular origin (Molina et al. Citation2003; Dal Bello et al. Citation2019). and has been used as a diagnostic, prognostic and follow-up biomarker in SCLC (Isgrò et al. Citation2015).
The study aimed to examine the serum levels of five protein biomarkers (Pro-GRP, NSE, CEA, SCC-Ag, and CYFRA 21-1) in patients with either lung cancer or nonmalignant lung disease NMLD. Additionally, it evaluated the diagnostic capacity of these biomarkers in differentiating patients with suspected lung cancer. Furthermore, the study assessed the sensitivity and specificity of these biomarkers for histological diagnosis.
Methods
Patient samples and determination of serum biomarkers
The patients included in this study consulted at Fundación Valle del Lili (FVL) between 2018 and 2020. FVL is a non-profit university hospital that serves as a reference healthcare facility for the Southwestern region of Colombia. Two groups of patients were included: 60 patients with malignant and 33 patients with benign lung lesions.
The inclusion criteria for patients with malignant pathology were: (1) age > 18 years old, (2) a lung lesion suggestive of malignancy and a high probability of being a primary lesion based on the clinical history and CT scan, and (3) patients scheduled for lung biopsy by thoracoscopy. For patients with benign pathology, the inclusion criteria were: (1) age > 18 years old, (2) a lung lesion with a high probability of being benign based on the clinical history and thoracic CT scan, and (3) patients with an indication for lung biopsy by thoracoscopy for histological classification. The following were the exclusion criteria: (1) Patients who declined to participate in the study and (2) patients with metastatic tumors.
Demographic, clinical, and laboratory variables were collected prospectively by a research assistant in the clinical research center at FVL, who was in charge of registering all the data into a database created with the BDClinic software. Median creatinine levels were measured considering the potential impact of altered renal function on biomarkers. All study participants gave written informed consent to participate in this study. The protocol for this study was previously approved by the Ethics Committee in biomedical research of FVL with the letter No. 018-2018.
Specimen collection and biomarker analysis
Blood samples were taken prior to the biopsy. All patients were tested for the following serum biomarkers: Pro-GRP, NSE, CEA, SCC-Ag, CYFRA 21-1, using the Cobas® 8000-Roche diagnostics system following manufacturer’s recommendations.
Histological analysis, considered the gold standard method for diagnosis, was performed on all patients. The tissue sample collected by thoracoscopy underwent histological classification using the BenchMark ULTRAÒ system-Roche diagnostics system. Tissue samples were subjected to immunohistochemistry (IHC) using specific biomarkers, including Anti-NSE, CEA31, Cytokeratin 19-A53-B/A2.26, anti-p40-BC28, and pro-GRP-mABM16. Once the tissue was prepared a pathologist analyzed the sample. Information regarding other diagnostic procedures, such as X-rays, bronchoscopy, and computed tomography, was available for all patients.
Statistical analysis
All statistical analyzes were performed using the R Project software version 4.1.3. Normality was evaluated in the continuous variables using Shapiro-Wilk’s test. For those variables that did not fit the normal distribution, the median with an interquartile range was used as a descriptive measure. Qualitative variables were described using absolute and relative frequencies. Comparisons between groups for the quantitative variables were performed using the Kruskal-Wallis H test and the Mann-Whitney U test in pairs. Differences for the categorical variables were evaluated using Pearson’s Chi-squared or Fisher’s exact test. P-values < 0.05 were considered statistically significant.
ROC (Receiver Operating Characteristic curve) curves were performed to compare the diagnostic efficacy of each biomarker in the discrimination between SCLC and NSCLC concerning the benign pulmonary disease. The optimal cut-off points for each biomarker were estimated using the values observed in the samples through the ROC analysis, using the method based on the Youden index. The results of the ROC analysis were tabulated as sensitivity, specificity, and precision (area under the curve, AUC) with 95% confidence. The diagnostic accuracy was evaluated based on the following criteria: AUC < 0.5, indicating no diagnostic accuracy; AUC between 0.5 and 0.7, indicating low diagnostic accuracy; AUC between 0.7 and 0.9, indicating moderate diagnostic accuracy; and AUC > 0.9, indicating high diagnostic accuracy.
Results
The study included a total of 93 patients, with 9 (9.70%) diagnosed with SCLC, 51 (54.8%) with NSCLC, and 33 (35.5%) with NMLD. Overall, lung cancer patients reported a smoking history in 66% of cases, with a median tumor size of 40 mm. Patients with NMLD had a median lesion size of 11.5 mm. Among the NSCLC cases, 38 patients had adenocarcinoma, ten had squamous cell carcinoma, and two had large cell carcinoma. One patient did not have a specific histological subtype. The three groups of patients showed median creatinine measurements below 1 mg/dL. The demographic and clinical characteristics of each group are detailed in .
Table 1. Demographic and clinical characteristics of the patients enrolled in the study.
Measurement of serum biomarkers
In and , the medians of the results of each biomarker under study are presented for the three populations of interest. Higher median serum levels of Pro-GRP, CEA, CYFRA 21-1, SCC-Ag, and NSE were found in patients with both SCLC and NSCLC compared to patients diagnosed with NMLD. However, no statistically significant differences were found for Pro-GRP (p = 0.116) and SCC-Ag levels (p = 0.234) between benign and malignant lesions, nor by lung cancer histological type. In NCSLC patients, elevated levels of CEA (5.84 ng/ml) and CYFRA 21-1 (4.27 ng/ml) biomarkers were observed (p < 0.01) compared to NMLD patients, with statistical significance. Patients with SCLC showed higher levels of NSE (41.9 ng/ml) (p < 0.01).
Figure 1. Distribution of pro-GRP, CEA, CYFRA 21-1, SCC-Ag and NSE levels in SCLC, NSCLC and NMLD (non-malignant lung disease).
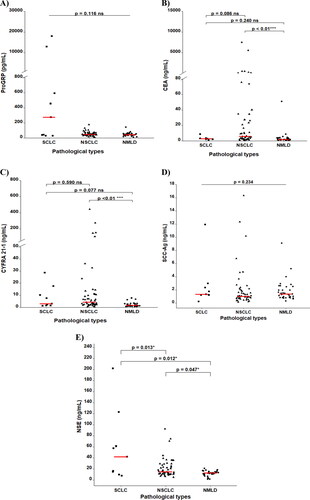
Table 2. Serum biomarkers of the patients enrolled in the study.
CEA levels were higher in NSCLC compared to NMLD (5.84 ng/mL vs. 1.82 ng/mL, p < 0.01). Similar results were observed for CYFRA 21-1 (NSCLC: 4.27 ng/mL vs. NMLD: 1.74 ng/mL, p < 0.01). Conversely, patients with SCLC exhibited higher NSE levels compared to NMLD (SCLC: 41.9 ng/mL vs. NMLD: 12.5 ng/mL, p = 0.012). Additionally, NSE levels were higher in patients with SCLC compared to NSCLC (41.9 ng/mL vs. 14.5 ng/mL, p = 0.013).
Overall accuracy of serum biomarkers for lung cancer diagnosis
The performance of individual biomarkers in diagnosing lung cancer is shown in , and stratified by histological subtype vs. NSCLC. CEA moderate diagnostic accuracy in distinguishing malignancy (NSCLC + SCLC) from NMLD, with an AUC of 76.3%, a sensitivity of 55.0%, and a specificity of 87.9%. Specifically, it showed moderate accuracy in discriminating NSCLC from NMLD, with an AUC of 78.6%, sensitivity of 60.8%, and specificity of 87.9%, this does not indicate the same diagnostic accuracy in distinguishing SCLC from NMLD.
Figure 2. ROC curve of each marker: CEA, CYFRA21-1, NSE, pro-GRP, SCC-Ag for NSCLC + SCLC with respect to non-malignant lung disease.
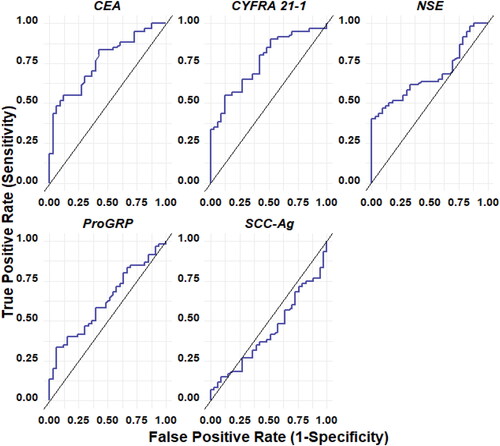
Figure 3. ROC curve of each marker: CEA, CYFRA21-1, NSE, pro-GRP, SCC-Ag for NSCLC with respect to non-malignant lung disease.
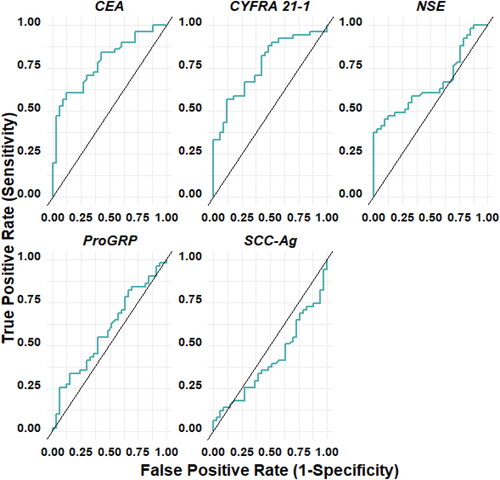
Figure 4. ROC curve of each marker: CEA, CYFRA21-1, NSE, pro-GRP, SCC-Ag for SCLC with respect to non-malignant lung disease.
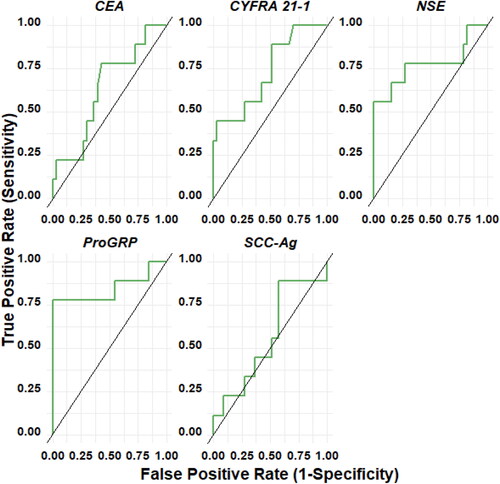
Similarly, CYFRA 21-1 exhibited moderate accuracy in differentiating malignancy (NSCLC + SCLC) from NMLD, with an AUC of 76.2%, a sensitivity of 55.1%, and a specificity of 87.9%. In comparison to NMLD, moderate accuracy was observed for each histology individually, with NSCLC vs. NMLD having an AUC of 76.7%, a sensitivity of 56.9%, and a specificity of 87.9%, and SCLC vs. NMLD having an AUC of 72.9%, a sensitivity of 44.4%, and a specificity of 97.0%. The SCC-Ag biomarker did not exhibit diagnostic accuracy. For Pro-GRP and NSE biomarkers, diagnostic accuracy was low when evaluated individually. However, NSE showed moderate accuracy in discriminating SCLC from NMLD, with an AUC of 77.4%, sensitivity of 55.6%, and specificity of 100%.
In , the performance of combined biomarkers in the diagnosis of lung cancer is described, stratified by histological subtype vs NMLD. Overall, it is observed that in all cases, the diagnostic accuracy for identifying lung cancer from all biomarkers improves when combined with others, achieving moderate to high performance. Combining Pro-GRP with other biomarkers, such as CEA and CYFRA 21-1, enhances the diagnostic value for NSCLC (AUC = 80.4%, sensitivity = 70.6%, and specificity = 81.8%), and further combining it with CEA, CYFRA 21-1, and NSE results in increased diagnostic accuracy (AUC = 81.1%, sensitivity = 74.5%, and specificity = 78.8%). These combinations outperform Pro-GRP alone (AUC = 58.7%, sensitivity = 25.5%, and specificity = 93.9%). For SCLC, the diagnostic efficacy of Pro-GRP is higher when when combined with other biomarkers, such as NSE and CYFRA 21-1, resulting in an AUC of 97.3%, sensitivity of 88.8%, and specificity of 98.9%.
Table 3. Performance different combinations of biomarkers in discriminating NSCLC and SCLC types with respect to non-malignant lung disease (NMLD).
Discussion
Lung cancer remains the predominant cause of cancer-related mortality globally. Despite significant progress in diagnostic modalities and treatments, many patients receive diagnoses at advanced stages, rendering surgical intervention useless and limiting curative options such as chemotherapy and radiotherapy. Screening studies utilizing radiography and sputum cytology have proven ineffective in reducing mortality associated with this disease (Patz et al. Citation2007). The concept of biomarkers is based on the understanding that cancer is a systemic disease. As it progresses, it releases proteins crucial for its growth and metastasis-enhancing capabilities (Patz et al. Citation2007).
Biomarkers have found extensive use in lung cancer, primarily for therapy monitoring and early detection of recurrences (Liu et al. Citation2017). Previous research has highlighted the challenges in using biomarkers for lung cancer diagnosis, including the low detection rate of true positives and the limitations of individual biomarkers due to inter-individual heterogeneity, diverse biochemical pathways, and tumor biology variations (Molina et al. Citation2009; Holdenrieder Citation2016). Consequently, biomarkers are more valuable in the context of differential diagnosis rather than as screening tools (Molina et al. Citation2009; Holdenrieder Citation2016). The use of serum markers for distinguishing between histological types of lung cancer remains controversial, primarily due to their low sensitivity (Mauro et al. Citation2019).
In the present study, patients diagnosed with SCLC exhibited significantly higher median levels of NSE and Pro-GRP than those with NSCLC or NMLD. Specifically, the median values of Pro-GRP were approximately 20 times higher in SCLC patients. At the same time, NSE levels were at least two times higher. This finding aligns with existing literature, which has consistently reported elevated median pro-GRP and NSE levels in SCLC, often ranging around 28 and 4.5 times higher, respectively (Cavalieri et al. Citation2018).
Furthermore, the association between elevated pro-GRP and NSE levels and the presence of SCLC supports the notion that as these biomarker levels increase, the probability of SCLC also rises. Pro-GRP levels exceeding 300 ng/L are considered 99% specific for detecting SCLC (Molina et al. Citation2009). It is important to note that in this study, all patients had normal creatinine levels, which indicates normal renal function. However, it is crucial to consider that serum biomarkers, particularly pro-GRP and CYFRA 21-1, can be elevated in patients with chronic renal impairment, potentially confounding the interpretation of these biomarkers (Nakahama et al. Citation1998).
The serum biomarkers analyzed in the study demonstrated varying predictive capacities for diagnosing lung cancer. Among them, CEA exhibited the highest accuracy; however, it is important to note that CEA levels can also increase in several benign and malignant medical conditions, limiting its specificity (Hammarström Citation1999; Holdenrieder Citation2016; Molina et al. Citation2016). On the other hand, although serum biomarkers such as CEA, CYFRA 21-1, and NSE showed low sensitivity when analyzed individually, they demonstrated high specificity (ranging from 87.9% to 100%). This high specificity makes them valuable in excluding cancer in patients with benign lesions.
Accurate histological subtyping, particularly distinguishing between SCLC and NSCLC, is essential for prognosis and identifying appropriate treatment strategies. Our observations indicate higher CEA and CYFRA 21.1 levels in patients with malignant pathology, particularly those with NSCLC, suggesting their potential as serum biomarkers for this specific histological type. However, we encountered several outliers during the study. It is worth mentioning that various studies have employed different combinations of biomarkers to differentiate between lung cancer patients.
Several biomarkers, including CYFRA 21.1, CEA, SCCA, tissue polypeptide antigen (TPA), and cancer antigen-125 (CA-125), have been investigated for their potential use in NSCLC. These biomarkers have also been associated with clinical outcomes. For instance, a recent meta-analysis found a significant correlation between positive CEA tests and nodal involvement and mortality, even in stage I NSCLC patients (Maeda et al. Citation2017; Nasralla et al. Citation2020). At the same time, NSE has shown promise for SCLC (Jørgensen et al. Citation1996; Molina et al. Citation2003; Cho Citation2007). Similarly, NSE has demonstrated utility as a prognostic biomarker for survival, treatment monitoring, and relapse prediction (Barak et al. Citation2010; Holdenrieder Citation2016).
To overcome the limitations of using individual serum biomarkers for lung cancer diagnosis, researchers have proposed the use of combined biomarker panels for improved accuracy. One promising biomarker, Pro-GRP, has been extensively studied and suggested as a valuable tool for differentiating between various histological types of lung cancer (Patz et al. Citation2007).
This study evaluated the diagnostic value of combining Pro-GRP, CYFRA 21.1, and CEA. The results were highly promising for NSCLC, with an impressive diagnostic value of 80.4% (AUC), a sensitivity of 70.6%, and a specificity of 81.8%. Likewise, for SCLC, combining Pro-GRP, CYFRA 21.1, and NSE yielded the highest diagnostic value of 97.3% (AUC), a sensitivity of 88.8%, and a specificity of 98.9%.
Interestingly, a recent publication that utilized the same biomarkers as our study reported similar findings. They demonstrated that combining these biomarkers improved diagnostic accuracy, achieving a sensitivity of 88.5% and a specificity of 82% (Molina et al. Citation2016). These results highlight the potential of a combined approach to enhance the accuracy of lung cancer diagnosis and emphasize the importance of utilizing multiple biomarkers in clinical practice.
Finally, the present study has several limitations that should be considered. Despite including a sample size of patients with malignant pathology that was twice the calculated number, the number of patients with SCLC identified during the study period was limited to only nine individuals. Although this distribution aligns with the prevalence of histological subtypes in the general population, it likely affected the study’s statistical power to detect a higher accuracy of biomarkers in distinguishing between SCLC and NSCLC, as previously reported in the literature. Furthermore, the study was not specifically designed to evaluate different cutoff values for the biomarkers or to conduct serial testing of multiple biomarkers to enhance their specificity. These aspects were not within the scope of the investigation.
Conclusions
In conclusion, serum biomarker measurement represents a non-invasive approach that can potentially assist in the differential diagnosis of lung cancer. However, in the present study, individual biomarkers alone did not demonstrate sufficient sensitivity and specificity to enable accurate differential diagnosis or histological subtyping. Nevertheless, distinct patterns were observed, as certain biomarkers showed higher levels in specific histological subtypes. Further investigation is warranted to explore the predictive capacity of combined serum biomarkers after serial testing. This approach may hold promise in improving the accuracy and reliability of biomarkers in differentiating between various histological subtypes of lung cancer.
Author’ contributions
All authors have read and approved the manuscript, and significantly contributed to this paper. LFS: Substantial contributions to the conception and design of the work, acquisition, analysis, and interpretation of data for the work, critical review of intellectual content, final approval of the version to be published. SJSG: Substantial contributions to the manuscript writing, analysis, and interpretation of data for the work, critical review of intellectual content, final approval of the version to be published. MN: Substantial contribution to work analysis and interpretation of data and critical review of intellectual content. MAAD: Substantial contribution to work analysis and interpretation of data, writing, critical review of intellectual content, final approval of the version to be published. LFT: Substantial contributions to the conception and design of the work, acquisition, analysis, and interpretation of data for the work, critical review of intellectual content, final approval of the version to be published.
Ethics approval and consent to participate
This manuscript was written in compliance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration. The protocol (IRB/EC protocol number 1210) for this study, was approved by Ethics Committee in biomedical research of the Fundacion Valle del Lili by letter IRB/EC No. 018-2018, which is available if needed with the Corresponding Author. All study participants gave written informed consent to participate in this study.
Funding support
This study was supported by ABBVIE S.A.S. [Grant No. 897-2017], resource management was carried out by the Clinical Research Center of the Fundacion Valle del Lili.
Acknowledgements
Thanks to the Clinical Research Center of the Fundación Valle del Lili and ABBVIE S.A.S. for sponsoring this study.
Disclosure statement
The authors declare that they have no competing interests. This manuscript has not been published and is not under consideration for publication elsewhere. Additionally, all the authors have approved the contents of this paper and have agreed to the journal’s submission policies.
Availability of data and materials
All data and material are available for sharing if needed with the corresponding author.
References
- Barak V, Holdenrieder S, Nisman B, Stieber P. 2010. Relevance of circulating biomarkers for the therapy monitoring and follow-up investigations in patients with non-small cell lung cancer. Cancer Biomark. 6(3-4):191–196. doi:10.3233/CBM-2009-0129
- Barata FJ, Costa AF. 2007. Carcinoma do pulmão de pequenas células – Estado da arte e perspectivas futuras. Pulmonol. 13(4):587–604.
- Bigbee WL, Gopalakrishnan V, Weissfeld JL, Wilson DO, Dacic S, Lokshin AE, Siegfried JM. 2012. A multiplexed serum biomarker immunoassay panel discriminates clinical lung cancer patients from high-risk individuals found to be cancer-free by CT screening. J Thorac Oncol. 7(4):698–708. Apr
- Cavalieri S, Morelli D, Martinetti A, Galli G, Nichetti F, de Braud F, Platania M. 2018. Clinical implications for pro-GRP in small cell lung cancer. A single center experience. Int J Biol Markers. 33(1):55–61. doi:10.5301/ijbm.5000305
- Cedrés S, Nuñez I, Longo M, Martinez P, Checa E, Torrejón D, Felip E. 2011. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 12(3):172–179.
- Cho WCS. 2007. Potentially useful biomarkers for the diagnosis, treatment and prognosis of lung cancer. Biomed Pharmacother. 61(9):515–519. doi:10.1016/j.biopha.2007.08.005
- Chu GCW, Lazare K, Sullivan F. 2018. Serum and blood based biomarkers for lung cancer screening: a systematic review. BMC Cancer. 18(1):181. doi:10.1186/s12885-018-4024-3
- Crosbie PAJ, Shah R, Summers Y, Dive C, Blackhall F. 2013. Prognostic and predictive biomarkers in early stage NSCLC: CTCs and serum/plasma markers. Transl Lung Cancer Res. 2(5):382–397. doi:10.3978/j.issn.2218-6751.2013.09.02
- Dal Bello MG, Filiberti RA, Alama A, Orengo AM, Mussap M, Coco S, Vanni I, Boccardo S, Rijavec E, Genova C, et al. 2019. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J Transl Med. 17(1):74. Mar 8 doi:10.1186/s12967-019-1828-0
- Fang R, Zhu Y, Khadka VS, Zhang F, Jiang B, Deng Y. 2018. The evaluation of serum biomarkers for non-small cell lung cancer (NSCLC) diagnosis. Front Physiol. 9:1710. doi:10.3389/fphys.2018.01710
- Hammarström S. 1999. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 9(2):67–81. Apr doi:10.1006/scbi.1998.0119
- Holdenrieder S. 2016. Biomarkers along the continuum of care in lung cancer. Scand J Clin Lab Invest Suppl. 245:S40–S45. doi:10.1080/00365513.2016.1208446
- Isgrò MA, Bottoni P, Scatena R. 2015. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 867:125–143. doi:10.1007/978-94-017-7215-0_9
- Jørgensen LG, Osterlind K, Genollá J, Gomm SA, Hernández JR, Johnson PW, Løber J, Splinter TA, Szturmowicz M. 1996. Serum neuron-specific enolase (S-NSE) and the prognosis in small-cell lung cancer (SCLC): a combined multivariable analysis on data from nine centres. Br J Cancer. 74(3):463–467. doi:10.1038/bjc.1996.383
- Keogh A, Finn S, Radonic T. 2022. Emerging biomarkers and the changing landscape of small cell lung cancer. Cancers. 14(15):3772. doi:10.3390/cancers14153772
- Liu L, Teng J, Zhang L, Cong P, Yao Y, Sun G, Zhijun L, Teng Y, Mingjun L. 2017. The combination of the tumor markers suggests the histological diagnosis of lung cancer. Biomed Res Int. 2017:2013989.
- Lokshin A, Bast RC, Rodland K. 2021. Circulating cancer biomarkers. Cancers. 13(4):802. doi:10.3390/cancers13040802
- Maeda R, Suda T, Hachimaru A, Tochii D, Tochii S, Takagi Y. 2017. Clinical significance of preoperative carcinoembryonic antigen level in patients with clinical stage IA non-small cell lung cancer. J Thorac Dis. 9(1):176–186. doi:10.21037/jtd.2017.01.30
- Mao Y, Yang D, He J, Krasna MJ. 2016. Epidemiology of lung cancer. Surg Oncol Clin N Am. 25(3):439–445. doi:10.1016/j.soc.2016.02.001
- Mauro C, Passerini R, Spaggiari L, Galetta D, Radice D, Lentati P, Sandri MT. 2019. New and old biomarkers in the differential diagnosis of lung cancer: Pro-gastrin-releasing peptide in comparison with neuron-specific enolase, carcinoembryonic antigen, and CYFRA 21-1. Int J Biol Markers. 34(2):163–167. doi:10.1177/1724600819834235
- Molina R, Agusti C, Filella X, Jo J, Joseph J, Giménez N, Ballesta AM. 1994. Study of a new tumor marker, CYFRA 21-1, in malignant and nonmalignant diseases. Tumour Biol. 15(6):318–325.
- Molina R, Agusti C, Mañe JM, Filella X, Jo J, Joseph J, Giménez N, Estapé J, Ballesta AM. 1994. CYFRA 21-1 in lung cancer: comparison with CEA, CA 125, SCC and NSE serum levels. Int J Biol Markers. 9(2):96–101. doi:10.1177/172460089400900206
- Molina R, Augé JM, Bosch X, Escudero JM, Viñolas N, Marrades R, Ramírez J, Carcereny E, Filella X. 2009. Usefulness of serum tumor markers, including progastrin-releasing peptide, in patients with lung cancer: correlation with histology. Tumour Biol. 30(3):121–129.
- Molina R, Auge JM, Filella X, Viñolas N, Alicarte J, Domingo JM, Ballesta AM. 2005. Pro-gastrin-releasing peptide (proGRP) in patients with benign and malignant diseases: comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung cancer. Anticancer Res. 25(3A):1773–1778.
- Molina R, Filella X, Augé JM, Fuentes R, Bover I, Rifa J, Moreno V, Canals E, Viñolas N, Marquez A, et al. 2003. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 24(4):209–218.
- Molina R, Holdenrieder S, Auge JM, Schalhorn A, Hatz R, Stieber P. 2010. Diagnostic relevance of circulating biomarkers in patients with lung cancer. Cancer Biomark. 6(3-4):163–178. doi:10.3233/CBM-2009-0127
- Molina R, Marrades RM, Augé JM, Escudero JM, Viñolas N, Reguart N, Ramirez J, Filella X, Molins L, Agustí A, et al. 2016. Assessment of a combined panel of six serum tumor markers for lung cancer. Am J Respir Crit Care Med. 193(4):427–437. doi:10.1164/rccm.201404-0603OC
- Nakahama H, Tanaka Y, Fujita Y, Fujii M, Sugita M. 1998. CYFRA 21-1 and ProGRP, tumor markers of lung cancer, are elevated in chronic renal failure patients. Respirology. 3(3):207–210. Sep doi:10.1111/j.1440-1843.1998.tb00123.x
- Nasim F, Sabath BF, Eapen GA. 2019. Lung cancer. Med Clin North Am. 103(3):463–473. doi:10.1016/j.mcna.2018.12.006
- Nasralla A, Lee J, Dang J, Turner S. 2020. Elevated preoperative CEA is associated with subclinical nodal involvement and worse survival in stage I non-small cell lung cancer: a systematic review and meta-analysis. J Cardiothorac Surg. 15(1):318. doi:10.1186/s13019-020-01353-2
- Navada S, Lai P, Schwartz AG, Kalemkerian GP. 2006. Temporal trends in small cell lung cancer: Analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. JCO. 24(18_suppl):7082–7082. doi:10.1200/jco.2006.24.18_suppl.7082
- Niklinski J, Furman M, Rapellino M, Chyczewski L, Laudanski J, Oliaro A, Ruffini E. 1995. CYFRA 21-1 determination in patients with non-small cell lung cancer: clinical utility for the detection of recurrences. J Cardiovasc Surg. 36(5):501–504. Oct
- Patz EF, Campa MJ, Gottlin EB, Kusmartseva I, Guan XR, Herndon JE. 2007. Panel of serum biomarkers for the diagnosis of lung cancer. J Clin Oncol. 25(35):5578–5583. Dec 10 doi:10.1200/JCO.2007.13.5392
- Schiller JH. 2001. Current standards of care in small-cell and non-small-cell lung cancer. Oncology. 61(Suppl 1):3–13. doi:10.1159/000055386
- Seijo LM, Peled N, Ajona D, Boeri M, Field JK, Sozzi G, Pio R, Zulueta JJ, Spira A, Massion PP, et al. 2019. Biomarkers in lung cancer screening: achievements, promises, and challenges. J Thorac Oncol. 14(3):343–357.
- Sertić Milić H, Franjević A, Bubanović G, Marušić A, Nikolić I, Puljić I. 2015. Size, edge, and stage of NSCLC determine the release of CYFRA 21-1 in bloodstream. Wien Klin Wochenschr. 127(11-12):465–471. Jun doi:10.1007/s00508-014-0678-2
- Siegel RL, Miller KD, Fuchs HE, Jemal A. 2021. Cancer Statistics, 2021. CA Cancer J Clin. 71(1):7–33. doi:10.3322/caac.21654
- Spira A, Ettinger DS. 2004. Multidisciplinary management of lung cancer. N Engl J Med. 350(4):379–392. doi:10.1056/NEJMra035536
- Stieber P, Hasholzner U, Bodenmüller H, Nagel D, Sunder-Plassmann L, Dienemann H, Meier W, Fateh-Moghadam A. 1993. CYFRA 21-1. A new marker in lung cancer. Cancer. 72(3):707–713. doi:10.1002/1097-0142(19930801)72:3<707::AID-CNCR2820720313>3.0.CO;2-X
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 71(3):209–249. May
