Abstract
Introduction
Hyponatremia, defined as a serum sodium concentration <135 mmol/l, is a frequent electrolyte disorder in patients presenting to an emergency department (ED). In this context, appropriate diagnostic and therapeutic management is rarely performed and challenging due to complex pathophysiologic mechanisms and a variety of underlying diseases.
Objective
To implement a feasible pathway of central diagnostic and therapeutic steps in the setting of an ED.
Methods
We conducted a narrative review of the literature, considering current practice guidelines on diagnosis and treatment of hyponatremia. Underlying pathophysiologic mechanisms and management of adverse treatment effects are outlined. We also report four cases observed in our ED.
Results
Symptoms associated with hyponatremia may appear unspecific and range from mild cognitive deficits to seizures and coma. The severity of hyponatremia-induced neurological manifestation and the risk of poor outcome is mainly driven by the rapidity of serum sodium decrease. Therefore, emergency treatment of hyponatremia should be guided by symptom severity and the assumed onset of hyponatremia development, distinguishing acute (<48 hours) versus chronic hyponatremia (>48 hours).
Conclusions
Especially in moderately or severely symptomatic patients presenting to an ED, the application of a standard management approach appears to be critical to improve overall outcome. Furthermore, an adequate work-up in the ED enables further diagnostic and therapeutic evaluation during hospitalization.
Epidemiology of hyponatremia in the emergency department
Hyponatremia, defined as a serum sodium concentration <135mmol/l, is the most common electrolyte disorder (Giordano et al. Citation2016, Spasovski et al., Citation2014). There is limited data from mostly retrospective cohort studies on the prevalence of hyponatremia in patients presenting to the emergency department (ED), with numbers varying between 3-10% (Arampatzis et al. Citation2012; Olsson et al. Citation2013; Imai and Shibagaki Citation2019; Lee et al. Citation2000). The prevalence is even higher in hospitalized patients, affecting up to 30% of hospitalized patients (Waikar et al. Citation2009; Hoorn et al. Citation2006). The risk of hyponatremia increases with age. In detail, the prevalence ranges from 2% in patients aged 16 – 21 years to 17% in patients aged >80 years in ED (Lindner et al. Citation2014). Higher prevalence was also found in patients with acute kidney injury (Woitok et al. Citation2020) and in patients on diuretic medication (especially thiazide-like diuretics) (Arampatzis et al. Citation2013). Importantly, hyponatremia is an independent risk factor of mortality and length of hospital stay (Waikar et al. Citation2009; Woitok et al. Citation2020; Corona et al. Citation2013). It has also been reported to be a potent predictor of poor outcome in patients with underlying chronic diseases, including heart failure and liver cirrhosis (Bettari et al. Citation2012; Balling et al. Citation2011; Jenq et al. Citation2010). Even mild hyponatremia, defined by a serum sodium concentration between 130-134 mmol/l (Spasovski et al., Citation2014) and often perceived ‘asymptomatic’ in clinical practice, has been associated with cognitive impairment and higher incidences of falls, bone fractures and osteoporosis (Renneboog et al. Citation2006; Soiza et al. Citation2014). Notably, a high proportion of iatrogenic or hospital-acquired hyponatremia has been reported, accounting for about two thirds of patients studied (Anderson et al. Citation1985).
A retrospective analysis of more than 200 000 patients presenting to a swedish ED has shown, that the frequency of adequate diagnostic testing in the ED was low (Olsson et al. Citation2013), with only 31% of patients with profound hyponatremia (serum sodium concentration <120mmol/l) being evaluated with a basic laboratory investigation. In this study, the majority of patients was treated with 0.9% sodium chloride infusion. In 27% of patients with severe hyponatremia, there was an overly rapid correction of hyponatremia. Also, studies on management of hyponatremia in hospitalized patients revealed that adequate work-up and treatment were rarely performed, as shown in an analysis of more than 3000 hospitalized patients of the Hyponatremia Registry in the United States and European Union (Greenberg et al. Citation2015). Inadequate work-up and treatment were itself associated with higher morbidity and mortality (Greenberg et al. Citation2015; Tzoulis et al. Citation2014; Huda et al. Citation2006). Despite the association with poor outcomes and availability of effective therapy, most patients with hyponatremia are discharged from hospital still hyponatremic (Greenberg et al. Citation2015). In contrast, following a simple algorithm for diagnosing and treating hyponatremia significantly improved management outcomes (Fenske et al. Citation2010).
Given that hyponatremia is associated with multiple underlying pathophysiological mechanisms, diseases, and treatment options, the differential diagnosis of hyponatremia is often challenging. Especially in an ED setting with limited resources and time restriction, a final diagnosis of the underlying etiology of hyponatremia might not always be possible. However, an initial adequate work-up, including adequate assessment of medical history, combined analysis of blood and urine samples and clinical assessment will form a substantial basis for further diagnostic and therapeutic evaluation during hospitalization. In the authors view, given existing data outlined above, the application of a standard approach for work-up of hyponatremic patients presenting to an ED seems to be critical to improve overall outcome of patients with hyponatremia.
Therefore, the aim of this narrative review is to reflect current practice guidelines on diagnosis and treatment of hyponatremia (Spasovski et al., Citation2014), hereby pointing out important diagnostic steps which are feasible to follow in the setting of an ED. We also outline indications for treatment initiation and risk of adverse treatment effects.
Pathophysiology and clinical manifestation
In general, different forms of hyponatremia can be distinguished according to tonicity, which is defined by the concentration of effective osmoles in the extracellular space. As sodium and its accompanying anions are the major effective extracellular solutes, hyponatremia usually refers to hypotonic hyponatremia. Importantly, marked elevations of either lipids or proteins in plasma can cause an artifactual decrease in serum sodium concentration (‘pseudohyponatremia’), which is associated with isotonicity. Acute hyperglycemia can induce iso- or even hypertonic hyponatremia, as glucose as additional effective solute next to sodium induces i) an osmotic shift of water from the intracellular to the extracellular fluid (ECF) compartment and ii) osmotic diuresis with consecutive dehydration. This dilutional decrease in serum sodium will automatically resolve with glucose lowering therapy. This so-called translocational hyponatremia can also occur in the presence of other additional serum osmoles, such as mannitol or glycine (e.g. if used as irrigation fluid in urological or gynecological surgery). However, the most common form of hyponatremia is hypotonic hyponatremia. Based on the patients ECF status, hypotonic hyponatremia can be classified in three sub-categories which inherit substantial differences in pathophysiology and therefore require differential therapeutic strategies: hypovolemic hyponatremia, euvolemic hyponatremia and hypervolemic hyponatremia. Next, we report four cases observed in our ED illustrating these sub-categories.
Case 1
A 77-year old woman presenting to the ED reported to have suffered from acute diarrhea one week earlier, followed by nausea (without vomiting), vertigo and weakness for five days. At admission, she reported a reduced oral fluid intake of less than one liter per day. Volume status was evaluated hypovolemic, although blood pressure was elevated with 160/80 mmHg. Her medication list revealed antidiabetic and antihypertensive medication, including torasemide 10 mg per day. There was no intake of thiazide-like diuretics. Torasemide had been added one week before on a neurologic ward for control of blood pressure, after having been diagnosed with subarachnoid hemorrhage five weeks before re-admission to hospital. Plasma sodium was 121 mmol/l, plasma osmolality was 249 mosmo/kg, urine sodium was 24 mmol/l and urine osmolality was 166 mosmo/kg. In ED, assuming symptomatic hypovolemic hyponatremia, 500 ml of balanced crystalloid solution was administered. Point-of-care based electrolyte analysis revealed increase of sodium level from 121 mmol/l to 124 mmol/l within three hours, which supported the diagnosis of hypovolemic hyponatremia. On ward, plasma sodium level normalized within seven days under continuous intravenous volume repletion and by withholding torasemide, as the latter might have aggravated volume depletion in the context of diarrhea and low oral fluid intake.
Case 2
A 35-year old woman was admitted to the ED with nausea (without vomiting) and vertigo by an endocrinologist with suspicion of adrenal crisis. She had undergone transsphenoidal resection of a macroprolactinoma nine days earlier. Post-operative sodium and basal cortisol levels had been within the reference range. She had been dismissed without prophylactic hydrocortisone substitution. At presentation to the ED, volume status was euvolemic with normotensive blood pressure of 120/90 mmHg. Low plasma sodium of 123 mmol/l was accompanied by low plasma osmolality (255 mosmo/kg), higher urine sodium (56 mmol/l) and urine osmolality (338 mosmo/kg). There was no intake of possibly interfering medication. Prior to admission, she had been administered an oral hydrocortisone dose of 30 mg by the endocrinologist. In ED, continuous intravenous hydrocortisone infusion was started immediately (100 mg/24h). The day after, plasma sodium had only increased by 1 mmol/l. According to the euvolemic status and laboratory constellation, postoperative syndrome of inappropriate antidiuresis (SIAD) was assumed. An oral fluid restriction by one liter per day was started. Within 24 hours, plasma sodium increased from 125 mmol/l to 135 mmol/l. The ongoing i.v. hydrocortisone administration was changed to oral intake (30 mg per day). Plasma sodium level further increased and remained stable under liberalization of oral fluid intake during the next days.
Comment by the authors: Postoperative hyponatremia may occur due to a SIAD, which typically occurs on day 7-10 after pituitary surgery and is a transient condition in most cases. Therefore, in house patients may benefit from a daily control of plasma sodium levels. Importantly, secondary adrenal insufficiency after pituitary surgery may also cause SIAD due to disinhibition of AVP suppression. Therefore, if there is any suspicion of adrenal crisis, continuous hydrocortisone treatment should be immediately started. Numerous patients require hydrocortisone treatment (e.g. hydrocortisone 30 mg/day) after resection of pituitary tumors. Several centers recommend a prophylactic hydrocortisone treatment in all patients over the next weeks. Patients should be well informed about symptoms and management of adrenal insufficiency including an emergency certificate and prescription of an emergency medical kit.
Case 3
A 54-year old man presented to the ED with progressive symmetric leg and arm edemas, nycturia, exertional dyspnea (NYHA III) as well as orthopnea.
He was a smoker with 30 pack years without other pre-known illnesses. He did not take any medication. Electrocardiogram showed normofrequent sinus rhythm (95/min) without signs of myocardial ischemia. Blood gas analysis showed normoglycemia and hyponatremia with 123 mmol/l. NTproBNP was elevated with 8366 ng/l (<386). There were no clinical and laboratory signs of an infection. Troponin T hs was 28 ng/l and 35 ng/l after 3 hours (<14 ng/l), with moderate elevation of creatine kinase (288 U/l and 231 U/l after 3 hours (<190 U/l)). Chest x-ray revealed bilateral pleural effusion (right > left side) and signs of central congestion. Transthoracic echocardiography showed tricuspid insufficiency grade 1-2 as well as signs of both pulmonary hypertension (SPAP 65 mmHg) and diastolic insufficiency. In the ED, right-sided pleural puncture was performed, draining 1.5 liters of serous pleural fluid. Intravenous diuretic therapy (furosemide) was initiated in the ED. Analysis of spot urine sodium and osmolality was not performed in the ED. Based on available information, hyponatremia was diagnosed as hypervolemic hyponatremia in cardiac decompensation. The plasma sodium level normalized through i.v.-diuretic therapy and further medication for cardiac insufficiency within the following days, consecutively leading to euvolemia with a total loss of 13 kg of excessive fluids. Combined left- and right-heart catheter exam revealed triple-vessel coronary artery disease as well as pulmonal-artery hypertension as underlying cause for cardiac decompensation.
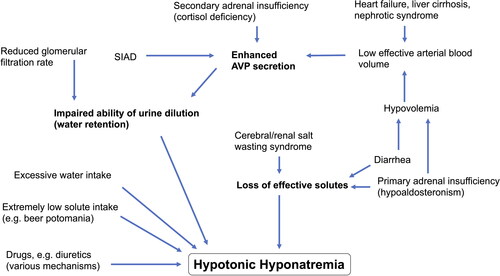
In physiologic condition, hyponatremia is prevented by maximal dilution of urine, except if there is excessive water intake exceeding physiologic renal water excretion (primary polydipsia, intense exercise, use of ‘Ecstasy’) or if the dietary solute intake is extremely low (severe malnutrition, beer potomania) (Sterns Citation2015). Apart from these scenarios, hypotonic hyponatremia is caused by the impaired ability of the body to dilute the urine, mostly based on plasma activity of arginine vasopressin (AVP). This mechanism will lead to hyponatremia if the person’s water intake exceeds the capacity of renal water excretion and thus can be aggravated in the context of a reduced glomerular filtration rate in acute kidney injury or chronic kidney disease. AVP secretion is stimulated by two mechanisms: 1) increased plasma osmolality via activation of osmoreceptors, located in anterior hypothalamus and 2) decrease in blood pressure via activation of baroreceptors (nonosmotic hemodynamic stimuli). Binding of AVP to the renal AVP V2 receptor subtype leads to reabsorption of water via AVP mediated insertion of Aquaporin-2 water channels in the collection duct. The nonosmotic hemodynamic stimuli for AVP secretion are predominantly responsible for water retention in hypovolemic states and in states of extended extravascular fluid but reduced intravascular fluid (e.g. decompensated heart failure, liver cirrhosis, nephrotic syndrome) (Anderson et al. Citation1985, Robertson Citation2006). The syndrome of inappropriate antidiuresis (SIAD), being the most common cause of euvolemic hyponatremia, describes a failure of AVP suppression in the absence of renal insufficiency, adrenal insufficiency (AI) or any recognized (osmotic or non-osmotic) stimulus for AVP secretion. It was originally described by Schwartz and Bartter as syndrome of inappropriate antidiuretic hormone secretion (SIADH) (Schwartz et al. Citation1957). The term SIAD was introduced later, when other pharmacologic and genetic factors were found to inappropriately increase water permeability in the collecting duct in the absence of AVP. In SIAD, endogenous AVP secretion can be either ectopic (from a tumor, e.g. small cell lung carcinoma) or eutopic (from neurohypophysis). It can be induced by a variety of malignant and pulmonary diseases, as well as disorders of the central nervous system, stress, nausea, or injury (Robertson Citation2006). Moreover, a large variety of medications are known to enhance AVP activity (drug-induced SIAD), including antidepressants, antipsychotics and anticonvulsive drugs (Liamis et al. Citation2008).
Apart from this, one of the most common forms of hyponatremia is diuretic-induced hyponatremia, which is almost exclusively caused by thiazides or thiazide-like drugs. There exist numerous implicated underlying pathophysiological mechanisms, which are still a matter of debate. Interestingly, the clinical manifestation of thiazide-induced hyponatremia typically resembles a SIAD-like pattern in terms of laboratory constellation and clinical euvolemia (Liamis et al. Citation2008). In contrast, loop diuretics rarely cause hyponatremia which might be due to differences in their tubular site of action, impairing both renal concentrating and diluting mechanisms as well as the responsiveness to AVP (Liamis et al. Citation2008; Szatalowicz et al. Citation1982). Case 4 illustrates a diuretic-induced hyponatremia observed in our ED.
Case 4
A 84-year old woman presented to the ED with nausea, vomiting and weakness for three days. Oral fluid intake had been 1.5 − 2 liter per day. Venous blood gas analysis revealed substantial hyponatremia (111 mmol/l) and hypokalemia (2.8 mmol/l), excluding hyperglycemia. The patient was awake (GCS 15). Volume status was estimated to be euvolemic. No intake of diuretic medication could be evaluated, although the patient did not recall one substance which had been added by the general practitioner three weeks before. Due to weekend, this could not be clarified. She received one liter of isotonic saline with substitution of potassium chloride, which raised sodium concentration by 2 mmol/l in one hour. The patient was then rapidly transferred to an intensive care unit (ICU) for further treatment. Urine sodium concentration was 100 mmol/l and urine osmolality was 284 mosmo/kg. On ICU, sodium and potassium levels were increased by administration of 500 ml isotonic saline, substitution of potassium chloride and administration of 1000 mg of oral sodium chloride. At the time of transfer to endocrinological ward, plasma sodium had stabilized at 119 mmol/l and patient was no longer symptomatic. Based on initial urine results, euvolemic status, the known absence of diuretics and normal kidney function, a SIAD could not be excluded despite the response to high doses of sodium administration. After exclusion of AI, fluid restriction (one liter per day) was started. As serum sodium concentration decreased during infusion to 115 mmol/l (asymptomatic), an SIAD was rather unlikely. This was also supported by the finding, that a subsequent administration of 500 ml of 0.9% sodium chloride saline was followed by an immediate increase of serum sodium levels. In the meantime, the general practitioner was contacted, which revealed that the newly added medication was chlorthalidone, which the patient had been taking due to intermittent leg edemas in summer. Under ad libitum oral fluid intake, the sodium concentration normalized slowly in the following days. The patient and general practitioner were informed that thiazide-like diuretics should be avoided in the future.
Comment by the authors: This case illustrates well the importance and the challenge of an accurate assessment of medication intake in ED. Furthermore, it shows that interpretation of urine results must always take into account (occult) intake of diuretic medication. Here, the decreased sodium and potassium level as well as elevated urine sodium and urine osmolality were caused by chlorthalidone intake. Vomiting may have aggravated hypokalemia. The increase of sodium concentration by correction of hypokalemia must also be considered. In the view of the authors, initial administration of 3% hypertonic sodium saline could have been evaluated in this case, as vomiting might already reflect severe neurological manifestation. However, clinicians might not have felt confident enough to attribute the symptoms to hyponatremia which justifies the less aggressive approach. It should have been definitely performed, if the patient had presented with reduced responsiveness.
Both primary and secondary AI induce hyponatremia, resulting from hypoaldosteronism (primary AI) or from disinhibited AVP release due to cortisol deficiency (secondary AI) (Spasovski et al., Citation2014). Clinicians must also be aware that depletion of potassium contributes to hyponatremia, since the serum sodium concentration is determined by total exchangeable sodium and potassium in total body water, according to a simplified version of the Edelman equation (Edelman et al. Citation1958). Conversely, oral or intravenous repletion of potassium will automatically increase serum sodium concentration (Nguyen and Kurtz Citation2004).
Hyponatremia may rarely occur in patients with severe hypothyroidism, predominantly in patients with myxedema. The hypothyroidism-induced decrease in cardiac output is seen as the main driver of elevated AVP levels leading to water retention and hyponatremia (Liamis et al. Citation2017). Renal and cerebral salt wasting are also rather rare causes of hyponatremia (Spasovski et al., Citation2014). Cerebral salt wasting, firstly described by Peters et al. in 1950, can occur in patients with intracranial disorders such as subarachnoid hemorrhage (Berendes et al. Citation1997, Peters et al. Citation1950). Renal salt wasting describes basically the same clinical manifestation in the absence of a cerebral disorder and can be observed in tubulopathies (e.g. chemotherapy-induced). Both forms are caused by profound natriuresis that is followed by volume depletion. The latter induces an ‘appropriate’ AVP secretion further driving hyponatremia. The underlying mechanisms are still not completely understood but may involve an impaired central regulation of sodium reabsorption caused by natriuretic factors (e.g. Brain Natriuretic Peptide) as well as a decreased sympathetic outflow to the kidney (Berendes et al. Citation1997, Oh and Shin Citation2014).
summarizes contributing factors to development of hyponatremia. Taken together, hyponatremia is mostly a phenomenon of dilution due to water retention and/or excess water intake, which is sometimes combined with loss of effective solutes (e.g. primary AI or diarrhea). Although sodium is even retained in hypervolemic hyponatremia through activation of the renin-angiotensin-aldosterone system, the non-osmotic AVP release outweighs sodium retention leading to hyponatremia. This is noteworthy, as oral sodium chloride supplementation would aggravate the vicious circle of ECF expansion and should therefore be avoided in hypervolemic hyponatremia. Understanding the phenomenon of dilution is also crucial for initial treatment of hypovolemic hyponatremia with accompanied increased AVP release, as oral intake of solute-free water can aggravate hyponatremia due to AVP-induced water retention. Therefore, only administration of isotonic saline infusion will adequately provide the needed increase in effective arterial blood volume to reduce AVP levels in hypovolemic hyponatremia. Equally, patients with renal/cerebral salt wasting will benefit from isotonic saline administration. In contrast, oral fluid restriction is effective in SIAD (normovolemic hyponatremia) treatment to counteract the dilutional effect.
Figure 1. Pathophysiology of hyponatremia.
Development of hyponatremia is mostly a phenomenon of dilution due to water retention and/or excess water intake, which is sometimes combined with loss of effective solutes. Mechanisms are simplified in order to create an overview. Abbreviations: SIAD = Syndrome of inappropriate antidiuresis; AVP = Arginine vasopressin.
Clinical manifestations of hyponatremia are primarily neurologic due to brain edema caused by entry of water into neurons to counteract plasma hypotonicity. Symptoms depend on the rapidity as well as the severity of the decline in plasma sodium concentration. In chronic hyponatremia (>48h), homeostatic compensatory mechanisms have allowed brain cells to adapt to changes in plasma osmolality over time. These adaptations include the fast release of electrolytes, as well as the slower release (within 24-48h) of organic solutes from brain cells, in order to maintain intracellular solute concentration equal to plasma osmolality. The brain cell adaptation explains why chronic hyponatremia is usually associated with milder and more unspecific clinical symptoms (like cognitive deficits or gait instability). Inversely, a patient with chronic hyponatremia is at greater risk of adverse effects of sodium overcorrection compared to acute hyponatremia, as the compensatory restoration of lost organic solutes in brain cells takes time. Moderately severe symptoms of hyponatremia include headache, confusion and nausea without vomiting (Spasovski et al., Citation2014).
In contrast, acute hyponatremia (<48h) usually causes more severe neurological symptoms such as vomiting, seizures and coma, as a result of osmotically induced cerebral edema, which can ultimately result in fatal transforaminal herniation, respiratory arrest, permanent brain damage and death (Spasovski et al., Citation2014, Adrogué et al. Citation2022). Seizures are partly promoted by acute compensatory release of the organic osmolyte glutamate (Sterns Citation2015). Therefore, acute hyponatremia frequently requires a faster recompensation. However, overly rapid correction of hyponatremia may cause severe neurologic impairment and death as a result of osmotic demyelination due to osmotic stress, which predominantly affects the central pons, but can also involve extrapontine structures (Sterns Citation2015). Typically, the course of such a osmotic demyelination syndrome (ODS) is biphasic with a symptom-free phase after sodium overcorrection and followed by ODS-induced secondary deterioration 1-7 days after the triggering factor (Adrogué et al. Citation2022). Pons myelinolysis can result in dysarthria, dysphagia, oculomotor dysfunction and variable degrees of quadriparesis (Sterns Citation2015). The course of the disease is highly variable and can be temporary, permanent and progressive, which appears to be unpredictable on the basis of initial presentation (Adrogué et al. Citation2022, Louis et al. Citation2012). However, the long-term prognosis is more favorable than thought so far, eventually resulting in full functional recovery despite initial severe manifestation (Louis et al. Citation2012). A systematic review on 541 patients with ODS, comprising all types of ODS etiology, reported favorable outcome in about half of cases and a mortality rate of about 25% (Singh et al. Citation2014). Recognized risk factors for development of osmotic demyelination are extreme chronic hyponatremia <105-110 mmol/l, alcohol use disorder, liver disease or transplant, malnutrition and hypokalemia (Adrogué et al. Citation2022, Singh et al. Citation2014, Verbalis et al. Citation2013). Importantly, after inadvertent sodium overcorrection, ODS can be prevented by relowering plasma sodium within 12-24 hours below the recommended limit of sodium correction (Sterns Citation2015, Ochiai and Uenishi Citation2018).
Diagnostic work-up in ED
Over the last years, numerous diagnostic pathways have been developed and successfully implemented. displays a diagnostic pathway which appears reasonable for use in ED. This is adapted from the European guideline (Spasovski et al., Citation2014), which is largely in line with the American Expert Panel Recommendations (Verbalis et al. Citation2013).
Figure 2. Diagnostic work-up of hyponatremic patients in the emergency department.
This figure displays the required diagnostic steps in differential diagnosis of hyponatremia, including laboratory tests in blood and urine samples and assessment of current medication and comorbidities. Abbreviations: SIAD = syndrome of inappropriate antidiuresis. The figure is adapted from: Spasovski et al., Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med 2014; 40: 320–331. (Spasovski et al., Citation2014).
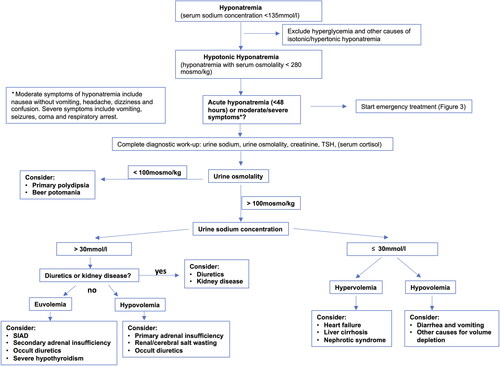
Beside accurate documentation of course of symptoms possibly attributable to hyponatremia, collection of anamnestic data should always include a critical review of current medication (especially diuretics and psychotropic drugs), recent surgeries or therapies (e.g. chemotherapy), as well as history of heart failure, liver cirrhosis, pulmonary diseases, psychiatric disorders or malignancies. If available, recent laboratory results should be checked. In clinical practice, the distinction between acute and chronic hyponatremia is often unclear, particularly for patients presenting to ED. Thus, the European guideline recommends to consider hyponatremia as being chronic, unless there are obvious reasons to assume it is acute (Spasovski et al., Citation2014) (e.g. documented laboratory results of the last 48 hours or onset of symptoms right after consumption of Ecstasy). One should be careful when attributing (moderately) severe symptoms to only slightly decreased plasma sodium levels. Here, clinical course and anamnestic data should be used to evaluate the causal relation between hyponatremia and a certain symptom (Spasovski et al., Citation2014).
Both pseudohyponatremia and hyperglycemia-induced hyponatremia should be first ruled out, because these forms do not require specific therapy. In the ED, pseudohyponatremia can be easily detected by use of point-of-care device to measure sodium concentration, as these are not subject to the laboratory artifact. To estimate the extent to which hyponatremia is caused by hyperglycemia, it is suggested to use an equation adding 2.4 mmol/l to the measured sodium concentration for every 100 mg/dl increase in (serum) glucose concentration above a standard serum glucose concentration of 100 mg/dl (Spasovski et al., Citation2014, Hillier et al. Citation1999). Measurement of serum osmolality is a useful tool to discriminate iso- or hypertonic hyponatremia from the hypotonic form. A measured serum osmolality < 280 mosmo/kg always confirms hypotonic hyponatremia. The osmolality can also be obtained by use of different formulas (mostly based on sodium, urea and glucose). However, a calculated hypoosmolality could be associated with hypotonic, isotonic or hypertonic hyponatremia depending on the used formula and on which osmotically active agents are present (Spasovski et al., Citation2014). Therefore, it is recommendable to be aware of the laboratory techniques used at one’s own clinic and to rather rely on measured serum osmolality. If measurement of serum osmolality is not possible in the acute setting, exclusion of relevant hyperglycemia appears to be a feasible second-line approach to assume hypotonic hyponatremia, as other causes for iso-/hypertonic hyperglycemia are far less common (e.g. mannitol intake).
Assuming hypotonic hyponatremia, the next step should focus on assessment of symptom severity and distinction of acute vs. chronic hyponatremia, as acute manifestation or severe symptoms require emergency treatment. Otherwise, analysis of urine osmolality and sodium in a spot urine sample should be performed in all patients, ideally before treatment initiation. Furthermore, correct interpretation of laboratory measurements requires contemporaneous collection of blood and urine samples.
The urine osmolality reflects AVP activity. A maximally diluted urine (characterized by an urine osmolality ≤ 100 mosmo/kg) in the context of serum hyponatremia usually reflects physiologically suppressed AVP levels. In this case, an excess water intake should be assumed as causal driver of hyponatremia (primary polydipsia, low solute intake, beer potomania). In the setting of hypotonic hyponatremia, an urine osmolality above 100mosmo/kg reflects insufficient AVP suppression/inadequate antidiuresis. Urine sodium concentration now helps to distinguish the stimulus for AVP suppression (inappropriate vs. non-osmotic hemodynamic stimulus). Elevated natriuresis (urine sodium concentration > 30 mmol/l) reflects inappropriate AVP secretion (SIAD). On the contrary, reduced natriuresis (urine sodium concentration ≤ 30 mmol/l) indicates reduced effective arterial blood volume (e.g. volume depletion, heart failure). Importantly, intake of diuretics or impaired kidney function should be taken into account at this step, too. Particularly thiazide-like diuretics induce natriuresis and can even resemble a SIAD-like pattern. Impaired kidney function can contribute to hyponatremia and often diminishes the kidney’s ability to regulate urine osmolality and urine sodium. Finally, after exclusion of diuretics and kidney disease, the clinical assessment of volume status, referring to ECF volume in the context of hyponatremia, is essential regarding differing pathophysiological processes and therapeutic needs, as outlined above. However, it has shown both low sensitivity and specifity for correctly identifiying the different forms of hyponatremia based on ECF volume alone (Chung et al. Citation1987). In contrast, an urine sodium concentration threshold of 30 mmol/l has proven high sensitivity and acceptable specificity in distinguishing hypovolemia from euvolemia or hypervolemia in several studies, even in patients on diuretics (Spasovski et al., Citation2014, Chung et al. Citation1987, Fenske et al. Citation2008). Therefore, interpretation of urine osmolality and urine sodium are prioritized over assessment of volume status in current guideline recommendation (Spasovski et al., Citation2014). In patients on diuretics (especially thiazides, spironolactone, amiloride) with a urine sodium concentration >30mmol/l, diuretics should always be considered a contributing factor to hyponatremia and should be promptly withheld (Spasovski et al., Citation2014).
Importantly, albeit being the most common cause of euvolemic hyponatremia, SIAD is a diagnosis of exclusion and is often misdiagnosed (Greenberg et al. Citation2015). Apart from euvolemia and the suitable urine constellation (urine osmolality >100mosmo/kg, urine sodium >30mmol/l) in the absence of diuretics, there should be no sign of AI, severe hypothyroidism and impaired renal function (see full list of diagnostic criteria in (Spasovski et al., Citation2014)). Therefore, the setting of an ED might not allow to correctly diagnose a SIAD immediately. However, all data assessed in ED will help to classify the type of hyponatremia afterwards.
From a theoretical point of view, the direct measurement of AVP could help in the diagnostic pathway of sodium related disorders, but is limited by complex pre-analytical requirements. In contrast, copeptin can be easily measured with commercially available assays and its levels mirror AVP concentration, being released by the same osmotic and non-osmotic stimuli (Balanescu et al. Citation2011). Copeptin has therefore evolved as a reliable surrogate marker of AVP (Refardt et al. Citation2019). Based on recent findings, copeptin is of high diagnostic value in differential diagnosis of polyuria-polydipsia syndrome (Refardt et al. Citation2019). In detail, high levels of randomly measured copeptin were found to be reliable for diagnosis of nephrogenic diabetes insipidus and hypertonic saline-stimulated copeptin levels differentiate between central diabetes insipidus and primary polydipsia with higher sensitivity and specifity as the indirect water deprivation test (Rosen and Ingelfinger Citation2018). On the contrary, in differential diagnosis of hyponatremia, the broad use of unstimulated copeptin measurement is currently not recommended by the Clinical Practice Guideline due to the observed wide overlap of copeptin levels in patients with all entities of hyponatremia as seen in different observational studies (Spasovski et al., Citation2014, Refardt et al. Citation2019, Fenske et al. Citation2016; Nigro et al. Citation2017; Fenske et al. Citation2009). Of note, in one prospective observational study of patients with profound hyponatremia, very high copeptin levels (>84pmol/l) identified patients with hypovolemic hyponatremia with high specifity of 91%, while very low copeptin levels (<3.9 pmol/l) were associated with primary polydipsia (specifity 90%) (Nigro et al. Citation2017). Mostly, an osmotic stimulation is required to use copeptin levels as a diagnostic marker. Importantly, acute conditions (e.g. heart failure) and stress in general can itself elevate copeptin levels, possibly confounding copeptin levels of patients presenting to the ED (Refardt et al. Citation2019).
Treatment of hyponatremia in ED
provides an overview of therapeutic management of hyponatremic patients presenting to the ED. The severity of neurological manifestation should be the first and most important factor within the therapeutic pathway. Importantly, there must be sufficient confidence in attributing (moderately) severe symptoms to hyponatremia, which is virtually never justified in the context of mild hyponatremia (130–134mmol/l). Generally, the treatment of symptomatic hyponatremia should be sufficient to prevent complications of untreated disease, while avoiding harmful, excessive therapy. Therefore, one has to distinguish therapeutic goal (desirable correction rate) from therapeutic limit (rates associated with harm that should be avoided). Aiming at this prompt, but limited correction of hyponatremia, animal data have shown that an increase in sodium concentration by 4-6 mmol/l is sufficient to prevent brain edema while minimizing the risk of ODS, even in chronic hyponatremia (Sterns Citation2018).
Figure 3. Therapeutic management of hyponatremic patients in the ED.
This figure provides an overview of therapeutic management of hyponatremic patients presenting to the ED, whereby the severity of neurological manifestation should be the first and most important factor to be considered within the pathway. In contrast to patients with (moderately) severe symptoms and/or acute onset of hyponatremia requiring emergency treatment, patients with ‘asymptomatic’ or mild symptoms of chronic hyponatremia may be dismissed home for ambulatory diagnostic work-up, unless they are hospitalized for other reasons.
Abbreviations: ED = emergency department, ICU = intermediate care unit. The figure is adapted from: Spasovski et al., Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med 2014; 40: 320–331. (Spasovski et al., Citation2014).
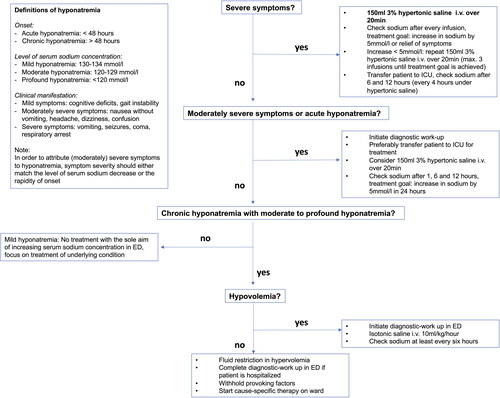
According to the European Guideline, patients presenting with severe symptoms should obtain immediate i.v. treatment with 150 ml hypertonic 3% saline over 20 min, checking the sodium concentration right after. Equal administration can be repeated (max. three times), checking sodium concentration after each infusion, until an increase of 5 mmol/l is achieved or symptoms have improved, whichever occurs first (Spasovski et al., Citation2014) (see ). The acute risk of herniation is prioritized over risk of ODS, regardless of whether hyponatremia is acute or chronic (Spasovski et al., Citation2014). If the symptoms have improved after a 5 mmol/l increase in serum sodium concentration, the hypertonic infusion should be stopped and followed by slow infusion of 0.9% saline until cause-specific treatment is started. If there is no improvement of symptoms despite a sodium increase of 5 mmol/l, a further increase of 1 mmol/l per hour by infusion of 3% hypertonic saline may be attempted while initiating additional diagnostic evaluation, as symptoms might not be related to hyponatremia alone. Infusion should be stopped if the correction limit of 10 mmol/l in the first 24 hours, or a sodium concentration of 130 mmol/l has been reached. However, in the European Guideline, vomiting is considered as a severe symptom, which is seen questionable by some experts due to lack of evidence for a benefit of aggressive treatment (Sterns Citation2018). The American Expert Panel limits the aggressive regimen, regardless of chronicity, to presence of seizures and coma, as well as to moderately/severely symptomatic hyponatremia due to self-induced water intoxication, postoperative hyponatremia or intracranial pathology (Verbalis et al. Citation2013).
In patients with moderately severe symptoms, the European Guideline suggests a less aggressive treatment with a single 3% hypertonic saline infusion over 20 minutes (Spasovski et al., Citation2014). In general, an increase of sodium concentration by 5 mmol/l within the first 24 hours should be aimed, while diagnostic steps and cause-specific treatment should be started at the same time.
In asymptomatic or mildly symptomatic patients with documented acute hyponatremia exceeding a decrease in serum sodium concentration >10mmol/l, the European Guideline suggests a single i.v. infusion of 150 ml 3% hypertonic saline over 20 minutes.
Interestingly, both rapid intermittent bolus therapy and continuous infusion therapy have recently been reported to be safe and equally effective (Baek et al. Citation2021).
Irrespective of symptom severity and initial therapeutic regimen, the increase in sodium concentration should never exceed a total of 10 mmol/l during the first 24 hours and 8 mmol/l every 24h thereafter until the serum sodium reaches 130 mmol/l (therapeutic limit) (Spasovski et al., Citation2014). Therefore, sodium concentration should be closely monitored, whereas the recommended frequency depends on symptom severity and course of treatment. In patients with severe symptoms and achieved symptom relief after an increase of 5 mmol/l, it should be checked after 6 and 12 hours. If hypertonic saline needs to be continued, it should be checked every 4 hours. In patients with moderately severe symptoms, it should be checked after 1, 6 and 12 hours. After the first 12 hours, daily controls should be performed to ensure stability of sodium level. Patients with severe hyponatremia and/or severe symptoms should be admitted to an intermediate care unit to guarantee close monitoring of changes in sodium concentration.
Iatrogenic sodium overcorrection should be treated as a medical emergency. In most cases, overcorrection results from the unexpected excessive water diuresis after resolution of the cause for impaired water excretion (e.g. normalization of effective arterial blood volume by i.v. hydratation) (Sterns and Hix Citation2009). If the therapeutic limit has been exceeded (>10 mmol/l within first 24 hours), administration of 5% dextrose in water in individual doses of 10 ml per kilogram body weight given over one hour, checking sodium concentration afterwards, can be used to return the sodium concentration below the therapeutic limits (Spasovski et al., Citation2014). If a patient is undergoing sudden water diuresis (>100ml/h), serum sodium concentration and urine output should be checked every two hours and administration of a single dose of 2 μg desmopressin could be an effective method to counteract inadvertent overcorrection (Spasovski et al., Citation2014, Perianayagam et al. Citation2008).
In patients with chronic hyponatremia without severe/moderately severe symptoms and moderate to profound hyponatremia, a cause-specific treatment should be aimed for (e.g. fluid restriction in SIAD and hypervolemic hyponatremia), while avoiding contributing factors (e.g. thiazide diuretics). Checks of sodium concentration should be performed every 6 hours holding on to the general therapeutic limits of sodium increase mentioned above. If chronic hyponatremia is only mild, initiation of treatment with the sole aim to increase serum sodium concentration is not recommended. This also accounts for moderate hyponatremia in patients with hypervolemic hyponatremia. The latter recommendations are based on the persistent lack of evidence for long-term improvement of patient outcomes through correction of hyponatremia alone (despite the reported long-term risk associated with chronic mild hyponatremia), which is outweighed by the risk of adverse treatment effects in such patients (Spasovski et al., Citation2014). As nausea is a potential stimulus for eutopic AVP release, it is sometimes challenging to determine whether nausea and vomiting are the cause or the consequence of SIAD. Hyponatremia-induced nausea can even perpetuate inappropriate ADH release in SIAD, which was initially triggered by a different disease. Therefore, consequent antiemetic treatment is crucial in hyponatremia treatment (Robertson Citation2006). If there is any suspicion of AI, serum cortisol should be checked (preferably at 8am) and clinicians should administer i.v. hydrocortisone (e.g. a bolus of 50 mg, followed by continuous infusion of 100 mg/24 hours), even if AI can be only excluded later after receiving the appropriate laboratory values (Case 2). If patients present with clear signs of hypovolemia, i.v. hydration with isotonic crystalloid should be started under frequent sodium controls (Case 1). An isotonic saline dose of 23 - 30 ml/kg/24h has shown to be safe and effective in hypovolemic hyponatremia (Ruiz-Sánchez et al. Citation2020). The European Guideline recommends i.v. infusion of 0.9% saline or a balanced crystalloid solution at 0.5-1ml/kg/h (Spasovski et al., Citation2014). Provoking factors should be withheld or replaced by a different drug if possible (e.g. thiazide-like diuretics) (Case 4). If diuretic treatment is necessary, loop diuretics may be administered as they rarely cause hyponatremia.
If neurological symptoms are mild or absent, the clinician in ED should focus on adequate initial diagnostic work-up to allow a cause-specific therapeutic regimen during hospitalization. Asymptomatic patients with mild hyponatremia may be dismissed from ED with recommendation for ambulatory work-up.
Of note, the selective oral vasopressin V2-receptor antagonist Tolvaptan is an approved and effective drug for treatment of moderate to profound hyponatremia in SIAD (., 2006), although the European Guideline recommends against its use due to reported higher rate of overly rapid sodium correction and holds on to oral fluid restriction as first-line therapy (Spasovski et al., Citation2014).
Ethical approval
This study, including the use of anonymized case reports, has been approved by the Institutional Review Board of the Charité-Universitätsmedizin Berlin (approval number EA4/034/24). Contact details of patients were not taken at the emergency department, therefore written informed consent could not be obtained. Every reasonable effort has been made to ensure anonymity of the patients discussed in the article.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Adrogué HJ, Tucker BM, Madias NE. 2022. Diagnosis and management of hyponatremia: a review. Jama. 328(3):280–291. doi: 10.1001/jama.2022.11176
- Anderson RJ, Chung HM, Kluge R, Schrier RW. 1985. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med. 102(2):164–168. doi: 10.7326/0003-4819-102-2-164
- Arampatzis S, Frauchiger B, Fiedler G-M, Leichtle AB, Buhl D, Schwarz C, Funk G-C, Zimmermann H, Exadaktylos AK, Lindner G, et al. 2012. Characteristics, symptoms, and outcome of severe dysnatremias present on hospital admission. Am J Med. 125(11):1125 e1121–1125 e1127. doi: 10.1016/j.amjmed.2012.04.041
- Arampatzis S, Funk G-C, Leichtle AB, Fiedler G-M, Schwarz C, Zimmermann H, Exadaktylos AK, Lindner G. 2013. Impact of diuretic therapy-associated electrolyte disorders present on admission to the emergency department: a cross-sectional analysis. BMC Med. 11:83. doi: 10.1186/1741-7015-11-83
- Baek SH, Jo YH, Ahn S, Medina-Liabres K, Oh YK, Lee JB, Kim S. 2021. Risk of overcorrection in rapid intermittent bolus vs slow continuous infusion therapies of hypertonic saline for patients with symptomatic hyponatremia: the SALSA randomized clinical trial. JAMA Intern Med. 181(1):81–92. doi: 10.1001/jamainternmed.2020.5519
- Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J. 2011. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar States. J Clin Endocrinol Metab. 96(4):1046–1052. doi: 10.1210/jc.2010-2499
- Balling L, Schou M, Videbaek L, Hildebrandt P, Wiggers H, Gustafsson F, et al. 2011. Prevalence and prognostic significance of hyponatraemia in outpatients with chronic heart failure. Eur J Heart Fail. 13:968–973.
- Berendes E, Walter M, Cullen P, Prien T, Van Aken H, Horsthemke J, Schulte M, von Wild K, Scherer R. 1997. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 349(9047):245–249.
- Bettari L, Fiuzat M, Shaw LK, Wojdyla DM, Metra M, Felker GM, et al. 2012. Hyponatremia and long-term outcomes in chronic heart failure–an observational study from the Duke Databank for Cardiovascular Diseases. J Card Fail. 18:74–81.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. 1987. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 83(5):905–908. doi: 10.1016/0002-9343(87)90649-8
- Corona G, Giuliani C, Parenti G, Norello D, Verbalis JG, Forti G, Maggi M, Peri A. 2013. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PLoS One. 8(12):e80451. doi: 10.1371/journal.pone.0080451
- Edelman IS, Leibman J, O’Meara MP, Birkenfeld LW. 1958. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 37(9):1236–1256. doi: 10.1172/JCI103712
- Fenske W, Maier SKG, Blechschmidt A, Allolio B, Störk S. 2010. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 123(7):652–657. doi: 10.1016/j.amjmed.2010.01.013
- Fenske W, Sandner B, Christ-Crain M. 2016. A copeptin-based classification of the osmoregulatory defects in the syndrome of inappropriate antidiuresis. Best Pract Res Clin Endocrinol Metab. 30(2):219–233. doi: 10.1016/j.beem.2016.02.013
- Fenske W, Störk S, Blechschmidt A, Maier SGK, Morgenthaler NG, Allolio B. 2009. Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab. 94(1):123–129. doi: 10.1210/jc.2008-1426
- Fenske W, Störk S, Koschker A-C, Blechschmidt A, Lorenz D, Wortmann S, Allolio B. 2008. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 93(8):2991–2997. doi: 10.1210/jc.2008-0330
- Giordano M, Ciarambino T, Castellino P, Malatino L, Di Somma S, Biolo G, et al. 2016. Diseases associated with electrolyte imbalance in the ED: age-related differences. Am J Emerg Med. 34:1923–1926.
- Greenberg A, Verbalis JG, Amin AN, Burst VR, Chiodo JA, 3rd, Chiong JR, et al. 2015. Current treatment practice and outcomes. Report of the hyponatremia registry. Kidney Int. 88:167–177.
- Hillier TA, Abbott RD, Barrett EJ. 1999. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 106(4):399–403. doi: 10.1016/s0002-9343(99)00055-8
- Hoorn EJ, Lindemans J, Zietse R. 2006. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 21(1):70–76. doi: 10.1093/ndt/gfi082
- Huda MSB, Boyd A, Skagen K, Wile D, van Heyningen C, Watson I, Wong S, Gill G. 2006. Investigation and management of severe hyponatraemia in a hospital setting. Postgrad Med J. 82(965):216–219. doi: 10.1136/pmj.2005.036947
- Imai N, Shibagaki Y. 2019. The prevalence of dysnatremia in the elderly patients without CKD. Am J Emerg Med. 37(3):499–501. doi: 10.1016/j.ajem.2018.12.004
- Jenq C-C, Tsai M-H, Tian Y-C, Chang M-Y, Lin C-Y, Lien J-M, Chen Y-C, Fang J-T, Chen P-C, Yang C-W, et al. 2010. Serum sodium predicts prognosis in critically ill cirrhotic patients. J Clin Gastroenterol. 44(3):220–226. doi: 10.1097/MCG.0b013e3181aabbcd
- Lee CT, Hr G, Chen JB. 2000. Hyponatremia in the emergency department. Am J Emerg Med. 18:264–268.
- Liamis G, Filippatos TD, Liontos A, Elisaf MS. 2017. Management of endocrine disease: hypothyroidism-associated hyponatremia: mechanisms, implications and treatment. Eur J Endocrinol. 176(1):R15–R20. doi: 10.1530/EJE-16-0493
- Liamis G, Milionis H, Elisaf M. 2008. A review of drug-induced hyponatremia. Am J Kidney Dis. 52(1):144–153. doi: 10.1053/j.ajkd.2008.03.004
- Lindner G, Pfortmüller CA, Leichtle AB, Fiedler GM, Exadaktylos AK. 2014. Age-related variety in electrolyte levels and prevalence of dysnatremias and dyskalemias in patients presenting to the emergency department. Gerontology. 60(5):420–423. doi: 10.1159/000360134
- Louis G, Megarbane B, Lavoué S, Lassalle V, Argaud L, Poussel J-F, Georges H, Bollaert PE. 2012. Long-term outcome of patients hospitalized in intensive care units with central or extrapontine myelinolysis. Crit Care Med. 40(3):970–972. doi: 10.1097/CCM.0b013e318236f152
- Nguyen MK, Kurtz I. 2004. Role of potassium in hypokalemia-induced hyponatremia: lessons learned from the Edelman equation. Clin Exp Nephrol. 8(2):98–102. doi: 10.1007/s10157-004-0281-3
- Nigro N, Winzeler B, Suter-Widmer I, Schuetz P, Arici B, Bally M, Blum CA, Nickel CH, Bingisser R, Bock A, et al. 2017. Evaluation of copeptin and commonly used laboratory parameters for the differential diagnosis of profound hyponatraemia in hospitalized patients: ‘The Co-MED Study. Clin Endocrinol (Oxf)). 86(3):456–462. doi: 10.1111/cen.13243
- Ochiai H, Uenishi E. 2018. Early relowering of serum sodium concentration overcomes disturbances in consciousness during hyponatremia overcorrection and prevents osmotic demyelination syndrome. Intern Med. 57(16):2353–2357. doi: 10.2169/internalmedicine.0299-17
- Oh JY, Shin JI. 2014. Syndrome of inappropriate antidiuretic hormone secretion and cerebral/renal salt wasting syndrome: similarities and differences. Front Pediatr. 2:146.
- Olsson K, Öhlin B, Melander O. 2013. Epidemiology and characteristics of hyponatremia in the emergency department. Eur J Intern Med. 24(2):110–116. doi: 10.1016/j.ejim.2012.10.014
- Perianayagam A, Sterns RH, Silver SM, Grieff M, Mayo R, Hix J, Kouides R. 2008. DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol. 3(2):331–336. doi: 10.2215/CJN.03190807
- Peters JP, Welt LG, Sims EA, Orloff J, Needham J. 1950. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Phys. 63:57–64.
- Refardt J, Winzeler B, Christ-Crain M. 2019. Copeptin and its role in the diagnosis of diabetes insipidus and the syndrome of inappropriate antidiuresis. Clin Endocrinol (Oxf). 91:22–32.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. 2006. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 119(1):71 e71–71.e8. doi: 10.1016/j.amjmed.2005.09.026
- Robertson GL. 2006. Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am J Med. 119(7 Suppl 1):S36–S42. doi: 10.1016/j.amjmed.2006.05.006
- Rosen CJ, Ingelfinger JR. 2018. A reliable diagnostic test for hypotonic polyuria. N Engl J Med. 379(5):483–484. doi: 10.1056/NEJMe1808195
- Ruiz-Sánchez JG, Meneses D, Álvarez-Escolá C, Cuesta M, Calle-Pascual AL, Runkle I. 2020. The effect of the dose of isotonic saline on the correction of serum sodium in the treatment of hypovolemic hyponatremia. J Clin Med. 9(11):9. doi: 10.3390/jcm9113567
- Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. 2006. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 355(20):2099–2112. doi: 10.1056/NEJMoa065181
- Schwartz WB, Bennett W, Curelop S, Bartter FC. 1957. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med. 23(4):529–542. doi: 10.1016/0002-9343(57)90224-3
- Singh TD, Fugate JE, Rabinstein AA. 2014. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol. 21(12):1443–1450. doi: 10.1111/ene.12571
- Soiza RL, Cumming K, Clarke JM, Wood KM, Myint PK. 2014. Hyponatremia: special considerations in older patients. J Clin Med. 3:944–958.
- Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, et al. 2014. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 170(3):G1–47. doi: 10.1530/EJE-13-1020
- Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, et al. 2014. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med. 40(3):320–331. doi: 10.1007/s00134-014-3210-2
- Sterns RH, Hix JK. 2009. Overcorrection of hyponatremia is a medical emergency. Kidney Int. 76(6):587–589. doi: 10.1038/ki.2009.251
- Sterns RH. 2015. Disorders of plasma sodium–causes, consequences, and correction. N Engl J Med. 372(1):55–65. doi: 10.1056/NEJMra1404489
- Sterns RH. 2018. Treatment of severe hyponatremia. Clin J Am Soc Nephrol. 13(4):641–649. doi: 10.2215/CJN.10440917
- Szatalowicz VL, Miller PD, Lacher JW, Gordon JA, Schrier RW. 1982. Comparative effect of diuretics on renal water excretion in hyponatraemic oedematous disorders. Clin Sci (Lond)). 62(2):235–238. doi: 10.1042/cs0620235
- Tzoulis P, Evans R, Falinska A, Barnard M, Tan T, Woolman E, Leyland R, Martin N, Edwards R, Scott R, et al. 2014. Multicentre study of investigation and management of inpatient hyponatraemia in the UK. Postgrad Med J. 90(1070):694–698. doi: 10.1136/postgradmedj-2014-132885
- Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ. 2013. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 126(10 Suppl 1):S1–S42. doi: 10.1016/j.amjmed.2013.07.006
- Waikar SS, Mount DB, Curhan GC. 2009. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 122(9):857–865. doi: 10.1016/j.amjmed.2009.01.027
- Woitok BK, Funk GC, Walter P, Schwarz C, Ravioli S, Lindner G. 2020. Dysnatremias in emergency patients with acute kidney injury: a cross-sectional analysis. Am J Emerg Med. 38(12):2602–2606. doi: 10.1016/j.ajem.2020.01.009
