ABSTRACT
There is increasing evidence that inter-individual interaction among conspecifics can cause population-level lateralization. Male–female and mother–infant dyads of several non-human species show lateralised position preferences, but such preferences have rarely been examined in humans. We observed 430 male–female human pairs and found a significant bias for males to walk on the right side of the pair. A survey measured side preferences in 93 left-handed and 92 right-handed women, and 96 left-handed and 99 right-handed men. When walking, and when sitting on a bench, males showed a significant side preference determined by their handedness, with left-handed men preferring to be on their partner’s left side and right-handed men preferring to be on their partner’s right side. Women did not show significant side preferences. When men are with their partner they show a preference for the side that facilitates the use of their dominant hand. We discuss possible reasons for the side preference, including males prefering to occupy the optimal “fight ready” side, and the influence of sex and handedness on the strength and direction of emotion lateralization.
Introduction
Lateralization of brain and behaviour is a fundamental property of humans and other species (Güntürkün, Ströckens, & Ocklenburg, Citation2020; Rogers, Citation2017). As humans are social in nature, often spending long periods of time interacting with other people, lateralized behaviours can also be expected to emerge in a variety of social interactions. This has been found to be the case in many non-human species, suggesting that lateralization may have the important adaptive function of helping co-ordinate and synchronize behaviours among conspecifics (Rogers, Rigosi, Frasnelli, & Vallortigara, Citation2013; Zaynagutdinova, Karenina, & Giljov, Citation2021; Vallortigara & Rogers, Citation2020). As proposed by Rogers (Citation1989, Citation2021), it is possible that individual lateralization developed first, because of the cognitive advantages that it conveys (Vallortigara & Rogers, Citation2005, Citation2020), and then, as sociality evolved in a species, the requirement to interact effectively led to patterns of population-level lateralization. Therefore, understanding the lateralization of social behaviours and inter-individual interactions may be crucial to understanding the origin and function of lateralization (Bisazza, Cantalupo, Capocchiano, & Vallortigara, Citation2000; Frasnelli, Iakovlev, & Reznikova, Citation2012; Frasnelli & Vallortigara, Citation2018; Rogers, Citation2021; Schnell, Jozet-Alves, Hall, Radday, & Hanlon, Citation2019).
In humans, the lateralization of motor behaviour during social interactions has been examined most extensively for types of social touch, including kissing, cradling and embracing (Barrett, Greenwood, & McCullagh, Citation2006; Güntürkün, Citation2003; Harris, Cárdenas, Spradlin Jr, & Almerigi, Citation2010; Manning, Heaton, & Chamberlain, Citation1994; Ocklenburg et al., Citation2018; Packheiser et al., Citation2021; Turnbull, Stein, & Lucas, Citation1995). Each of these behaviours typically involves an emotional connection between individuals, and their lateralization has been found to be influenced by handedness and by the emotional context (Ocklenburg et al., Citation2018; Packheiser, Rook, et al., Citation2019). The aim of this research was to examine the lateralization of another type of social behaviour between humans, namely the side that members of a male–female pair, or couple, prefer to occupy when together. Female–male and mother–infant pairs of many non-human species show lateralized side preferences (Karenina, Giljov, Ingram, Rowntree, & Malashichev, Citation2017; Zaynagutdinova et al., Citation2021) but they have rarely been explored in human pairs. Borden and Homleid (Citation1978) found side preferences in heterosexual couples were influenced by the handedness of males, particularly when touching, though this may have been due to many of the observed couples leaving a building, with the woman often emerging first.
One explanation for why side preferences are exhibited by dyads of different species derives from hemispheric asymmetries in emotional processing. Many species have a preference to keep social partners in their left visual field (Bisazza et al., Citation2000; Giljov, Karenina, & Malashichev, Citation2018; Hauser & Akre, Citation2001; Rogers, Citation2021; Salva, Daisley, Regolin, & Vallortigara, Citation2009; Quaresmini, Forrester, Spiezio, & Vallortigara, Citation2014; Vallortigara & Andrew, Citation1994). This applies to male–female pairs (Zaynagutdinova et al., Citation2021) and mother–infant interactions in a wide range of mammalian species, including sheep, red kangaroo, orca, muskox, horse, and reindeer (Karenina et al., Citation2017). This left-looking bias is believed to originate from the right hemisphere’s greater specialization in recognizing and interpreting emotions (Gainotti, Citation2019), thereby facilitating social interactions and perhaps monitoring the intentions of conspecifics (Quaresmini et al., Citation2014; Rogers, Citation2021). Research with humans has also provided strong evidence for the right hemisphere’s greater role in emotion processing (Borod, Zgaljardic, Tabert, & Koff, Citation2001; Gainotti, Citation2019), including vocal emotion perception (Grimshaw, Citation1998; Grimshaw, Séguin, & Godfrey, Citation2009; Rodway & Schepman, Citation2007) and facial emotion perception (Ley & Bryden, Citation1979; Borod et al., Citation2001; but see Prete, Laeng, Fabri, Foschi, & Tommasi, Citation2015 for evidence of valence-specific asymmetries).
The greater role of the RH in emotional processing may also account for why socially driven emotions affect the lateralization of motor behaviours in humans and non-human species (see Boulinguez-Ambroise, Aychet, & Pouydebat, Citation2022). Gorillas and Chimpanzees (Forrester, Leavens, Quaresmini, & Vallortigara, Citation2011; Forrester, Quaresmini, Leavens, Spiezio, & Vallortigara, Citation2012) show different hand preferences for unimanual actions to inanimate and animate objects (e.g., self, conspecifics). It is suggested that the handedness preference reflects hemispheric functional specialization, with the left-hand (RH) more suited to the manipulation of emotive (animate) objects (Forrester et al., Citation2012). In humans, social emotions also influence the expression of lateralized motor behaviours. Side preferences for kissing are affected by whether the kiss is romantic or parental (Sedgewick & Elias, Citation2016), and for embracing by whether the embrace is emotional or neutral (Packheiser, Rook, et al., Citation2019). The left-side maternal cradling bias present in humans (Packheiser, Schmitz, Berretz, Papadatou-Pastou, & Ocklenburg, Citation2019) and other species (Boulinguez-Ambroise, Pouydebat, Disarbois, & Meguerditchian, Citation2020; Giljov et al., Citation2018; Manning & Chamberlain, Citation1990), which results in the infant in the mother’s left visual field, is also believed to be due to the RH’s greater specialization for emotion processing.
A body of evidence therefore suggests that lateralized side preferences can be influenced by the social emotional context, with preferences favouring greater involvement of the right hemisphere. Consequently, when members of a couple are with their partner, they may prefer to have their partner to their left, so that verbally and visually communicated information preferentially goes to the right hemisphere, as this arrangement may facilitate social interaction. It is possible, however, that this may be less pronounced in left-handers than right-handers because motor behaviours (McManus, Van Horn, & Bryden, Citation2016; Rodway, Thoma, & Schepman, Citation2022) and hemispheric functional asymmetries (Johnstone, Karlsson, & Carey, Citation2021; Knecht et al., Citation2000) are less consistently lateralized in left-handers. Moreover, while some research suggests that emotion perception is lateralized similarly in left and right-handers (Rodway, Wright, & Hardie, Citation2003; Van Strien & Van Beek, Citation2000), or that left-handers show RH specialization but less strongly than right-handers (Elias, Bryden, & Bulman-Fleming, Citation1998; Badzakova-Trajkov, Häberling, Roberts, & Corballis, Citation2010), other studies have found a reversed pattern (Willems, Peelen, & Hagoort, Citation2010; see also Schrammen, Grimshaw, Berlijn, Ocklenburg, & Peterburs, Citation2020). This indicates that left-handers might show a weaker preference to be on their partner’s right side compared to right-handers.
A further reason why members of mixed-sex couples could have side preferences is because of the possibility of agonistic inter-individual encounters, which require an individual to be in an effective fighting (or defending) position. For example, a right-handed individual might prefer to be on their partner’s right, and a left-handed individual on their partner’s left (from their perspective), so that their dominant hand is free to fight a potential opponent. Considerable evidence indicates that the sexual selection of human males has been strongly influenced by intra-sexual competition, with the selection of male traits that favour fighting ability, such as upper body strength (Kordsmeyer, Hunt, Puts, Ostner, & Penke, Citation2018; Puts, Citation2010; Richardson & Gilman, Citation2019). It is relevant that gelada baboons have a left visual field preference for agonistic interactions (Casperd & Dunbar, Citation1996), and inter-individual position biases appear important for displays of aggression in other species (Frasnelli & Vallortigara, Citation2018; Lemaire, Viblanc, & Jozet-Alves, Citation2019). Moreover, several species show left-right asymmetries in predatory behaviour, including toads (Robins & Rogers, Citation2004), lizards (Hews, Castellano, & Hara, Citation2004), and whales (Canning et al., Citation2011). Conceivably, therefore, side preferences in mixed-sex pairs of humans could arise from the potential for agonistic inter-individual interactions.
The ability to fight effectively is clearly adaptive (Puts, Citation2010) and it has been hypothesized that the persistence of a stable minority of left-handers in human populations is due to a fighting advantage of left-handed males (Raymond, Pontier, Dufour, & Møller, Citation1996). The fighting hypothesis suggests that the surprise advantage that left-handedness conveys to males, when fighting in a population of predominantly right-handed males, increases their chances of surviving and reproducing (Groothuis, McManus, Schaafsma, & Geuze, Citation2013). This form of frequency-dependent selection requires a low frequency of left-handed males in a population for the advantage to be present, because the surprise advantage would be eliminated if left-handedness became relatively common. It is thought that this is genetically supported by a polymorphism (Billiard, Faurie, & Raymond, Citation2005) promoting survival and/or reproductive success via the father’s fighting advantage.
Evidence in favour of the fighting hypothesis is the finding that left-handed males are over-represented in fighting sports (Grouios, Tsorbatzouidis, Alexandris, & Barkoukis, Citation2000, Citation2002; Pollet, Stulp, & Groothuis, Citation2013; Raymond et al., Citation1996), compared to indirect contact sports (Groothuis et al., Citation2013), and left-handed fighters have greater fighting success (Richardson & Gilman, Citation2019). Other evidence for the theory is somewhat mixed (Groothuis et al., Citation2013; Vallortigara & Rogers, Citation2020), such as a suggested link between left-handedness and health problems (Porac, Citation2015; Groothuis et al., Citation2013). In addition, the proposed relationship between the frequency of homicide and left-handedness in non-industrial societies has not received strong support, partly because of alternative interpretations of the data and difficulties in obtaining accurate measures of left-handedness in non-industrial societies (Groothuis et al., Citation2013). Moreover, an examination of the fighting hypothesis in male fiddler crabs (genus Uca) found no evidence for a frequency-dependent fighting advantage in left-clawed crabs (Backwell et al., Citation2007). Despite this, the fighting hypothesis remains influential, and suggests that left-handedness is a sexually selected trait in males (Billiard et al., Citation2005; Richardson & Gilman, Citation2019), and is compatible with evidence that sexual selection of human males has been strongly influenced by intra-sexual competition, such as fighting (Kordsmeyer et al., Citation2018; Richardson & Gilman, Citation2019).
If hand dominance for fighting is as important as the fighting hypothesis suggests, then it could influence the side that left and right-handed men prefer to occupy when with their partner because it could affect a man’s ability to fight, protect a female partner, and their likelihood of reproducing. The fighting hypothesis proposes that in human societies it is men that engage in fighting (see also Puts, Citation2010; Sell et al., Citation2009). Therefore the desire to occupy the optimal “fight ready” side should apply to men rather than women. If men prefer a particular side, so that they are better prepared to fight, right-handed men should prefer to be on the right side of their partner, whereas left-handed men should prefer to be on the left of their partner, because these positions would better enable the use of their dominant hand for fighting.
A further possible cause of side preferences is hand dominance, independently of any thoughts of threats from other humans. A person’s desire to have their dominant hand free to be used in different ways, rather than being restricted by the potentially close presence of a partner, may cause people to want to have their dominant hand on the outside of the couple. In this case, right-handed individuals can be expected to prefer to be on the right side and left-handed individuals on the left side. This would be the same for both men and women.
To examine whether side preferences were more compatible with an emotional communication, fighting readiness, or hand dominance explanation, we conducted an observational study and a survey. In the observational study we observed male–female pairs walking together and noted the side occupied, from their perspective, of the male–female members of a pair, with pairs defined as two people walking together, without necessarily being a couple. The survey measured side preferences in different situations, in right-handed and left-handed men and women who were members of a couple (defined as someone who was in a romantic heterosexual relationship). The two methods had complementary strengths. The observational data allowed us to observe actual behaviour that took place when a man and woman walked together. The self-report survey data indicated individual preferences from people who were in a romantic relationship, and whose handedness was known based on self-report and as checked via the Edinburgh Handedness Inventory (Oldfield, Citation1971). The survey also allowed us to ask about preferences in different situations (walking, sitting on a bench, and lying in bed, all with their partner).
The inclusion of the preferred position in bed served as a useful check on the accuracy of the reported preferences, because we expected that people would have a strong preference to be on the left or right, and very few would have no preference. Additionally, because people typically spend long periods of time in bed sleeping together, it enabled the examination of whether this habitual arrangement influenced side preferences in other circumstances. That is, would the side habitually occupied by members of a couple when in bed, a spatial arrangement that is longer lasting than most other situations, determine side preferences when together in other circumstances, such as walking and sitting? Finally, it also allowed us to examine preferences for a situation where the man’s preferred position was, arguably, less likely to be influenced by his preparedness to fight with his dominant hand. This is because lying down in a bed would be an unlikely location to fight another man and the bed would usually be in a location of shelter.
For the observational data to be more consistent with the fighting readiness hypothesis we would expect a significantly higher proportion of men than women to walk on the right from the pair’s perspective. This is based on the fact that there are more right-handed than left-handed men, and on the evidence-based assumption that the fighting drive resides in men, rather than in women (Billiard et al., Citation2005). The RH emotional communication hypothesis and the hand-dominance hypothesis both predicted that the majority of people would have a preference to walk on the right. Because it is not spatially possible in pairwise walking arrangements for both members of the pair to fulfil a hypothesized drive to be on the right, these two hypotheses may lead to a situation in which there is a balance in walking sides across the sexes, due to the walking sides being determined by factors other than sex.
For the survey data to be consistent with the fighting readiness hypothesis, we would expect to find a difference in side preference for men only, with left-handed men preferring to be on the left side more often than right-handed men, and right-handed men preferring to be on the right side more often than left-handed men, but only in the public locations (walking, sitting on a bench). For the survey data to be consistent with the right-hemisphere hypothesis, individuals should show a preference to be on the right in all situations, regardless of sex. As some evidence suggests that left-handers are less strongly right-hemisphere lateralized for emotional processing it was possible that the preference to be on the right would be weaker in left-handed men and women. Finally, for data to be consistent with the dominant hand hypothesis, individuals should show a preference to occupy the side that has their dominant hand on the outside of the pair, regardless of sex.
Study 1: Observation
This was a naturalistic observational study of male–female pairs of adults walking along a riverside walkway popular with recreational visitors.
Method
Ethical arrangements
The research was approved by the School of Psychology Ethics Committee at the University of Chester and complied with the British Psychological Society Code of Ethics. Because the data were based on anonymous observations in a public place where people could expect to be observed, and because the observations made were not intrusive or sensitive, participants were not required to give informed consent.
The observed, sample size calculation, and impact on inferencing
We observed 430 pairs of people in which one member presented as a man and the other as a woman. The sample size was determined via a sample size analysis for one-sample proportions, conducted on SPSS 27, based on an initial subsample of 164 observations, with desired power set at .8 (and in fact, using the final data, observed power was calculated as .83).
This type of power calculation using interim data to estimate how many observations are needed to gain sufficient power for a stable effect is named “Peeking for Power” and is used in medical research outside clinical trials, in real-world environments, where effect sizes and samples sizes may be difficult to estimate until trials are underway (Wasser, Citation2021). Planned “peeks for power” do not require “alpha spending” (adjusting alpha to accommodate the increased risk of a type I error, or false positive). However, in our study, a full peek for power protocol was not planned a priori, so an alpha adjustment was made to protect the final result against the risk of a type I error. This was done using the Bonferroni correction. This has been classed as common by Wasser (Citation2021), and as valid yet conservative by Lakens, Pahlke, and Wassmer (Citation2021). Thus, in light of one “peek”, the final alpha was set at .05/2 = .025. To provide additional inferencing in a different tradition we also report Bayesian Statistics (see e.g., Dienes, Citation2011). This, in part, helped quantify the strength of the evidence.
Procedure
The observed pairs were walking on a largely pedestrianized road with only occasional local vehicular traffic called “The Groves” along the River Dee in Chester, UK. Participants walked in either direction, in a target area delimited by Snugbury’s ice cream parlour (coordinates: 53.188288, −2.884253) and the Grosvenor Rowing Club (coordinates: 53.188707105699905, −2.882302682399487). We judged their status as pairs, as opposed to random co-walkers or members of larger groups, based on the distance from other walkers. We only included participants if they had shown a stable period of walking together on the same side of each other. Pairs were included if both members were judged without doubt to be aged 18 or over, and excluded if they appeared under 18. Care was taken to avoid including the same pair more than once, e.g., if they traversed the target area more than once.
Because a number of factors could have impacted on their preferred walking side, pairs were not included in the sample if one or more of the following exclusion criteria applied: (1) if the pair walked with other people (adults/children); (2) if the pair walked with one or more dogs; (3) if one or both members of the pair pushed a pram, bike, or walking frame; (4) if one or both members of the pair walked with crutches or with a walking stick; (5) if one or both members of the pair carried or pulled one or more large items of baggage.
The observers were both authors, who sat on one of the many benches available in the target area with their back to the walking area, looking to the left and right, and partly over their shoulders. The observations were made unobtrusively, and the walkers were not aware that they were being observed in an observational study. Following a brief trial of individual and collaborative observations, the authors settled on full collaborative observation to optimize accuracy. This meant that the observers collaboratively decided on inclusion decisions and apparent sex. They then each recorded their observations in a small notebook with a black pen. They recorded the apparent sex of the member of the pair walking on the left from the observers’ perspective while the pair’s faces were in the observers’ view. Observations took place in the afternoons of four separate days with pleasant summer weather in July and August 2021, in full daylight. The total time spent observing was 259 min (4 h:19 m), with an average rate of observing target pairs of 1.66 pairs per minute.
Upon recoding for statistical analysis, male walkers who walked on the left from the observers’ perspective were coded as 1, and female walkers as 0. Data were entered in the order in which they were observed. A Wald–Wolfowitz runs test was performed to check whether there were any signs of non-randomness in the order of the observations, and this test found no such non-random patterning in the data, test value = 0.5, cases < test value = 187, cases > test value = 243, total number of cases = 430, number of runs = 209, Z = 0.33, p = .74. Thus, the ordering of the data was random, confirming the important assumption of non-patterning, independent observations.
Results and discussion
Of the 430 participants, 243 (57%) men and 187 (43%) women were observed walking on the right from the pair’s perspective, which was significant, p = .008 (less than the Bonferroni-corrected alpha of .025). The effect size was Cohen’s h = .13.
For readers who may not feel satisfied with Bonferroni-corrected frequentist statistics, we also supply Bayesian statistics (Dienes, Citation2011). A Bayesian binomial test was conducted (JASP team, Citation2022) to establish whether the number of men walking on the right was robustly above the chance proportion of .5. The 95% Credible Interval around the median of .57 was [.52, .61], BF+0 = 4.62. The credible interval was fully above .5, and the Bayes Factor showed that the data were 4.62 times more probable under the alternative hypothesis (H1) than the null hypothesis (H0), which constituted moderate evidence (3 < BF10 < 10) for H1 over H0. Thus, the Bayesian statistics showed a very similar outcome to that of the frequentist Null Hypothesis Significance Testing outcome, suggesting good calibration.
The results of this observation study did not include information about handedness, and did not include preferences that individuals expressed separately. For those reasons, survey data were collected to allow us to evaluate the theoretical positions against richer data.
Study 2: Survey
Method
An online survey was conducted on Qualtrics.
Ethical arrangements and participants
Ethical approval for the study was given by the School of Psychology Ethics Committee at the University of Chester, UK, in turn complying with Ethical Guidelines of the British Psychological Society. Participants gave their informed consent electronically.
A sample of 400 participants took part (100 left-handed women, 100 right-handed women, 100 right-handed men, and 100 left-handed men). They were recruited online via Prolific.co, and invited to take part only if they were UK residents over the age of 18, had self-reported their handedness and sex in the Prolific demographic pre-screening data, had indicated that their sexual orientation was heterosexual, and had confirmed that they were in a relationship, engaged, or married. Participants who matched these criteria were invited to take part via a criteria-selective recruitment message on Prolific, and were then free to opt in.
At data processing, data from 20 participants were removed because their handedness/gender/relationship status did not match the information provided via Prolific pre-screening. This raised questions about the reliability of their data, and out of caution, we removed them. We hereby lost 7 left-handed women, 8 right-handed women (non-matching handedness), 4 left-handed men (1 not in a relationship, 3 non-matching handedness of which one also had non-matching gender), and 1 right-handed man (non-matching gender), leaving 380 participants in the sample for analysis. The mean age of the remaining sample was 33.21 years, SD = 11.77, range 18–75, with six participants not providing their age. The participants’ current relationship had lasted, on average, 9.5 years, SD = 10.33, range = 0.8–55 years, with no missing data.
Materials and procedure
Participants read a Participant Information Sheet which briefly explained the task and ethical arrangements (right to withdraw, right to withhold data, data processing, data sharing, advantages, risks, contact details, standard disclaimers, and informed consent), following which they gave electronic consent.
They were asked for age and gender, and they were asked whether they had a partner, and how long they had been in their current relationship. They were then asked which hand they used for writing, left hand, right hand, or both left hand and right hand. Then, they were administered a modified version of the short 10-item Edinburgh Handedness Inventory (Oldfield, Citation1971) in which they were asked which hand they used in ten different tasks. Response options were: “always left”, “usually left”, “both hands equally”, “usually right”, and “always right”. The tasks were: Writing, Drawing, Throwing, Using scissors, a toothbrush, knife (without a fork), spoon, broom (upper hand), Striking a match (match), and Opening a box (lid), with the items in brackets indicating which object was the focus of the question.
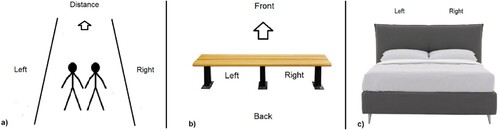
Following that, they were shown an image of silhouette figures walking into the distance (, panel a), with “Left” and “Right” clearly marked, and were asked “Imagine that the picture above is of you and your partner, walking into the distance, in the direction of the arrow. Please indicate the side that you would prefer to walk on. (Left / Right as shown in the picture)”. The following question displayed a bench (, panel b) again with sides clearly marked and an arrow indicating the facing direction, with instructions “Imagine that the picture above is of a bench that you and your partner sit on, facing towards the front, in the direction of the arrow. Please indicate the side of the bench that you would prefer to sit on. (Left / Right as shown in the picture)”. The final item showed a double bed pictured from the foot end perspective (, panel c), again with sides clearly marked, with the instruction “Imagine that the bed above is the bed that you share with your partner. Please indicate the side of the bed that you would prefer to sleep on. (Left / Right as shown in the picture)”. Response options for these were as for the modified Edinburgh Handedness Inventory, but with “hand(s)” replaced with “side(s)”. Note that the bed had the reverse perspective from the other two images. Participants were paid a small sum for their participation, in line with Prolific tariffs.
Missing data, design, analysis strategy, and pre-testing
There were very few missing data. There were 11/3800 = 0.2% missing data for the modified Edinburgh Handedness Inventory, and the overall scores were taken over the remaining items. There were no missing data for the walking or bench question, and one missing data point (0.26%) for the bed question.
For the main analysis of the location choice dependent variables “always left” and “usually left” were scored as 0; “usually right” and “always right” as 1; “both sides equally” as 2, except in the case of the bed, where left and right scores were reverse-scored, because the image of the bed was presented from the viewer’s perspective. Changing it to the sleeper’s perspective when lying on their back ensured that the orientation matched the other two position preference measures.
The modified Edinburgh Handedness Inventory was scored as a Laterality Quotient using Oldfield’s method of assigning 2 (++) to “always” responses, 1 (+) to “usually” responses, separately on the left and right, and then entering those scores into the formula: Laterality Quotient (LQ) = ((Right − Left) / (Right + Left)) *100. The modified Edinburgh Handedness Inventory score was a calibrating variable via which we tested whether self-declared left- and right-handed samples obtained mean scores that confirmed their self-declared status. We treated modified Edinburgh Handedness scores as parametric scale data because they were calculated over multiple items. Descriptive statistics for the Laterality Quotients derived from the modified Edinburgh Handedness Inventory can be found in .
Table 1. Laterality quotients for the modified Edinburgh handedness inventory.
We used a 2 × 2 (Sex, Handedness) independent-samples ANOVA for the analysis of the Laterality Quotients derived from the modified Edinburgh Handedness Inventory. These showed a significant main effect of Handedness, F (1, 376) = 3237.21, p < .001, ηp2 = .90, but no significant main effect of Sex, F (1, 376) = 0.31, p = .58, ηp2 = .001, and no significant interaction, F (1, 376) = 1.04, p = .31, ηp2 = .003. Thus, self-declared handedness was clearly confirmed in the modified Edinburgh Handedness Inventory scores, with no sign of asymmetries between the sexes.
We present the full side preference survey data representing all three coded responses (left, right, both equally) in all three settings for descriptive purposes. However, we performed our main inferential statistical analysis on the left vs. right preferences only, removing the less informative “both sides equally” responses. In addition, we subsequently double-checked whether the pattern was the same when all three response categories were included. The measures with focal relevance to the hypotheses were the side preferences related to walking and sitting on a bench.
Data for sleeping in a bed were treated separately, because they formed control data. To check whether analysing the bed setting separately in this way was statistically justified, we ran Kendall’s tau b correlations between all responses in the three settings using “left”, “both sides equally”, and “right” responses, coded 0, 1, and 2, respectively just for this analysis. There was a significant positive correlation between walking and bench, τb = .30, N = 380, p < .001, but there were no significant correlations between bed and walking, τb = −.001, N = 379, p = .98, nor bed and bench, τb = −.034, N = 379, p = .48. These analyses showed that the bed data differed from the walking and bench data, justifying its separate analysis.
The left and right choices for walking and bench data were analysed using Generalized Mixed Model using GAMLj (Gallucci, Citation2019) on jamovi 2.0.0 (jamovi team, Citation2021) in turn running R (R Core Team, Citation2021). For the main analysis, we used a logistic model type, with the logit link function, and the binomial distribution. In line with Barr, Levy, Scheepers, and Tily (Citation2013), we used the maximal model compatible with the design, which had Sex (Female = 0, Male = 1) and Handedness (Left-handed = 0, Right-handed = 1) as between-subjects fixed factors, and Setting (Walking = 0, Bench = 1) as a within-subjects fixed factor, with Subjects (participants) as the random factor. Sex and Handedness were nested in Subjects, and random slopes could therefore not be estimated. There were too few observations for further random intercept or slopes to be identified.
We followed reporting standards proposed by Meteyard and Davies (Citation2020). As part of this, we tested the maximal model against alternative reduced models (see ) and found that the overall fit was significantly worse without the fixed factors, and could not be significantly bettered by removing the less hypothesis-relevant effect of Setting, so we settled on the maximal model as our final model. Diagnostics showed no evidence of overdispersion Χ2 / df = 0.53; (<1), which confirmed the suitability of the binomial distribution. We report Odds Ratios [OR = Exp(B)] as effect sizes, labelling OR < 1.68 as very small, 1.68 ≤ OR < 3.47 as small, 3.47 ≤ OR < 6.71 as medium, OR ≥ 6.71 as large, in line with Chen, Cohen, and Chen (Citation2010).
Table 2. Model selection table for Study 2.
Results
Frequency scores for the three measures (walking, bench, bed) as a function of the independent variables are shown in , respectively. Full data are available via Supplementary Information in the interest of Open Science.
Figure 2. Study 2 (Survey): Preferred walking side data. Note: Frequencies (raw counts) for preferred walking side as a function of Sex and Handedness. Side is the side from the walker’s perspective (e.g., “Left” means the participant preferred to be on the left of their partner when walking). “Both” indicates the participants chose “Both sides equally”.
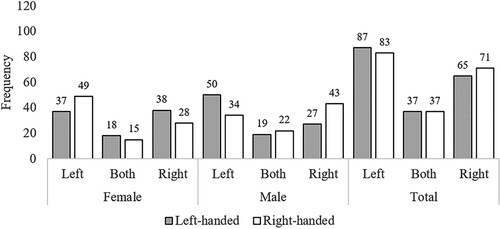
Figure 3. Study 2 (Survey): Preferred bench sitting side data. Note: Frequencies (raw counts) for preferred side when sitting on a bench as a function of Sex and Handedness. Side is the side from the sitter’s perspective (e.g., “Left” means the participant preferred to be on the left of their partner when sitting on a bench). “Both” indicates that the participants chose “Both sides equally”.
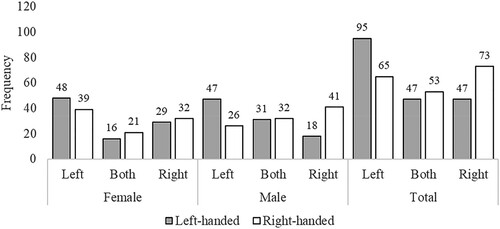
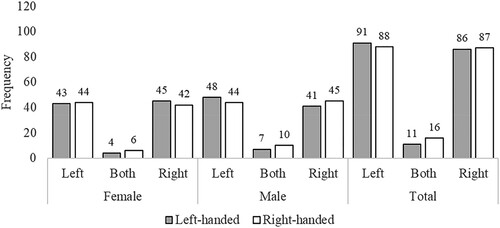
Figure 4. Study 2 (Survey): Preferred side when lying on a bed. Note: Frequencies (raw counts) for preferred side when lying on a bed as a function of Sex and Handedness. Side is the side of the bed from the perspective of the participant in relation to their partner when both are lying on their backs (e.g., “Left” means the participant preferred to be on the left of their partner when they are both lying in bed on their backs). “Both” indicates that the participants chose “Both sides equally”.
Walking and sitting
The results from the Generalized Mixed Model for left- and right-side preferences from the couple’s perspective (Left side = 0, Right side = 1) for walking and sitting on a bench are presented in (following Meteyard & Davies, Citation2020). Overall, the mean across all retained observations was .44, showing a slight overall preference to be on the left. This was similar across males (.45) and females (.42), with no significant overall effect of Sex (p = .67, OR = 1.121, very small effect size). The overall slight left preference was significantly influenced by two factors and two two-way interactions. There was a significant main effect of Handedness (p = .008, OR = 2.088, small effect size), with left-handers showing a mean of .38, and right-handers of .49, indicating a greater left-side preference for left-handers. This main effect was qualified by a significant interaction between Sex and Handedness (p < .001, OR = 6.388, medium effect size), with females showing a more modest difference as a function of handedness (Mleft-handed female = .44, Mright-handed female = .41) than males (Mleft-handed male = .32, Mright-handed male = .58). Bonferroni-corrected post hoc tests examining this Sex × Handedness interaction showed that left-handed males differed significantly from right-handed males, Z = 3.99, pBonferroni < .001, but females did not show a significant effect of handedness, Z = .52, pBonferroni = 1. In addition, right-handed females differed significantly from right-handed males, Z = 2.69, pBonferroni = .042. No other pairwise comparisons in this set were significant.
Table 3. Final (maximal) generalized mixed model for Study 2.
Although not of focal interest with respect to the hypotheses, the interaction between Setting (Walking, Bench) and Handedness was also significant (p = 0.017, OR = 2.813, small effect size) with a more modest impact of Handedness for walking (Mleft-handed walking = .43, Mright-handed walking = .46) than for sitting on a bench (Mleft-handed bench = .33, Mright-handed bench = .53). Bonferroni-corrected post hoc tests showed a significant effect of handedness for the bench, Z = 3.40, pBonferroni = .004, but not walking. There were no further significant pairwise differences in this set.
As stated, we double-checked the significance of the factors in an additional model, to ensure that the key observations reported above were not artefacts of the removal of the “Both sides equally” responses. Thus we built a maximal Generalized Mixed Model with the same factors, this time coding Left = 0, Both sides equally = 1, Right = 2, with a Poisson-family distribution and a log link function (Bono, Alarcón, & Blanca, Citation2021), including all 760 walking and bench observations from the 380 participants. This model did not show overdispersion (Χ2 / df = .77, < 1).
In this model, there was a significant main effect of Handedness, Z = 2.28, p = .023, and a significant interaction between Sex and Handedness, Z = 3.03, p = .002, matching the pattern reported for the left vs. right responses above. Further, the Bonferroni-corrected pairwise comparison between male left- and right-handers was significant, Z = −3.82, pBonferroni < .001, but that between female left- and right-handers was not, Z = 0.52, pBonferroni = 1.00. The interaction between Setting and Handedness (which was of less interest in relation to the hypotheses) was not significant in this analysis, Z = 1.80, p = .072. No other effects or interactions reached significance. Thus, the key patterns of interest were replicated in this additional analysis, suggesting a robust pattern.
Bed
The bed data were analysed separately. We built a Generalized Mixed Model (binomial, logistic, logit link function) for the bed setting, to test the impact of our factors on side preferences (left vs. right), using the same factors as for the walking and bench data, but without the factor of Setting. The model ran on 352 data points, with 28 excluded “both equally” responses or missing data. All means were very close to .5 (Mleft-handed female = .51; Mright-handed female = .49, Mleft-handed male = .46, Mright-handed male = .51). The main effect of Sex was not significant, Z = .31, p = .753, OR = .935, nor was the main effect of Handedness, Z = .21, p = .836, OR = 1.045, nor the interaction, Z = .64, p = .524, OR = 1.313, with very small effect sizes for all three.
In sum, the walking and bench data showed that males showed a significant difference as a function of handedness. The means indicated that men preferred to walk or sit on the dominant-hand side. This difference was not significantly present in females. The side preferences for the bed were not impacted by Sex or Handedness, nor an interaction between these two factors.
Discussion
In the observational study it was found that in 57% of pairs, men walked on the right side of the pair, and women in 43%. The proportion of men walking on the right was robustly above the chance value of 50%, based on Bonferroni-corrected frequentist inferencing and on Bayesian credible intervals and the Bayes Factor. This shows that humans, like many other species (Regaiolli, Spiezio, Ottolini, Sandri, & Vallortigara, Citation2021; Zaynagutdinova et al., Citation2021), exhibit lateralized position preferences when in a pair. In addition, the finding adds to the examples of other human social behaviours, such as kissing, cradling and embracing, which show patterns of lateralization. The greater number of men on the right side is compatible with the readiness to fight hypothesis which predicted that men would want to walk with their dominant hand on the outside of the formation, and because right-handed men are more frequent in a population, there would be more men on the right side of a pair. We evaluate this interpretation in more depth shortly.
For the survey data, the prediction from the readiness to fight hypothesis that men, but not women, would show a preference to have their dominant hand on the outside of the formation, was supported for walking and sitting on a bench. In contrast, there was no evidence for an overall preference to be on the right of a pair, and thus the data were not compatible with a right-hemisphere hypothesis that applies universally to all. The dominant-hand hypothesis also did not receive clear support because the preference to be on the right, when walking and sitting, applied to men but not women. Despite this, the dominant-hand hypothesis could account for the observational and survey data if it is assumed that men get to be on their preferred side more often, perhaps by having a stronger side preference and / or being more assertive. For example, manipulating objects might be more important to men than women, and so men might have a stronger preference to have their dominant hand on the outside of a pair, so it is free to manipulate objects (we are grateful to Dr Tucker Gilman for this suggestion). The stronger effect of handedness for side preferences in bench sitting compared to walking could be related to this. Further research is required to examine these possibilities. Taken together, without including additional moderating influences, the observational data and survey data are more compatible with a readiness to fight hypothesis than with a universal right-hemisphere hypothesis, or the dominant-hand hypothesis.
In the side of the bed preferences, the survey data showed that participants largely avoided “both sides equally” responses. Individual preferences for the side of the bed were not associated with sex or handedness. The bed question is helpful in the interpretation of the data. First, it showed that participants were not responding in a mindless or heuristic way, simply selecting the same response for each question. This provides confidence in the walking and bench data, because it suggests that participants expressed genuine preferences. Second, it showed that the typically habitual, long-lasting, and daily arrangement of a couple in bed did not determine the preferred side when walking and sitting on a bench. Third, although this is debatable, lying in bed is not usually a situation in which a man is required to fight a foe, and therefore this question served as a useful control condition. The observed pattern is compatible with the predictions from a fighting readiness hypothesis, if fighting-related behaviours are context-specific and only instantiated in relevant settings where fighting may be required. However, the bed data may contain some noise, because people tend not to sleep on their backs, and rotating into other positions can reverse the left and right sides, so some caution is required in the interpretation of the bed data.
Some researchers have discussed whether a fighting drive is still relevant in modern Western societies. For example, Faurie and Raymond (Citation2013) suggest that, rather than fighting leading to survival of the individual involved in the fight, in modern non-violent societies, fighting, along with sporting achievements, may instead serve as a ritualized display aimed at attracting mates and promoting procreation. However, evolutionary theorizing accommodates vestigial behaviours that may have conferred Darwinian fitness in the past but that are no longer adaptive (see e.g., Rognini, Citation2018). A drive to be prepared to fight, even if it is unlikely to be necessary, may still be present in men as a behavioural trait at an implicit level, even if, in most situations, it is not necessary to exhibit the fighting for which the man readies himself.
Other potential explanations of these findings cannot be discounted. One interpretation is that they were caused by sex and handedness differences in emotional lateralization, which caused a stronger side preference in right and left-handed males. There is evidence that males are more strongly lateralized than females (Hirnstein, Hugdahl, & Hausmann, Citation2019), and stronger leftward perceptual asymmetries in males have been reported with line bisection (Jewell & McCourt, Citation2000) and the chimeric faces task (Innes, Burt, Birch, & Hausmann, Citation2016). There is also evidence for sex differences in the lateralization of emotion perception (Bourne, Citation2005; Burton & Levy, Citation1989; Rodway et al., Citation2003; Van Strien & Van Beek, Citation2000; but see Borod et al., Citation2001), and in social behaviours involving emotional connections, such as cradling (Packheiser, Schmitz, et al., Citation2019). It is possible that stronger emotional asymmetries in men may predispose them to more strongly prefer to occupy one side of the pair, because it aids social interaction or the monitoring threats from other males. In relation to this, Marzoli, Prete, and Tommasi (Citation2014) propose that the leftward gaze bias could facilitate the monitoring of the dominant hand of other people, either for aiding communication or for monitoring potentially aggressive acts. A stronger leftward gaze to the hands than to other body parts, when looking at angry bodily postures, is in accord with this suggestion (Calbi, Langiulli, Siri, Umiltà, & Gallese, Citation2021; see also Lucafò et al., Citation2021). In addition, men have been found to be more strongly lateralized than women when looking at facial emotions of threat in male faces (Rahman & Anchassi, Citation2012). However, for stronger emotional lateralization in males to account for the current findings, it would have to be assumed that left-handed males have opposite emotional asymmetries to right-handed males. Some research has found this reversal in left-handers (Willems et al., Citation2010) while other evidence indicates weaker right hemisphere emotional lateralization, but not a reversal (Elias et al., Citation1998). In sum, it is apparent that an explanation of the side preference in terms of differences in lateralized emotion processing, due to sex and handedness, is consistent with the current findings and other research.
More research is needed to determine which of these alternative explanations is supported by the evidence. The fighting readiness explanation predicts that men will show a stronger side preference in more threatening environments, such as when it is dark, or when walking through a crowd of unfamiliar people. In contrast, a sex and handedness difference in lateralized emotion processing would not predict a stronger side preference in men under more threatening circumstances, if the asymmetry was operating to facilitate social communication with a partner. However, if it was functioning to facilitate the monitoring of the environment for potential threats from aggressive conspecifics, then a stronger side preference could be expected. In this case, the men’s preference for the side that facilitates the use of their dominant hand, and the monitoring of threats in the environment, could be the manifestation of a general behaviour to be “fight ready”.
Small effect sizes for some of our data could be said to be a limitation of the findings. For example, in the observational study, we observed a Cohen’s h of .13 which is a very small effect size. However, the preference was statistically robust, and the proportion of men on the right (.57) was meaningfully higher than chance (.50). The small effect size is most probably a reason why this subtle bias had not yet been discovered, because it is not noticeable via everyday non-systematic observation. If the effect had been larger, it is likely that people would have noticed the bias routinely in their daily lives. The survey data showed a medium effect size for the key interaction between Sex and Handedness for the walking and bench data (OR = 6.388). This may be because in the survey, people could express their preferences, which were not diluted to the same extent as the observational data, where additional random effects and conflicting inter-individual preferences could interfere with individual preferences. In this respect, the observational data and survey data are most usefully considered together because they complement each other’s limitations.
There are other limitations with the present research. First, it is unclear whether the side preferences in the survey reflect actual preferences, or the side that a person typically occupies, reflecting a memory rather than a preference, or a mixture of these influences. Clarifying this will be relevant to understanding the cause of the side bias. Second, the observational study was conducted in a particular location in the UK. While we have no reason to believe that the findings do not generalize to other locations where male–female pairs are able to walk freely, this requires testing. However, it can be noted that the survey data were from participants from throughout the UK, and the side preferences complemented those obtained in the observational study. This provides confidence in the generalizability of the observational data. Finally, it would have been desirable for all survey images to be matched for the presence of stick figures, so that all conditions were matched along that dimension.
In sum, our observations show that in male–female pairs more men walk to the right of a female than to the left from the pair’s perspective. Unlike women, men report significant side preferences when walking or sitting with a female partner, and this preference is dependent on their handedness. The side that men prefer, when with a partner, facilitates the use of their dominant hand and this might be because men want to be in the best position to fight effectively. There are other plausible explanations of the side preference exhibited by men, including an effect of sex and handedness on the strength and direction of emotional lateralization, or a stronger desire in males to be able use their dominant hand. Further research is desirable to clarify the cause of the side preference in human dyads.
Acknowledgements
We would like to thank the editor, Dr. Tucker Gilman, and an anonymous reviewer for their helpful comments on an earlier version the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Supplemental data for this manuscript can be accessed at: https://doi.org/10.6084/m9.figshare.20057021.v1.
Additional information
Funding
References
- Backwell, P. R. Y., Matsumasa, M., Double, M., Roberts, A., Murai, M., Keogh, J. S., & Jennions, M. D. (2007). What are the consequences of being left-clawed in a predominantly right-clawed fiddler crab? Proceedings of the Royal Society B: Biological Sciences, 274(1626), 2723–2729. doi:10.1098/rspb.2007.0666
- Badzakova-Trajkov, G., Häberling, I. S., Roberts, R. P., & Corballis, M. C. (2010). Cerebral asymmetries: Complementary and independent processes. PLoS One, 5(3), e9682. doi:10.1371/journal.pone.0009682
- Barr, D. J., Levy, R., Scheepers, C., & Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. doi:10.1016/j.jml.2012.11.001
- Barrett, D., Greenwood, J. G., & McCullagh, J. F. (2006). Kissing laterality and handedness. Laterality: Asymmetries of Body, Brain and Cognition, 11(6), 573–579. doi:10.1080/13576500600886614
- Billiard, S., Faurie, C., & Raymond, M. (2005). Maintenance of handedness polymorphism in humans: A frequency-dependent selection model. Journal of Theoretical Biology, 235, 85–93. doi:10.1016/j.jtbi.2004.12.021
- Bisazza, A., Cantalupo, C., Capocchiano, M., & Vallortigara, G. (2000). Population lateralisation and social behaviour: A study with 16 species of fish. Laterality: Asymmetries of Body, Brain and Cognition, 5(3), 269–284. doi:10.1080/713754381
- Bono, R., Alarcón, R., & Blanca, M. J. (2021). Report quality of generalized linear mixed models in psychology: A systematic review. Frontiers in Psychology, 12, 1345. doi:10.3389/fpsyg.2021.666182
- Borden, R. J., & Homleid, G. M. (1978). Handedness and lateral positioning in heterosexual couples: Are men still strong-arming women? Sex Roles, 4(1), 67–73. doi:10.1007/BF00288377
- Borod, J. C., Zgaljardic, D., Tabert, M. H., & Koff, E. (2001). Asymmetries of emotional perception and expression in normal adults. In G. Gainotti (Ed.), Emotional behavior and its disorders (2nd ed., Vol. 5, pp. 181–205). Oxford: Elsevier Science.
- Boulinguez-Ambroise, G., Aychet, J., & Pouydebat, E. (2022). Limb preference in animals: New insights into the evolution of manual laterality in hominids. Symmetry, 14(1), 96. doi:10.3390/sym14010096
- Boulinguez-Ambroise, G., Pouydebat, E., Disarbois, É, & Meguerditchian, A. (2020). Human-like maternal left-cradling bias in monkeys is altered by social pressure. Scientific Reports, 10(1), 1–8. doi:10.1038/s41598-020-68020-3
- Bourne, V. J. (2005). Lateralised processing of positive facial emotion: Sex differences in strength of hemispheric dominance. Neuropsychologia, 43(6), 953–956. doi:10.1016/j.neuropsychologia.2004.08.007
- Burton, L. A., & Levy, J. (1989). Sex differences in the lateralized processing of facial emotion. Brain and Cognition, 11(2), 210–228. doi:10.1016/0278-2626(89)90018-3
- Calbi, M., Langiulli, N., Siri, F., Umiltà, M. A., & Gallese, V. (2021). Visual exploration of emotional body language: A behavioural and eye-tracking study. Psychological Research, 85(6), 2326–2339. doi:10.1007/s00426-020-01416-y
- Canning, C., Crain, D., Eaton, T. S., Nuessly, K., Friedlaender, A., Hurst, T., … Weinrich, M. (2011). Population level lateralized feeding behaviour in north atlantic humpback whales, megaptera novaeangliae. Animal Behaviour, 82, 901–909. doi:10.1016/j.anbehav.2011.07.031
- Casperd, J. M., & Dunbar, R. I. M. (1996). Asymmetries in the visual processing of emotional cues during agonistic interactions by gelada baboons. Behavioural Processes, 37(1), 57–65. doi:10.1016/0376-6357(95)00075-5
- Chen, H., Cohen, P., & Chen, S. (2010). How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Communications in Statistics—Simulation and Computation®, 39(4), 860–864. doi:10.1080/03610911003650383
- Dienes, Z. (2011). Bayesian versus orthodox statistics: Which side are you on? Perspectives on Psychological Science, 6(3), 274–290. doi:10.1177/1745691611406920
- Elias, L. J., Bryden, M. P., & Bulman-Fleming, M. B. (1998). Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia, 36(1), 37–43. doi:10.1016/S0028-3932(97)00107-3
- Faurie, C., & Raymond, M. (2013). The fighting hypothesis as an evolutionary explanation for the handedness polymorphism in humans: Where are we? Annals of the New York Academy of Sciences, 1288(1), 110–113. doi:10.1111/nyas.12159
- Forrester, G. S., Leavens, D. A., Quaresmini, C., & Vallortigara, G. (2011). Target animacy influences gorilla handedness. Animal Cognition, 14(6), 903–907. doi:10.1007/s10071-011-0413-6
- Forrester, G. S., Quaresmini, C., Leavens, D. A., Spiezio, C., & Vallortigara, G. (2012). Target animacy influences chimpanzee handedness. Animal Cognition, 15(6), 1121–1127. doi:10.1007/s10071-012-0536-4
- Frasnelli, E., Iakovlev, I., & Reznikova, Z. (2012). Asymmetry in antennal contacts during trophallaxis in ants. Behavioural Brain Research, 232(1), 7–12. doi:10.1016/j.bbr.2012.03.014
- Frasnelli, E., & Vallortigara, G. (2018). Individual-level and population-level lateralization: Two sides of the same coin. Symmetry, 10(12), 739. doi:10.3390/sym10120739
- Gainotti, G. (2019). Emotions and the right hemisphere: Can new data clarify old models? The Neuroscientist, 25(3), 258–270. doi:10.1177/1073858418785342
- Gallucci, M. (2019). GAMLj: General analyses for linear models. [jamovi module]. Retrieved from https://gamlj.github.io/.
- Giljov, A., Karenina, K., & Malashichev, Y. (2018). Facing each other: Mammal mothers and infants prefer the position favouring right hemisphere processing. Biology Letters, 14. doi:10.1098/rsbl.2017.0707
- Grimshaw, G. M. (1998). Integration and interference in the cerebral hemispheres: Relations with hemispheric specialization. Brain and Cognition, 36(2), 108–127. doi:10.1006/brcg.1997.0949
- Grimshaw, G. M., Séguin, J. A., & Godfrey, H. K. (2009). Once more with feeling: The effects of emotional prosody on hemispheric specialisation for linguistic processing. Journal of Neurolinguistics, 22(4), 313–326. doi:10.1016/j.jneuroling.2008.10.005
- Groothuis, T. G., McManus, I. C., Schaafsma, S. M., & Geuze, R. H. (2013). The fighting hypothesis in combat: How well does the fighting hypothesis explain human left-handed minorities? Annals of the New York Academy of Sciences, 1288(1), 100–109. doi:10.1111/nyas.12164
- Grouios, G., Koidou, I., Tsorbatzouidis, H., & Alexandris, K. (2002). Handedness in sports. Journal of Human Movement Studies, 43, 347–362.
- Grouios, G., Tsorbatzouidis, H., Alexandris, K., & Barkoukis, V. (2000). Do left-handed competitors have an innate superiority in sports? Perceptual Motor Skills, 90, 1273–1282. doi:10.2466/pms.2000.90.3c.1273
- Güntürkün, O. (2003). Adult persistence of head-turning asymmetry. Nature, 421(6924), 711. doi:10.1038/421711a
- Güntürkün, O., Ströckens, F., & Ocklenburg, S. (2020). Brain lateralization: A comparative perspective. Physiological Reviews, 100(3), 1019–1063. doi:10.1152/physrev.00006.2019
- Harris, L. J., Cárdenas, R. A., Spradlin Jr, M. P., & Almerigi, J. B. (2010). Why are infants held on the left? A test of the attention hypothesis with a doll, a book, and a bag. Laterality: Asymmetries of Body, Brain and Cognition, 15(5), 548–571. doi:10.1080/13576500903064018
- Hauser, M. D., & Akre, K. (2001). Asymmetries in the timing of facial and vocal expressions by rhesus monkeys: Implications for hemispheric specialization. Animal Behaviour, 61(2), 391–400. doi:10.1006/anbe.2000.1588
- Hews, D. K., Castellano, M., & Hara, E. (2004). Aggression in females is also lateralized: Left-eye bias during aggressive courtship rejection in lizards. Animal Behaviour, 68, 1201–1207. doi:10.1016/j.anbehav.2003.11.024
- Hirnstein, M., Hugdahl, K., & Hausmann, M. (2019). Cognitive sex differences and hemispheric asymmetry: A critical review of 40 years of research. Laterality: Asymmetries of Body, Brain and Cognition, 24(2), 204–252. doi:10.1080/1357650X.2018.1497044
- Innes, B., Burt, D. M., Birch, Y. K., & Hausmann, M. (2016). A leftward bias however you look at it: Revisiting the emotional chimeric face task as a tool for measuring emotion lateralization. Laterality: Asymmetries of Body, Brain and Cognition, 21(4-6), 643–661. doi:10.1080/1357650X.2015.1117095
- jamovi project. (2021). jamovi. (Version 2.0.0) [Computer Software]. Retrieved from https://www.jamovi.org.
- JASP Team. (2022). JASP (Version 0.16.1) [Computer software].
- Jewell, G., & McCourt, M. E. (2000). Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia, 38(1), 93–110. doi:10.1016/s0028-3932(99)00045-7
- Johnstone, L. T., Karlsson, E. M., & Carey, D. P. (2021). Left-handers are less lateralized than right-handers for both left and right hemispheric functions. Cerebral Cortex, 31(8), 3780–3787. doi:10.1093/cercor/bhab048
- Karenina, K., Giljov, A., Ingram, J., Rowntree, V. J., & Malashichev, Y. (2017). Lateralization of mother–infant interactions in a diverse range of mammal species. Nature Ecology & Evolution, 1, 0030. doi:10.1038/s41559-016-0030
- Knecht, S., Dräger, B., Deppe, M., Bobe, L., Lohmann, H., Flöel, A., … & Henningsen, H. (2000). Handedness and hemispheric language dominance in healthy humans. Brain, 123(12), 2512–2518. doi:10.1093/brain/123.12.2512
- Kordsmeyer, T. L., Hunt, J., Puts, D. A., Ostner, J., & Penke, L. (2018). The relative importance of intra-and intersexual selection on human male sexually dimorphic traits. Evolution and Human Behavior, 39(4), 424–436. doi:10.1016/j.evolhumbehav.2018.03.008
- Lakens, D., Pahlke, F., & Wassmer, G. (2021). Group sequential designs: A tutorial. doi:10.31234/osf.io/x4azm
- Lemaire, B. S., Viblanc, V. A., & Jozet-Alves, C. (2019). Sex-specific lateralization during aggressive interactions in breeding king penguins. Ethology, 125(7), 439–449. doi:10.1111/eth.12868
- Ley, R. G., & Bryden, M. P. (1979). Hemispheric differences in processing emotions in faces. Brain and Language, 7, 127–138. doi:10.1016/0093-934X(79)90010-5
- Lucafò, C., Marzoli, D., Zdybek, P., Malatesta, G., Smerilli, F., Ferrara, C., & Tommasi, L. (2021). The bias toward the right side of others is stronger for hands than for feet. Symmetry, 13(1), 146. doi:10.3390/sym13010146
- Manning, J. T., & Chamberlain, A. T. (1990). The left-side cradling preference in great apes. Animal Behaviour, 39(6), 1224–1227. doi:10.1016/S0003-3472(05)80800-0
- Manning, J. T., Heaton, R., & Chamberlain, A. T. (1994). Left-side cradling: Similarities and differences between apes and humans. Journal of Human Evolution, 26(1), 77–83. doi:10.1006/jhev.1994.1005
- Marzoli, D., Prete, G., & Tommasi, L. (2014). Perceptual asymmetries and handedness: A neglected link? Frontiers in Psychology, 5, 163. doi:10.3389/fpsyg.2014.00163
- McManus, I. C., Van Horn, J. D., & Bryden, P. J. (2016). The tapley and Bryden test of performance differences between the hands: The original data, newer data, and the relation to pegboard and other tasks. Laterality: Asymmetries of Body, Brain and Cognition, 21(4–6), 371–396. doi:10.1080/1357650X.2016.1141916
- Meteyard, L., & Davies, R. A. I. (2020). Best practice guidance for linear mixed-effects models in psychological science. Journal of Memory and Language, 112, 2–22. doi:10.1016/j.jml.2020.104092
- Ocklenburg, S., Packheiser, J., Schmitz, J., Rook, N., Güntürkün, O., Peterburs, J., & Grimshaw, G. M. (2018). Hugs and kisses – The role of motor preferences and emotional lateralization for hemispheric asymmetries in human social touch. Neuroscience & Biobehavioral Reviews, 95, 353–360. doi:10.1016/j.neubiorev.2018.10.007
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
- Packheiser, J., Berretz, G., Rook, N., Bahr, C., Schockenhoff, L., Güntürkün, O., & Ocklenburg, S. (2021). Investigating real-life emotions in romantic couples: A mobile EEG study. Scientific Reports, 11(1), 1–12. doi:10.1038/s41598-020-80590-w
- Packheiser, J., Rook, N., Dursun, Z., Mesenhöller, J., Wenglorz, A., Güntürkün, O., & Ocklenburg, S. (2019). Embracing your emotions: Affective state impacts lateralisation of human embraces. Psychological Research, 83(1), 26–36. doi:10.1007/s00426-018-0985-8
- Packheiser, J., Schmitz, J., Berretz, G., Papadatou-Pastou, M., & Ocklenburg, S. (2019). Handedness and sex effects on lateral biases in human cradling: Three meta-analyses. Neuroscience & Biobehavioral Reviews, 104, 30–42. doi:10.1016/j.neubiorev.2019.06.035
- Pollet, T. V., Stulp, G., & Groothuis, T. G. (2013). Born to win? Testing the fighting hypothesis in realistic fights: Left-handedness in the ultimate fighting championship. Animal Behaviour, 86(4), 839–843. doi:10.1016/j.anbehav.2013.07.026
- Porac, C. (2015). Laterality: Exploring the enigma of left-handedness. Academic Press.
- Prete, G., Laeng, B., Fabri, M., Foschi, N., & Tommasi, L. (2015). Right hemisphere or valence hypothesis, or both? The processing of hybrid faces in the intact and callosotomized brain. Neuropsychologia, 68, 94–106. doi:10.1016/j.neuropsychologia.2015.01.002
- Puts, D. A. (2010). Beauty and the beast: Mechanisms of sexual selection in humans. Evolution and Human Behavior, 31(3), 157–175. doi:10.1016/j.evolhumbehav.2010.02.005
- Quaresmini, C., Forrester, G. S., Spiezio, C., & Vallortigara, G. (2014). Social environment elicits lateralized behaviors in gorillas (Gorilla gorilla gorilla) and chimpanzees (Pan troglodytes). Journal of Comparative Psychology, 128(3), 276–284. doi:10.1037/a0036355
- Rahman, Q., & Anchassi, T. (2012). Men appear more lateralized when noticing emotion in male faces. Emotion, 12(1), 174–179. doi:10.1037/a0024416
- Raymond, M., Pontier, D., Dufour, A. B., & Møller, A. P. (1996). Frequency-dependent maintenance of left handedness in humans. Proceedings of the Royal Society of London. Series B: Biological Sciences, 263(1377), 1627–1633. doi:10.1098/rspb.1996.0238
- R Core Team. (2021). R: A Language and environment for statistical computing. (Version 4.0) [Computer software]. Retrieved from https://cran.r-project.org. (R packages retrieved from MRAN snapshot 2021-04-01).
- Regaiolli, B., Spiezio, C., Ottolini, G., Sandri, C., & Vallortigara, G. (2021). Behavioural Laterality in two species of flamingos: Greater flamingos and Chilean flamingos. Laterality, 26(1-2), 34–54. doi:10.1080/1357650X.2020.1781877
- Richardson, T., & Gilman, R. T. (2019). Left-handedness is associated with greater fighting success in humans. Scientific Reports, 9(1), 1–6. doi:10.1038/s41598-019-51975-3
- Robins, A., & Rogers, L. J. (2004). Lateralized prey-catching responses in the cane toad, bufo marinus: Analysis of complex visual stimuli. Animal Behaviour, 68(4), 767–775. doi:10.1016/j.anbehav.2003.12.014
- Rodway, P., & Schepman, A. (2007). Valence specific laterality effects in prosody: Expectancy account and the effects of morphed prosody and stimulus lead. Brain and Cognition, 63(1), 31–41. doi:10.1016/j.bandc.2006.07.008
- Rodway, P., Thoma, V., & Schepman, A. (2022). The effects of sex and handedness on masturbation laterality and other lateralized motor behaviours. Laterality, 27(3), 324–352. doi:10.1080/1357650X.2021.2006211
- Rodway, P., Wright, L., & Hardie, S. (2003). The valence-specific laterality effect in free viewing conditions: The influence of sex, handedness, and response bias. Brain and Cognition, 53(3), 452–463. doi:10.1016/S0278-2626(03)00217-3
- Rogers, L. J. (1989). Laterality in animals. International Journal of Comparative Psychology, 3, 5–25.
- Rogers, L. J. (2017). A matter of degree: Strength of brain asymmetry and behaviour. Symmetry, 9(4), 57. doi:10.3390/sym9040057
- Rogers, L. J. (2021). Brain lateralization and cognitive capacity. Animals, 11(7), 1996. doi:10.3390/ani11071996
- Rogers, L. J., Rigosi, E., Frasnelli, E., & Vallortigara, G. (2013). A right antenna for social behaviour in honeybees. Scientific Reports, 3(1), 1–4. doi:10.1038/srep02045
- Rognini, P. (2018). Vestigial drifting drives in homo sapiens. Biological Theory, 13(3), 199–211. doi:10.1007/s13752-018-0297-7
- Salva, O. R., Daisley, J. N., Regolin, L., & Vallortigara, G. (2009). Lateralization of social learning in the domestic chick, gallus gallus domesticus: Learning to avoid. Animal Behaviour, 78(4), 847–856. doi:10.1016/j.anbehav.2009.06.021
- Schnell, A. K., Jozet-Alves, C., Hall, K. C., Radday, L., & Hanlon, R. T. (2019). Fighting and mating success in giant Australian cuttlefish is influenced by behavioural lateralization. Proceedings of the Royal Society B, 286(1898), 20182507. doi:10.1098/rspb.2018.2507
- Schrammen, E., Grimshaw, G. M., Berlijn, A. M., Ocklenburg, S., & Peterburs, J. (2020). Response inhibition to emotional faces is modulated by functional hemispheric asymmetries linked to handedness. Brain and Cognition, 145, 105629. doi:10.1016/j.bandc.2020.105629
- Sedgewick, J. R., & Elias, L. J. (2016). Family matters: Directionality of turning bias while kissing is modulated by context. Laterality: Asymmetries of Body, Brain and Cognition, 21(4-6), 662–671. doi:10.1080/1357650X.2015.1136320
- Sell, A., Cosmides, L., Tooby, J., Sznycer, D., Von Rueden, C., & Gurven, M. (2009). Human adaptations for the visual assessment of strength and fighting ability from the body and face. Proceedings of the Royal Society B: Biological Sciences, 276(1656), 575–584. doi:10.1098/rspb.2008.1177
- Turnbull, O. H., Stein, L., & Lucas, M. D. (1995). Lateral preferences in adult embracing: A test of the “hemispheric asymmetry” theory of infant cradling. The Journal of Genetic Psychology, 156(1), 17–21. doi:10.1080/00221325.1995.9914802
- Vallortigara, G., & Andrew, R. (1994). Differential involvement of right and left hemisphere in individual recognition in the domestic chick. Behavioural Processes, 33, 41–57. doi:10.1016/0376-6357(94)90059-0
- Vallortigara, G., & Rogers, L. (2005). Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences, 28(4), 575–589; discussion 589–633. doi:10.1017/S0140525X05240104
- Vallortigara, G., & Rogers, L. J. (2020). A function for the bicameral mind. Cortex, 124, 274–285. doi:10.1016/j.cortex.2019.11.018
- Van Strien, J. W., & Van Beek, S. (2000). Ratings of emotion in laterally presented faces: Sex and handedness effects. Brain and Cognition, 44(3), 645–652. doi:10.1006/brcg.1999.1137
- Wasser, T. (2021). Using adaptive research design to define the proper methodology to Use a data peek for power: Step by step process. Advances in Clinical Medical Research and Healthcare Delivery, 1(3). doi:10.53785/2769-2779.1035
- Willems, R. M., Peelen, M. V., & Hagoort, P. (2010). Cerebral lateralization of face-selective and body-selective visual areas depends on handedness. Cerebral Cortex, 20(7), 1719–1725. doi:10.1093/cercor/bhp234
- Zaynagutdinova, E., Karenina, K., & Giljov, A. (2021). Lateralization in monogamous pairs: Wild geese prefer to keep their partner in the left hemifield except when disturbed. Current Zoology, 67(4), 419–429. doi:10.1093/cz/zoaa074