ABSTRACT
Research in Parkinson’s or Alzheimer’s disease suggests that hand function is affected by neurodegenerative diseases. However, little is known about the relationship between hand function and mild cognitive impairment (MCI). Therefore, we conducted a kinematic analysis of unimanual hand movements in MCI patients to answer whether manual asymmetries and manual dexterity are affected or preserved in this condition. Forty-one MCI patients and fifty healthy controls were tested with the Purdue Pegboard test. All participants were right-handed. Kinematic analyses (by hand) were calculated for path length, angle, and linear and angular velocities during reaching, grasping, transport and inserting. Group differences were tested by with factorial MANOVAs and laterality indexes (LI) were assessed. Groups were compared on “Right–Left” hand correlations to identify kinematics that best single-out patients. Kinematics from grasping and inserting were significantly more deteriorated in the MCI group, while outcomes for reaching and transport denoted superior performance. LIs data showed symmetry of movements in the MCI group, during reaching and transport. Comparisons of “Right–Left” hand correlations revealed that kinematics in reaching and transport were more symmetrical in patients. This study showed a deterioration of fine manual dexterity, an enhancement in gross dexterity of upper-limbs, and symmetrical movements in MCI patients.
Hemispheric asymmetries exist at the cerebral level across several species including vertebrates and invertebrates (Gunturkun et al., Citation2020). Such asymmetries are thought to underlie lateralized functioning of the brain and expression of behavior. From a phylogenetic perspective, humans are usually regarded as extreme examples of lateralized specialization due to our language abilities (Gunturkun et al., Citation2020). At this respect, probably the best example of functional lateralization is the phenomenon of handedness. Handedness is an umbrella term, which embraces two facets: (a) the human preference for using one hand over the other, most often associated with writing (Lubben et al., Citation2021; Papadatou-Pastou, Citation2018), and (b) hand skill, which concerns how efficient, fast and strong a hand is compared to the other (Papadatou-Pastou, Citation2018). Notably, research has demonstrated that many motor tasks are performed faster and more accurately with the preferred hand, as compared to the non-preferred, including aiming, finger tapping, and peg moving tasks (Benson et al., Citation2021; Francis & Spirduso, Citation2000; Teixeira, Citation2008). In healthy, right-handed individuals, this right-hand advantage is reflected by asymmetrical movements of the preferred and non-preferred hands, respectively. For example, during performance of the Purdue Pegboard Test, faster movements have been reported in the right hand of both young and healthy older right-handers as compared to their left hand (Vasylenko et al., Citation2018).
Even though much research has been conducted on dexterity and manual asymmetries, the issue of how they are impacted by pathological aging remains largely unknown. According to a recent review (Lubben et al., Citation2021), the association between behavioral-brain asymmetries and neurodegenerative diseases has not received enough attention from the scientific community. Still, some studies have reported a relationship between use of the preferred hand and appearance of lateralized symptoms in neurodegenerative conditions such as in Parkinson’s disease (e.g., Uitti et al., Citation2005) or amyotrophic lateral sclerosis (Turner et al., Citation2011). Other data from studies in Alzheimer’s disease (AD) reveal the existence of asymmetrical brain degeneration in patients suffering this condition (e.g., Chen et al., Citation2020) as well as higher number of left-handers (Ryan et al., Citation2020; Seltzer et al., Citation1984) and ambidextrous (Cherbuin et al., Citation2011) in the early onset of AD. On this line of research, it has been reported a higher prevalence of left-handers among patients with various degrees of cognitive impairment (Riggio et al., Citation2020).
In addition to the above-mentioned studies suggesting that particular manual asymmetries are related to neurodegeneration, there are also data pointing to impaired hand dexterity in patients suffering neurodegenerative conditions. For example, manual dexterity is affected by Parkinson’s disease and various dementia types (e.g., Liou et al., Citation2020; de Paula et al., Citation2016). In addition, several studies show that declines in manual dexterity strongly correlate with dementia severity (e.g., de Paula et al., Citation2016; Yan et al., Citation2008). Thus, such impairments are suggested to help in the differential diagnosis of the syndrome (Fritz et al., Citation2016) and impaired dexterity has been proposed as a risk factor for neurodegenerative diseases (Darweesh et al., Citation2017).
Of especial interest at this regard, is the study conducted by Tamaru et al. (Citation2020). In this study, the authors addressed the issue of whether hand dysfunction in AD occurred in parallel for both hands. Results showed that dexterity deterioration was more evident in the preferred hand, which agrees with earlier studies reporting stronger motor declines in the preferred hand of Parkinson patients (e.g., Uitti et al., Citation2005). This specific study is noteworthy as it investigated asymmetric deterioration and dexterity decline in the same sample of patients. Most often, studies have addressed one of this topics independently from the other (dexterity vs. manual asymmetries). Thus, taken together the above reports, it seems justified to go one step further and conduct a detailed evaluation of hand function including both manual asymmetries and dexterity at the earliest stages of disease development, for instance, in mild cognitive impairment (MCI), which is the preclinical stage of dementia.
A core interest in conducting such investigation relies on the importance of hand function for activities of daily living (ADL), which are decisive aspects in the diagnosis of dementia (Arvanitakis et al., Citation2019). To date, it is acknowledged that hand movements are strongly related to ADL function (de Paula et al., Citation2016) and a recent review suggests hand movement assessment as a way to differentiate healthy aging from MCI (Ilardi et al., Citation2022). Likewise, the same review also emphasizes the need to conduct further research to better characterize the declines on hand function proper to MCI. For this reason, we intend to assess manual asymmetries as well as deteriorations in manual dexterity in older adults with MCI. By doing so, we will contribute with new knowledge on hand function in MCI, which eventually may be useful in the identification of individuals at increased risk of neurodegeneration. To our knowledge, there is no previous research reporting a comprehensive assessment of dexterity and manual asymmetries in MCI. As suggested by Tamaru et al. (Citation2020), in order to obtain detailed information on dexterity performance, a kinematic assessment of unimanual ability is required since simple measurements based on performance times cannot unveil asymmetries of hand function. Therefore, the present investigation aims to conduct a detailed kinematic analysis of dexterity in MCI patients during performance of the Perdue Pegboard Test (PPT). Importantly, the PPT has shown to be a good instrument for assessing the risk of neurodegenerative diseases (Darweesh et al., Citation2017). The main goal of the present study is to obtain a fine-grained description of hand function in MCI, including manual asymmetries and manual dexterity, which will represent a step towards explaining how hand function is affected by this condition.
Methods
Participants
Fifty healthy older adults and 41 patients with MCI participated in this study. Healthy older participants, 24 female, M (SD)age = 71.04 (6.12), were randomly selected from the sample previously described in Vasylenko, et al. (19). That original sample consisted of 55 healthy, community-dwelling older adults, M(SD)age = 70.6 (7.32) years, 25 female. MCI patients, 21 female, M(SD)age = 72.49 (8.73), were referred to the study by the University Hospital of North Norway. The patient group underwent standard clinical assessments at the hospital (i.e., cognitive/laboratory tests, brain imaging) and the MCI diagnosis was established by a senior geriatrician or a senior neurologist following criteria on the ICD-10.
Several screening measures were conducted as well as a short interview to both the healthy and the MCI groups. At the interview we obtained demographic and health information, including self-reported handedness. Participants were asked whether they considered themselves as being right- or left-handers. Their responses were further corroborated by the assessment of hand preference with the Briggs-Nebes Handedness Inventory (Briggs & Nebes, Citation1975), which consists of 12 items requiring subjects to respond whether they perfom different actions with right or left hand, ranging from “always left” to “always right”. Total score is calculated by allocating from −2 to +2 points for each item and the final score is obtained by adding together the points from all 12 items. The final score ranges from −24 to +24, whereof negative numbers reflect left-hand preferences and positive numbers right-hand preferences. Additionally, we employed the Mini-Mental State Examination (MMSE) (Folstein et al., Citation1975) for the assessment of global cognitive status and the Beck Depression Inventory, 2nd ed. (Beck et al., Citation1996) for the assessment of possible depressive symptoms. The exclusion criteria for all participants were being a non-native Norwegian speaker, recent injuries of arms or hands, current hand pain, left-handedness as indicated by the Briggs-Nebes Handedness Inventory (i.e., scores < +9), and being depressed as indicated by the Beck Depression Inventory, (i.e., scores > 13). An additional exclusion criterion for the healthy control group was an MMSE score < 28.
All participants recruited to the study received thorough information and gave informed consent prior to the procedure. Moreover, all underwent brain imaging assessments as well as a broad neuropsychological evaluation in three cognitive domains: executive function/working memory (EF/WM), memory, and visuospatial ability (VA). To calculate the EF/WM domain, we relied on four tests: Digit Span backwards from the Wechsler Adult Intelligence Scale (WAIS-IV, Wechsler, Citation2008), the interference part of the Stroop test (Golden, Citation1978), Part B of the Trail Making Test (TMT B, Reitan & Wolfson, Citation1993), and the Controlled Oral Word Association Test (COWAT, Benton, Citation1967). For the memory domain, the following tests were employed: Logical memory I and II from Wechsler Memory Scale, 3rd edition (Wechsler, Citation1997), Digits Span forward from Wechsler Adult Intelligence Scale (WAIS-IV, Wechsler, Citation2008), and semantic fluency test (Newcombe, Citation1969). Finally, three tests were used to calculate the visuospatial ability domain: Block Design from WAIS-IV (Wechsler, Citation2008), the Clock Drawing Test (CDT, Shulman, Citation2000), and Trail Making Test A (TMT A, Reitan & Wolfson, Citation1993). Cognitive scores for both groups are summarized in Supplementary Table 1. Based on neuropsychological data, the patients were classified into three subgroups: amnestic if impairment was on tests belonging to the memory domain, non-amnestic if patients did not present an impairment in the memory domain, and multiple domain if impairment existed in various cognitive domains. Impaired performance on any or various domains greater than >1 SD below corrected normative means was the criterion employed for assignment to subgroups (Jak et al., Citation2009; Bondi et al., Citation2014). The study was approved by the Regional Research Ethics Committee and carried out in accordance with the Helsinki guidelines.
Measures
Purdue pegboard test (PPT) and motion capture
From the four standard subtests of the PPT, we selected a unimanual task in which participants are required to pick up pins from the top cups, one by one, and insert them into a specific row of holes in the middle of the pegboard starting at the top. When assessing the right hand, the right row was used, while the left row was utilized in left hand assessment. The task was first performed with the preferred hand (right) and thereafter with the non-preferred hand (left). Some adaptations were done before conducting kinematic analyses. First, the pegboard was painted black and the pins red to increase contrast with the reflective markers. Second, to obtain equal amounts of data from each participant, we asked them to insert 10 pins in each task, instead of inserting pins for 30 s as indicated in the standardized administration procedure.
Overall dexterity performance and errors
Overall performance was measured by the time spent on each task and the number of errors made in each task. The most common errors were dropping a pin and picking up two pins at the same time.
Types of movements for kinematic analysis
After recording, all videos were manually subdivided into four movement types: reaching, grasping, transporting, and inserting of pins. The detailed procedure for this subdivision has been described in a previous study (Vasylenko et al., Citation2018). Kinematic measures were obtained and analyzed for each of the four movement types. Among the different movement types, reaching and transport of pins represent gross dexterity, and grasping and inserting represent fine dexterity.
Kinematic measures and their connotation
Linear velocity, path length, angle, angular velocity, and coefficients of variation (CVs) in linear velocity, angle, and angular velocity were measured. Specifications for registration and calculation of these variables has been reported elsewhere (Vasylenko et al., Citation2018). Higher linear velocity represents faster hand movement. Shorter path represents smoother movements with more direct trajectories to the target. Angles represent position of the hand. In 2D images, larger angles represent a less pronated position of the hand. Angular velocity was computed from angles. Higher angular velocity represents faster hand rotation. All within-trial CVs were computed as SD/M ratios from their respective kinematic measures. Higher variability in velocity and angle represents more adjustments to the speed and position of the hand, respectively. All kinematics were averaged across the 10 repetitions of each type of movement and the mean values were entered into statistical analyses.
Procedure
The study took place at the Department of Psychology, University of Tromsø. An interview and screening measures were conducted firstly, followed by the dexterity assessment with the PPT. A demonstration of the required task was given to the participants who also were asked to practice until able to correctly insert three pins. Afterwards, they were instructed to perform the task as quickly and accurately as possible at the experimenter’s signal.
Statistical analyses
Group differences in demographics and overall dexterity performance were assessed with independent-samples t-tests. Group differences in kinematics were analyzed by two-factor MANOVAs with repeated measures on the within-subjects factor Hand and with Group as the between-subjects factor. Separate MANOVAs were conducted for each of the four types of movement. The dependent variables were the seven kinematic measures. Significant omnibus tests were followed up by univariate ANOVAs for each kinematic measure. Greenhouse-Geisser correction was used in case the sphericity assumption was not met. Significant main effects and interactions were followed up by pairwise comparisons with Bonferroni correction. To further explore the nature of group differences in manual asymmetries, additional analyses were performed. First, laterality indices (LIs) were calculated with the formula LI = (R−L) / (R + L) and group differences in LIs were analyzed by MANOVAs with Group as the between-subjects factor and the seven LIs as dependent variables. Separate MANOVAs were performed for each of the four movement types and Bonferroni correction was used for planned comparisons. Second, associations between kinematics and number of errors were assessed by Pearson correlation coefficient. Finally, additional Pearson correlations were conducted separately on each group between right and left values of each kinematic variable to explore the extent of asymmetries by group. Thereafter, we compared the correlations between groups to find out whether specific variables differentiate patients from controls. All statistical analyses were performed with SPSS version 27.
Results
Demographics, handedness, and cognitive status
The healthy control (HC) and the MCI groups did not differ in age, M(SD)HC = 71.0 (6.12), M(SD)MCI = 72.49 (8.73), but the MCI group had fewer years of education, M(SD)MCI = 11.93 (3.98), M(SD)HC = 13.46 (3.07), t(89) = 2.08, p = .041, 95% CI [.07, 3.0]. All participants scored above +9 on the Briggs-Nebes Handedness Inventory (range 12-24) and hand preference scores did not differ significantly between the groups, M(SD)HC = 20.71 (3.24), M(SD)MCI = 21.33 (4.70). Regarding MMSE results, the MCI group had significantly lower score than controls, M(SD) MCI = 25.07 (3.39), M(SD) HC = 29.58 (0.70), t(88) = 9.16, p = <.001, 95% CI [3.52, 5.48].
Neuropsychological assessment results are summarized in Supplementary Table 1. Classification of the MCI group resulted in 17 patients in the amnestic subgroup, 16 in the multiple domain, and 8 in the non-amnestic subgroup.
Group differences in overall dexterity performance and errors
The MCI group spent significantly longer time than the HC group, both on the right-hand task, M(SD)MCI = 29.21(6.04), M(SD)HC = 26.64(5.57), t(89) = 2.57, p = .038, 95% CI [.14, 4.99], and the left-hand task, M(SD)MCI = 31.53(6.99), M(SD)HC = 27.33(5.35), t(89) = 4.20, p = .002, 95% CI [1.63, 6.77]. The MCI group made significantly more errors than controls in the right-hand task, M(SD)MCI = .98(1.21), M(SD)HC = .42(.70), t(89) = 2.60, p = .012, 95% CI [.128, .984], while no differences were observed in the left-hand task, M(SD)MCI = .71(1.03), M(SD)HC = .38(.64), t(89) = 1.78, p = .081.
Group differences in kinematics
presents kinematics of both hands for the four movement types: reaching, grasping, transport, and inserting. The ANOVAs revealed significant effects of Group, Hand, and Group × Hand interactions for all four types of movement. For clarity in presentation of group differences, only main and simple effects that involve Group are summarized in the text; the effects of Hand are presented in Supplementary Table 2. Multivariate effects on kinematics are presented in Supplementary Table 3.
Table 1. Means and Standard Deviations of kinematics in both tasks by type of movement.
Reaching
Main effects of Group were found for angular velocity, F(1,89) = 13.39, p < .001, η2p = .131, CV of angular velocity, F(1,89) = 51.51, p < .001, η2p = .367, and angle, F(1,89) = 11.21, p < .001, η2p = .112. Angular velocity was lower in the MCI group, M = 57.34, SD = 14.47, compared to the HC group, M = 68.50, SD = 14.48. CV of angular velocity was also lower in the MCI group, M(SD)MCI = .51(.10), M(SD)HC = .67(.11). Angle was larger in the MCI group, M(SD)MCI = 44.25(8.22), M(SD)HC = 38.46(8.20).
Group × Hand interactions were found for linear velocity, F(1,89) = 35.98, p < .001, η2p = .288, CV of linear velocity, F(1,89) = 61.23, p < .001, η2p = .408, and path length, F(1,89) = 12.47, p < .001, η2p = .123. For linear velocity, simple effects revealed that the MCI group was faster when reaching with the left hand, M(SD)MCI = 42.12(7.27), M(SD)HC = 35.46(6.00), p < .001, η2p = .238. CV of linear velocity was lower in the MCI group, both when reaching with the right hand, M(SD)MCI = .28(.07), M(SD)HC = .32(.07), p = .009, η2p = .074, and the left, M(SD)MCI = .32(.08), M(SD)HC = .48(.08), p < .001, η2p = .547. Path was shorter in the MCI group, both when reaching with the right hand, M(SD)MCI = 12.72(1.60), M(SD)HC = 13.74(1.59), p = .003, η2p = .094, and the left, M(SD)MCI = 13.58(1.81), M(SD)HC = 15.89(1.80), p < .001, η2p = .291.
Grasping
Main effects of Group were significant for CV of angular velocity, F(1,89) = 13.16, p < .001, η2p = .129, angle, F(1,89) = 18.50, p < .001, η2p = .172, and CV of angle, F(1,89) = 10.39, p = .002, η2p = .105. CV of angular velocity was lower in the MCI group, M = .69, SD = .08, compared to the HC group, M = .75, SD = .08. Angle was larger in the MCI group, M(SD)MCI = 43.13(9.92), M(SD)HC = 34.14(9.92). CV of angle was also larger in the MCI group, M(SD)MCI = .29(.10), M(SD)HC = .22(.11).
Group × Hand interactions were significant for linear velocity, F(1,89) = 11.19, p = .001, η2p = .112, CV of linear velocity, F(1,89) = 34.21, p < .001, η2p = .278, and path length, F(1,89) = 9.03, p = .003, η2p = .092. For linear velocity, simple effects showed that the MCI group was faster but only with the left hand, M(SD)MCI = 8.10(1.74), M(SD)HC = 6.49(1.59), p < .001, η2p = .207. CV of linear velocity was larger in the MCI group when grasping with the left hand, M(SD)MCI = .69(.10), M(SD)HC = .54(.09), p < .001, η2p = .401. Path was longer in the MCI group when grasping with the left hand, M(SD)MCI = 7.6(1.95), M(SD)HC = 5.2(2.72), p < .001, η2p = .277.
Transport
Main effects of Group were found for CV of angular velocity, F(1,89) = 82.36, p < .001, η2p = .481, angle, F(1,89) = 26.23, p < .001, η2p = .228, and CV of angle, F(1,89) = 5.50, p = .021, η2p = .058. CV of angular velocity was lower in the MCI group, M(SD)MCI = .58(.11), M(SD)HC = .79(.11). Angle was larger in the MCI group, M(SD)MCI = 59.86(7.59), M(SD)HC = 51.68(7.58). CV of angle was lower in the MCI group, M(SD)MCI = .10(.04), M(SD)HC = .12(.04).
Group × Hand interactions were found for linear velocity, F(1,89) = 15.39, p < .001, η2p = .147, CV of linear velocity, F(1,89) = 44.03, p < .001, η2p = .331, and path length, F(1,89) = 11.01, p = .001, η2p = .110. For linear velocity, simple effects revealed that the MCI group was slower compared to the HC group, but only with the right hand, M(SD)MCI = 32.01(5.71), M(SD)HC = 34.84(5.71), p = .012, η2p = .058. CV of linear velocity was lower in the MCI group, but only for the left hand, M(SD)MCI = .33(.06), M(SD)HC = .44(.06), p < .001, η2p = .427. Path was shorter in the MCI group, both for the right hand, M(SD)MCI = 9.64(1.37), M(SD)HC = 10.72(1.37), p < .001, η2p = .136, and the left, M(SD)MCI = 10.42(1.88), M(SD)HC = 12.79(1.87), p < .001, η2p = .288.
Inserting
There were significant main effects of Group for CV of angular velocity, F(1,89) = 44.37, p < .001, η2p = .333, angle, F(1,89) = 22.18, p < .001, η2p = .199, and CV of angle, F(1,89) = 5.23, p = .025, η2p = .056. CV of angular velocity was lower in the MCI group, M(SD)MCI = .75(.13), M(SD)HC = .93(.13). Angle was larger in the MCI group, M(SD)MCI = 52.75(8.82), M(SD)HC = 43.99(8.82). CV of angle was larger in the MCI group, M(SD)MCI = .25(.09), M(SD)HC = .21(.09).
Significant Group × Hand interactions were found for linear velocity, F(1,89) = 5.60, p = .020, η2p = .059, CV of linear velocity, F(1,89) = 18.04, p < .001, η2p = .169, path length, F(1,89) = 18.00, p < .001, η2p = .168, and angular velocity, F(1,89) = 7.39, p = .008, η2p = .077. Simple effects of Group were nonsignificant for linear velocity. CV of linear velocity was larger in the MCI group but only for the left hand, M(SD)MCI = .74(.08), M(SD)HC = .63(.08), p < .001, η2p = .298. Path was longer in the MCI group when inserting with the left hand, M(SD)MCI = 8.34(2.20), M(SD)HC = 55.48(2.19), p < .001, η2p = .291. Simple effects of Group for angular velocity were nonsignificant.
In summary, kinematic results revealed distinctive patterns of group differences for the actions of reaching and transport, and the actions of grasping and inserting, respectively. Specifically, during reaching and transport the MCI group had shorter path and lower variability in linear and angular velocities, suggesting better gross dexterity compared to the HC group. Conversely, during grasping and inserting, the MCI group consistently had longer path and higher variability in linear velocity, suggesting worse fine dexterity compared to the HC group. These differences were more pronounced in left-hand movements.
Association between kinematics and errors
Right hand task
In the MCI group, greater number of errors was significantly correlated with lower CV of angular velocity during grasping, r(41) = −.32, p = .041, and transport, r(41) = −.32, p = .041, and with higher CV of angle during inserting, r(41) = .38, p = .014. In the HC group, greater number of errors in this task was significantly associated with higher linear velocity during reaching, r(50) = .33, p = .019, and transport, r(50) = .38, p = .006, and with longer path during reaching, r(50) = .33, p = .018.
Left hand task
In the MCI group, greater number of errors correlated with higher linear velocity during reaching, r(41) = .40, p = .009, and transport, r(41) = .39, p = .010, lower CV of angular velocity during transport, r(41) = -.31, p = .046, and higher CV of angle during inserting, r(41) = .43, p = .005. In the HC group, greater number of errors was associated with longer path during grasping, r(50) = .38, p = .007, lower CV of linear velocity, r(50) = −.28, p = .047, and lower CV of angular velocity, r(50) = −.29, p = .043, during transport.
Overall, the correlation analysis indicated that the kinematic pattern found in the MCI group was associated with greater number of errors and, thus, with less accurate performance of the tasks.
Group differences in laterality indices
LIs for all kinematics are summarized in and portrayed in and . Positive LI values represent higher linear and angular velocity, longer path, larger angle, and more variability in right-hand movements compared to the left-hand movements; negative LI values represent the correspondent pattern of outcomes for left-hand. The absolute value represents the extent of symmetry in movement between the two hands, and values proximal to zero indicate more symmetrical movements.
Figure 1. Note. Mean ± SE laterality indices for kinematic variables during Reaching (A) and Transport (B) showing significant group differences. HC = healthy controls. MCI = mild cognitive impairment. LinV = linear velocity (cm/s). PL = path length (cm). AngV = angular velocity (°/s). CV = coefficient of variation. p < 0.01**; p < 0.001***.
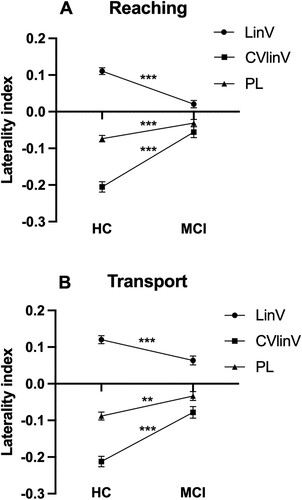
Figure 2. Note. Mean ± SE laterality indices for kinematic variables during Grasping (A) and Inserting (B) showing significant group differences. HC = healthy controls. MCI = mild cognitive impairment. LinV = linear velocity. PL = path length. AngV = angular velocity. CV = coefficient of variation. p < .05*; p < 0.01**; p < 0.001***.
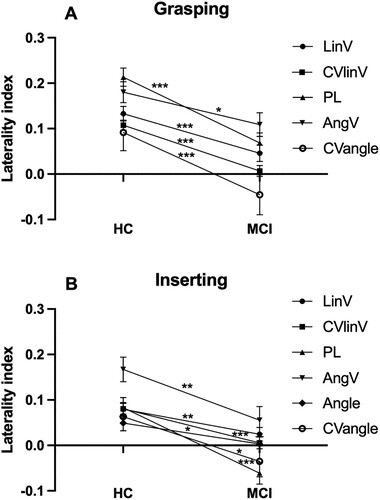
Table 2. Group differences in laterality indices of kinematics.
The series of MANOVAs revealed that absolute values of LIs were consistently closer to zero in the MCI group compared to the HC group (see ). This was true for linear velocity, CV of linear velocity, and path length in all four types of movements. Specifically, LIs of angular velocity and CV of angle were closer to zero in the MCI group during grasping and inserting, as well as LIs of angle during inserting (see ). However, symmetric outcomes were also evident during reaching and transport, which strongly contrasts with LIs from controls who presented clearly asymmetric results (see ). Thus, the LI analysis revealed that most kinematics with group differences also were more similar between the two hands in the MCI group. Speed and trajectory of movement of the two hands were more symmetrical in the MCI group compared to the HC group during all four types of movements, and, additionally, rotation and position of the hands were more symmetrical in the MCI group compared to the HC group during grasping and inserting.
Group differences in correlations between right–left kinematic values
The comparisons between correlations of right–left outcomes for each group showed group differences for three kinematics in transport of pins and for path length in reaching and grasping (see ). Mainly, there were stronger significant correlations in the MCI group than in the HC group for transport regarding angular velocity, CV of angular velocity, and CV of angle. These variables together with path length during reaching singled-out the strongest symmetric variables in the patient group. However, one peculiar difference in R–L correlations was found for path length during grasping, where the outcome showed a stronger correlation for the HC group compared to the MCI group.
Table 3. Bivariate correlations between right–left hand kinematic outcomes and comparisons by group.
Discussion
The detailed kinematic assessment showed significant differences between MCI patients and controls in several variables across the four actions analyzed. Interestingly, the majority of the significant findings point to stronger kinematic outcomes of the non-preferred hand in patients compared to controls during reaching and transport of pins, which primarily involve arm movements. Considering only these results, it would be tempting to assert that the MCI group displays a better upper-limb ability than controls. However, results from grasping and inserting, which primarily rely on movements of fingers and hands, indicate impaired fine motor function in patients compared to controls. This observation is strengthened by the higher commission of errors in the MCI group. Even though, errors occurred across all four actions, their incidence (dropping pins or picking various pins) relied on inappropriate movements of fingers. Thus, the kinematic and error data conjointly show that patients display a general deterioration in fine motor functions together with improved gross motor ability, notably on the non-preferred upper-limb. The deterioration in fine dexterity is in line with MCI/dementia studies reporting diminished ability of finger movements and wrist rotation (Yan et al., Citation2008) as well as higher error commission (e.g., Carment et al., Citation2018). Notwithstanding, improved motor function in MCI has, to our knowledge, only been reported once (Kubicki et al., Citation2016). In this study, anticipated motor command and activation of trunk muscles associated to arm-lifting was observed in MCIs, specifically concerning muscles of the left side. This piece of evidence coincides with the ameliorated non-preferred upper-limb ability displayed by our MCI participants.
Other data relevant to our findings have been reported by Van Deun et al. (Citation2019). These authors reported a reduction in preferred hand superiority in patients with moderate and mild degrees of AD during execution of the PPT (standard version). These patients presented equal dexterity in both hands, which agrees with our laterality indices (LI) in which values closely approach zero. In fact, the LI results indicate a symmetric unimanual performance in MCI patients between preferred and non-preferred hands in practically all movements. The most symmetric outcomes were observed during reaching and transport, i.e., gross dexterity, but also on several kinematics of inserting and grasping, i.e., fine dexterity. In an effort to identify which of the kinematic variables best single-out the MCI group, we compared correlations of right- and left-hand results for each kinematic variable between MCI and controls. Results showed that kinematic features related to angle and angular velocity during transport and path length during reaching were discernibly more symmetrical in patients. At this regard, a recent study evaluating reduced incidence of non-right handers among AD patients suggests that some of these individuals might “lose hand dominance as the disease progress” (Ryan et al., Citation2020). Our data and various other studies reporting reduced dexterity in the preferred hand of AD patients (Sakamoto et al., Citation2006, Citation2007; Yamada et al., Citation2003) provide good support for this hypothesis and the reduction of manual asymmetries across the different stages of cognitive decline associated to dementia.
In sum, the present study reveals two main findings. First, we found a differentiation on ability of upper-limb movements in MCI patients in which fine dexterity expressed by finger and hand movements during grasping and inserting was compromised, while gross dexterity related to arm action in reaching and transport of pins was superior to controls. The decline in fine motor function in AD and MCI has long been acknowledged (de Paula et al., Citation2016), and it is explained by degeneration of integrative cerebral networks vital for cognition and motricity (Liou et al., Citation2020), as well as by the emergence of paratonia (Van Deun et al., Citation2019). In contrast, ameliorated gross dexterity among MCI patients is a novel finding, difficult to interpret. Still, in line with Kubicki et al. (Citation2016), we put forward the hypothesis that such phenomenon occurs as a compensation for impaired fine dexterity of the hand. Fine motor control requires higher mental abilities that deteriorate rapidly and at a more important extent in early stages of dementia (Ocklenburg, Citation2017). In this context, the ability to control musculature underlying gross motor abilities seems to be preserved and even enhanced to balance up for fine manual deterioration.
Similar findings to those obtained in our study have also been reported by Kluger et al. (Citation1997). These authors found deteriorated fine motor functions and preserved gross motor skills in MCI individuals, and they proposed that the reason for the decline in fine motor functions was related to higher involvement of cortical and subcortical networks. In fact, more recent studies have equally proposed that some of the neural mechanisms possibly underlying dexterity decline in MCI are due to disconnection of the fronto-parietal pathway (Camarda et al., Citation2007; Koppelmans et al., Citation2022), and degeneration in the dopaminergic system (Reeves et al., Citation2010). The presence of white matter hyperintensities has also been related to dexterity decline in various older populations (Riaz et al., Citation2020) and thus, it might be a potential factor implicated in degeneration of hand function in MCI. Still, no research has been conducted on the subject yet. Moreover, dexterity is tightly related to visuomotor coordination, which becomes impaired in AD (Verheij et al., Citation2012), and accordingly the deterioration of networks supporting visuomotor integration needs to be emphasized. Various studies in both AD and prodromal stages of the disease suggest that the posterior parietal cortex (Ilardi et al., Citation2022) and the cerebellum (Toniolo et al., Citation2018) deteriorate in the dementia continuum. Currently, sparse data exist about the neurodegeneration of brain systems subserving visuomotor action in MCI, but it is highly probable that these systems are involved in dexterity deterioration at this stage of neurodegeneration and additional research is warranted.
The second and more striking finding is the reduction of asymmetry in MCI patients. We demonstrated more symmetric movements in this group, reflected by equal kinematic outcomes between hands in several variables. To the best of our knowledge, only Van Deun et al. (Citation2019) have reported symmetric hand movements in AD, remarkably also during execution of the PPT. This finding can be interpreted as either a decline of the preferred hand, a developed superiority of non-preferred hand or both. However, based on the current cross-sectional data, we cannot reach conclusions and only a longitudinal follow-up assessment of dexterity performance in MCI patients may shed light into this issue. As for the possible neural mechanisms behind the reduction of manual asymmetry, it is hypothesized that in degenerative diseases one brain hemisphere is more vulnerable to pathology (Uitti et al., Citation2005), and in AD, the left hemisphere seems to be most vulnerable to neurodegeneration, which would explain the decline of the preferred hand (Lubben et al., Citation2021). At this regard, the review by Lubben et al. (Citation2021) provides a timely overview of the current hypotheses explaining the existence of asymmetric brain deterioration in neurodegenerative diseases. Essentially, the review suggests that epigenetic factors involved early in the development of normal brain asymmetries predispose one hemisphere of the brain to higher risk for neurodegeneration. Later in life, additional asymmetries present on peripheral systems of the body (e.g., vagal nerve, blood-CSF barrier), might be responsible for allowing unilateral entrance of environmental triggers, such as infections, that promote asymmetric brain degeneration.
Limitations and strengths
Some limitations of the study need to be acknowledged. The first one refers to the exclusion of left-handed participants from the study. In order to reach conclusions about the declines on hand function in MCI, all types of individuals should be included. Notwithstanding, since the present study is part of a broader research project on motor functions and cognition in aging, the decision of focusing on right-handers was convenient as it helped to minimize heterogeneity in older adults. Future investigations are strongly advised to evaluate hand motor functions as those assessed in our study on both right- and left-handed participants. A second potential limitation regards the fix order in which the PPT was conducted. Because we wished to respect the standard version of the task, we conducted the unimanual assessment accordingly. That is, the right hand was first tested and then the left hand. Thus, it is possible that some training effect occurred due to this fixed order from one hand to the other. As we observed better dexterity performance on the non-preferred hand, the issue of possible learning effects associated to the tasks’ fixed order should be addressed in future investigations. Finally, we should mention the limitation related to the sample size of our groups, especially that of the patients. The number of participants included in our samples can be considered as relatively small and thus, results cannot be generalized to the entire MCI population. Notwithstanding, we also need to highlight that the composition of our patient group is clinically trustworthy as these participants were recruited and referred from the University Hospital with a clinical diagnosis of MCI. In addition, patients were categorized according to strict neuropsychological criteria, which contrasts with many aging studies in which older adults are recruited from the community and considered as individuals with MCI based on simple cognitive measures. Thus, in spite of the relative small sample size, we regard our patient group as properly classified. Moreover, the type of collected data relies on precise methodology, which in turn provides objective data of unimanual hand function. Therefore, we are certain that our study contributes with new and worthy evidence of the changes and declines occurring on the upper-extremities of older adults with MCI.
Conclusion
Collectively, the overall findings of this investigation diverge from recommendations of the CCDTD5 (Montero-Odasso et al., Citation2020) that discard hand function as a non-cognitive marker of dementia. Our data together with several recent studies (Liou et al., Citation2020; de Paula et al., Citation2016; Darweesh et al., Citation2017; Suzumura et al., Citation2016; Beeri et al., Citation2021) suggest the contrary and all of them highlight a nonnegligible deterioration in dexterity at different levels of the dementia continuum. Such deterioration is crucial for ADL functioning (de Paula et al., Citation2016), which is a decisive aspect in the diagnosis of dementia. In conclusion, this investigation demonstrates that important psychomotor changes occur in hand function in right-handed individuals with an MCI diagnosis. These findings need to be further corroborated in longitudinal research and with left-handed participants on concrete MCI subtypes to assert whether deficits in hand function displayed during execution of the PPT can be utilized as clinical markers of early dementia and even define MCI subtypes. Likewise, complementary research looking at neurobiological factors underlying dexterity decline and reduction of manual asymmetries in MCI is warranted.
Author contributions
PI, conception, design of the study and analyses: CRA; design, processing and analyses: OV; recruitment and logistics: CRA, MMG, KW; interpretation of data: OV, CRA; drafting the article: OV, CRA; critical revision of the article: OV, CRA, MMG, KW.
Supplementary_Laterality.docx
Download MS Word (18.3 KB)Acknowledgments
We thank the Departments of Geriatrics and Neurology at the University Hospital of North Norway for help with the recruitment of the patients. Special thanks to chief geriatricians Elena Kamycheva and Gunhild Ag for their support in this research. We also thank Maja Bergman, Heidi Almhaug, Amie-Christine Evjen and Barbro Abrahamsen for help with data collection and data processing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets for this article cannot be made publicly available due to restrictions of the Norwegian Research Ethics Committee. However, upon reasonable request, summary statistics can be obtained from the corresponding author (CRA).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Arvanitakis, Z., Shah, R. C., & Bennett, D. A. (2019). Diagnosis and management of dementia: Review. JAMA-Journal of the American Medical Association, 322(16), 1589–1599. https://doi.org/10.1001/jama.2019.4782.
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the beck depression inventory-II. Psychological Corporation.
- Beeri, M. S., Leurgans, S. E., Bennett, D. A., Barnes, L. L., & Buchman, A. S. (2021). Diverse motor performances are related to incident cognitive impairment in community-dwelling older adults. Frontiers in Aging Neuroscience, 13. https://doi.org/10.3389/fnagi.2021.717139.
- Benson, S. S. M., Williams, N., Tucker, J., & Bryden, P. J. (2021). How far will you go before switching hands? Handedness on the long pegboard across the lifespan. Developmental Psychobiology, 63(5), 1109–1119. https://doi.org/10.1002/dev.22112
- Benton, A. L. (1967). Problems of test construction in the field of aphasia. Cortex, 3(1), 32–58. https://doi.org/10.1016/S0010-9452(67)80005-4
- Bondi, M. W., Edmonds, E. C., Jak, A. J., Clark, L. R., Delano-Wood, L., McDonald, C. R., Nation, D. A., Libon, D. J., Au, R., Galasko, D., & Salmon, D. P. (2014). Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimers Disease, 42(1), 275–289. https://doi.org/10.3233/JAD-140276
- Briggs, G. G., & Nebes, R. D. (1975). Patterns of hand preference in a student population. Cortex, 11(3), 230–238. https://doi.org/10.1016/S0010-9452(75)80005-0
- Camarda, R., Camarda, C., Monastero, R., Grimaldi, S., Camarda, L. K. C., Pipia, C., Caltagirone, C., & Gangitano, M. (2007). Movements execution in amnestic mild cognitive impairment and Alzheimer's disease. Behavioural Neurology, 18(3), 135–142. https://doi.org/10.1155/2007/845914
- Carment, L., Abdellatif, A., Lafuente-Lafuente, C., Pariel, S., Maier, M. A., Belmin, J., … Lindberg, P. G. (2018). Manual dexterity and aging: A pilot study disentangling sensorimotor from cognitive decline. Frontiers in Neurology, 9. https://doi.org/10.3389/fneur.2018.00910
- Chen, H. F., Sheng, X. N., Qin, R. M., Luo, C. M., Li, M. C., Liu, R. Y., Zhang, B., Xu, Y., Zhao, H., & Bai, F. (2020). Aberrant white matter microstructure as a potential diagnostic marker in Alzheimer's disease by automated fiber quantification. Frontiers in Neuroscience, 14. https://doi.org/10.3389/fnins.2020.570123.
- Cherbuin, N., Sachdev, P. S., & Anstey, K. J. (2011). Mixed handedness is associated with greater age-related decline in volumes of the hippocampus and amygdala: The PATH through life study. Brain and Behavior, 1(2), 125–134. https://doi.org/10.1002/brb3.24
- Darweesh, S. K. L., Wolters, F. J., Hofman, A., Stricker, B. H., Koudstaal, P. J., & Ikram, M. A. (2017). Simple test of manual dexterity can help to identify persons at high risk for neurodegenerative diseases in the community. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(1), 75–81. https://doi.org/10.1093/gerona/glw122
- de Paula, J. J., Albuquerque, M. R., Lage, G. M., Bicalho, M. A., Romano-Silva, M. A., & Malloy-Diniz, L. F. (2016). Impairment of fine motor dexterity in mild cognitive impairment and Alzheimer's disease dementia: Association with activities of daily living. Revista Brasileira de Psiquiatria, 38(3), 235–238. https://doi.org/10.1590/1516-4446-2015-1874
- Folstein, M. F., Folstein, S. F., & McHugh, P. R. (1975). Mini mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
- Francis, K. L., & Spirduso, W. W. (2000). Age differences in the expression of manual asymmetry. Experimental Aging Research, 26(2), 169–180. https://doi.org/10.1080/036107300243632
- Fritz, N. E., Kegelmeyer, D. A., Kloos, A. D., Linder, S., Park, A., Kataki, M., Adeli, A., Agrawal, P., Scharre, D. W., & Kostyk, S. K. (2016). Motor performance differentiates individuals with Lewy body dementia, Parkinson's and Alzheimer's disease. Gait & Posture, 50, 1–7. https://doi.org/10.1016/j.gaitpost.2016.08.009
- Golden, C J. (1978). Stroop color and word test. A manual for clinical and experimental uses. Wood Dale, IL: Stoelting.
- Gunturkun, O., Strockens, F., & Ocklenburg, S. (2020). Brain lateralization: A comparative perspective. Physiological Reviews, 100(3), 1019–1063. https://doi.org/10.1152/physrev.00006.2019
- Ilardi, C. R., Chieffi, S., Iachini, T., & Iavarone, A. (2022). Neuropsychology of posteromedial parietal cortex and conversion factors from mild cognitive impairment to Alzheimer’s disease: Systematic search and state-of-the-art review. Aging Clinical and Experimental Research, 34(2), 289–307. https://doi.org/10.1007/s40520-021-01930-y
- Ilardi, C. R., Iavarone, A., La Marra, M., Iachini, T., & Chieffi, S. (2022). Hand movements in mild cognitive impairment: Clinical implications and insights for future research. Journal of Integrative Neuroscience, 21(2), 067. https://doi.org/10.31083/j.jin2102067.
- Jak, A. J., Bondi, M. W., Delano-Wood, L., Wierenga, C., Corey-Bloom, J., Salmon, D. P., & Delis, D. C. (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry, 17(5), 368–375. https://doi.org/10.1097/jgp.0b013e31819431d5.
- Kluger, A., Gianutsos, J. G., Golomb, J., Ferris, S. H., George, A. E., Franssen, E., & Reisberg, B. (1997). Patterns of motor impairment in normal aging, mild cognitive decline, and early Alzheimer's disease. The Journals of Gerontology: Psychological Sciences, 52B(1), P28–P39. https://doi.org/10.1093/geronb/52B.1.P28
- Koppelmans, V., Silvester, B., & Duff, K. (2022). Neural mechanisms of motor dysfunction in mild cognitive impairment and Alzheimer's disease: A systematic review. Journal of Alzheimer's Disease Reports, 6(1), 307–344. https://doi.org/10.3233/ADR-210065
- Kubicki, A., Fautrelle, L., Bourrelier, J., Rouaud, O., & Mourey, F. (2016). The early indicators of functional decrease in mild cognitive impairment. Frontiers in Aging Neuroscience, 8. https://doi.org/10.3389/fnagi.2016.00193
- Liou, W. C., Chan, L., Hong, C.-T., Chi, W.-C., Yen, C.-F., Liao, H.-F., … Liou, T.-H. (2020). Hand fine motor skill disability correlates with dementia severity. Archives of Gerontology and Geriatrics, 90, 104168. https://doi.org/10.1016/j.archger.2020.104168.
- Lubben, N., Ensink, E., Coetzee, G. A., & Labrie, V. (2021). The enigma and implications of brain hemispheric asymmetry in neurodegenerative diseases. Brain Communications, 3(3). https://doi.org/10.1093/braincomms/fcab211
- Montero-Odasso, M., Pieruccini-Faria, F., Ismail, Z., Li, K., Lim, A., Phillips, N., … Camicioli, R. (2020). CCCDTD5 recommendations on early non cognitive markers of dementia: A Canadian consensus. Alzheimers & Dementia-Translational Research & Clinical Interventions, 6(1). https://doi.org/10.1002/trc2.12068.
- Newcombe, F. (1969). Missile wounds of the brain: A study of psychological deficits. Oxford University Press.
- Ocklenburg, S. (2017). Tachistoscopic viewing and dichotic listening. In L. Rogers, & G. Vallortigada (Eds.), Lateralized brain functions (pp. 3–28). Humana Press. https://doi.org/10.1007/978-1-4939-6725-4_1.
- Papadatou-Pastou, M. (2018). Handedness and cognitive ability: Using meta-analysis to make sense of the data. In G. S. Forrester, W. D. Hopkins, K. Hudry, & A. Lindell (Eds.), Cerebral lateralization and cognition: Evolutionary and developmental investigations of behavioral biases (pp. 179–206). Academic Press. https://doi.org/10.1016/bs.pbr.2018.06.008.
- Reeves, S., Mehta, M., Howard, R., Grasby, P., & Brown, R. (2010). The dopaminergic basis of cognitive and motor performance in Alzheimer's disease. Neurobiology of Disease, 37(2), 477–482. https://doi.org/10.1016/j.nbd.2009.11.005
- Reitan, R. M., & Wolfson, D. (1993). The halstead-reitan neuropsychological test battery: Theory and clinical interpretation. Neuropsychology Press.
- Riaz, M., Vangberg, T.R., Vasylenko, O., Castro-Chavira, S., Gorecka, M.M., Waterloo, K., Rodríguez-Aranda, C. (2020). What does hand motor function tell us about our aging brain in association with WMH? Aging Clinical and Experimental Research, 33(6), 1577–1584. https://doi.org/10.1007/s40520-020-01683-0.
- Riggio, F., Nuovo, S., & Zappala, G. (2020). Aging, cognitive decline, and manual preference: Descriptive and correlational analyses. Life Span and Disability, 23(2), 283–302.
- Ryan, J. J., Kreiner, D. S., & Paolo, A. M. (2020). Handedness of healthy elderly and patients with Alzheimer's disease. International Journal of Neuroscience, 130(9), 875–883. https://doi.org/10.1080/00207454.2019.1707824.
- Sakamoto, M., Kikuchi, E., & Shigeta, M. (2006). The levels of recognition and hand function of patients with Alzheimer's disease: A pilot study based on the simple test for evaluating hand function. Japanese Journal of Geriatrics, 43, 612–621. https://doi.org/10.3143/geriatrics.43.616.
- Sakamoto, M., Kikuchi, E., & Shigeta, M. (2007). Relationship between hand dexterity and severity of dementia in Alzheimer's disease: Changes in handedness superiority in the course of progression. Japanese Journal of Rehabilitation Medical, 44, 391–397. https://doi.org/10.2490/jjrmc.44.391.
- Seltzer, B., Burres, M. J. K., & Sherwin, I. (1984). Left-handedness in early and late onset dementia. Neurology, 34(3), 367–369. https://doi.org/10.1212/WNL.34.3.367
- Shulman, K. I. (2000). Clock-drawing: Is it the ideal cognitive screening test? International Journal of Geriatric Psychiatry, 15(6), 548–561. https://doi.org/10.1002/1099-1166(200006)15:6<548::AID-GPS242>3.0.CO;2-U
- Suzumura, S., Osawa, A., Nagahama, T., Kondo, I., Sano, Y., & Kandori, A. (2016). Assessment of finger motor skills in individuals with mild cognitive impairment and patients with Alzheimer's disease: Relationship between finger-to-thumb tapping and cognitive function. Japanese Journal of Comprehensive Rehabilitation Science, 7(0), 19–28. https://doi.org/10.11336/jjcrs.7.19
- Tamaru, Y., Tanaka, H., Ueda, M., Sumino, H., Imaoka, M., Matsugi, A., Nishikawa, T., Ishii, R., & Naito, Y. (2020). Effect of Alzheimer's disease severity on upper limb function. Psychogeriatrics, 20(5), 802–804. https://doi.org/10.1111/psyg.12585
- Teixeira, L. A. (2008). Categories of manual asymmetry and their variation with advancing age. Cortex, 44(6), 707–716. https://doi.org/10.1016/j.cortex.2006.10.002
- Toniolo, S., Serra, L., Olivito, G., Marra, C., Bozzali, M., & Cercignani, M. (2018). Patterns of cerebellar gray matter atrophy across alzheimers disease progression. Frontiers in Cellular Neuroscience, 12, https://doi.org/10.3389/fncel.2018.00430
- Turner, M. R., Wicks, P., Brownstein, C. A., Massagli, M. P., Toronjo, M., Talbot, K., & Al-Chalabi, A. (2011). Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. Journal of Neurology Neurosurgery and Psychiatry, 82(8), 853–854. https://doi.org/10.1136/jnnp.2010.208413
- Uitti, R. J., Baba, Y., Whaley, N. R., Wszolek, Z. K., & Putzke, J. D. (2005). Parkinson disease – handedness predicts asymmetry. Neurology, 64(11), 1925–1930. https://doi.org/10.1212/01.WNL.0000163993.82388.C8
- Van Deun, B., Van Den Noortgate, N., Van Bladel, A., Palmans, T., & Cambier, D. (2019). The impact of paratonia on fine and gross motor function in older adults With mild and moderate dementia. Alzheimer Disease & Associated Disorders, 33(1), 54–61. https://doi.org/10.1097/WAD.0000000000000278
- Vasylenko, O., Gorecka, M. M., & Rodriguez-Aranda, C. (2018). Manual dexterity in young and healthy older adults. 1. Age- and gender-related differences in unimanual and bimanual performance. Developmental Psychobiology, 60(4), 407–427. https://doi.org/10.1002/dev.21619
- Verheij, S., Muilwijk, D., Pel, J. J. M., van der Cammen, T. J. M., Mattace-Raso, F. U. S., &, van der Steen, J. (2012). Visuomotor impairment in early-stage Alzheimer's disease: Changes in relative timing of eye and hand movements. Journal of Alzheimers Disease, 30(1), 131–143. https://doi.org/10.3233/JAD-2012-111883
- Wechsler, D. (2008). Wechsler adult intelligent scale – fourth edition (WAIS-IV). Psychological Corporation.
- Wechsler, D. A. (1997). Wechsler memory scale-III. Psychological Corporation.
- Yamada, T., Yamada, K., & Yamaguchi, M. (2003). Study on healthy elderly people and people with Alzheimer's dementia by using subtest on simple test for evaluation of hand function. Health Care, 45, 921–927.
- Yan, J. H., Rountree, S., Massman, P., Doody, R. S., & Li, H. (2008). Alzheimer's disease and mild cognitive impairment deteriorate fine movement control. Journal of Psychiatric Research, 42(14), 1203–1212. https://doi.org/10.1016/j.jpsychires.2008.01.006.
