?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Meta-analyses have shown subtle, group-level asymmetries of spatial attention in adults favouring the left hemispace (pseudoneglect). However, no meta-analysis has synthesized data on children. We performed a random-effects meta-analysis of spatial biases in children aged ≤16 years. Databases (PsycINFO, Web of Science & Scopus) and pre-print servers (bioRxiv, medRxiv & PsyArXiv) were searched for studies involving typically developing children with a mean age of ≤16, who were tested using line bisection. Thirty-three datasets, from 31 studies, involving 2101 children, were included. No bias was identified overall, but there was a small leftward bias in a subgroup where all children were aged ≤16. Moderator analysis found symmetrical neglect, with right-handed actions resulting in right-biased bisections, and left-handed actions in left-biased bisections. Bisections were more leftward in studies with a higher percentage of boys relative to girls. Mean age, hand preference, and control group status did not moderate biases, and there was no difference between children aged ≤7 and ≥7 years, although the number of studies in each moderator analysis was small. There was no evidence of small study bias. We conclude that pseudoneglect may be present in children but is dependent on individual characteristics (sex) and/or task demands (hand used).
Registration: Open Science Framework (https://osf.io/n68fz/)
Introduction
Pseudoneglect is characterized by a small, but consistent, lateralized asymmetry of visuospatial attention, where space and objects on the left-hand side are preferred, or overestimated, in magnitude, relative to those on the right (Bowers & Heilman, Citation1980). Leftward biases are typically attributed to a right hemispheric dominance for spatial attention, particularly within the parieto-occipital cortex, which manifests in this attentional preference for the contralateral left hemispace (Çiçek et al., Citation2009; Fink et al., Citation2000; Foxe et al., Citation2003; Zago et al., Citation2017).
The majority of studies investigating pseudoneglect have tested healthy younger adults, usually aged 18-50, but changes in the direction and magnitude of lateralized biases are also commonly reported in children and in older adults. Specifically, older adults have variously been reported to have a reduced leftward bias relative to young adults, or a bias that is reversed, whereby the right hemispace is favoured, possibly due to changes in hemispheric lateralization in older age. These scenarios have been explained by different theoretical models, such as the hemispheric asymmetry reduction in older adults (HAROLD) model (Cabeza, Citation2002), where activity is generally less lateralized for cognitive functions in older people relative to young adults. This model could explain findings of a reduced or eliminated pseudoneglect bias. A second theory, which would accommodate a shift into the right hemispace (i.e., reversed pseudoneglect) is the right hemi-aging model, where the right hemisphere experiences age-related decline prior to (or to a greater extent than) the left hemisphere (Goldstein & Shelly, Citation1981). The extent to which these models are relevant to spatial attention remains unclear (Mańkowska et al., Citation2020). In any case, our recent meta-analysis of line bisection and landmark task performance in healthy adults aged over 50 indicates that a small group-level leftward bias may remain present in older age (Learmonth & Papadatou-Pastou, Citation2021).
In children, leftward line bisection biases are often observed from around 5-7 years of age (Bradshaw et al., Citation1988; De Agostini et al., Citation1999; Failla et al., Citation2003; Hoyos et al., Citation2020), but there is little evidence of a bias to either side of space in children younger than this (Girelli et al., Citation2017; Patro et al., Citation2018). Patro et al. (Citation2018) aimed to identify the age at which spatial bias emerges by testing children aged 3-7 on a horizontal line bisection task. No bias was identified in 3-4-year-olds, but a leftward bias emerged in 5-6-year-olds. Similarly, Girelli et al. (Citation2017) tested 3-, 5-, and 8-year-olds, who bisected lines, words, and strings of geometric figures. No line bisection bias was identified in 3-year-olds, with only a trend towards the left in 5-year-olds, although both groups bisected words and figures to the right. The leftward line bisection bias only emerged consistently in the 8-year-olds. The emergence of pseudoneglect during childhood is therefore likely to be a result of an interplay of factors, such as the development of low-level perceptual and attentional systems during this period, in conjunction with improvements in the dexterity and motor co-ordination that is required to bisect a horizontal line (Girelli et al., Citation2017). These findings also serve to highlight that variations in experimental methodology and stimulus features can influence the direction of the spatial bias observed in the line bisection task.
In very young children, line bisections that are performed using the left hand tend to produce leftward biases and right-handed bisections tend to produce rightward biases (“symmetrical neglect”; Bradshaw et al., Citation1987, Citation1988; Hausmann et al., Citation2003). Symmetrical neglect is generally eliminated in older children and adults, with more consistent leftward biases produced as a result of both left- and right-handed bisections (although left-handed bisections give rise to marginally larger leftward biases than right) (Jewell & McCourt, Citation2000). The gradual maturation and myelination of the corpus callosum during childhood has been touted as an explanation for this phenomenon, a structure which promotes the integration of perceptual information across the left and right cerebral hemispheres (Pulsipher et al., Citation2009). In younger children, the left hemisphere directs attention towards the right visual field and the right hemisphere to the left visual field (Hausmann et al., Citation2003). In adults and older children, the mature corpus callosum facilitates the transfer of visual information predominately towards the right hemisphere, leading to the observed leftward pseudoneglect bias (Yazgan et al., Citation1995). Pulsipher et al. (Citation2009) found no association between callosal volume (as a proxy for myelination) and line bisection bias in older children and adolescents aged 8-18, however structural imaging in children younger than this, coinciding with the critical period of emergence of pseudoneglect at 5-7 years old, is lacking.
Finally, the development of pseudoneglect in later childhood may also, at least partially, be related to the development of reading skills during this period of childhood, and to cultural factors, such as reading direction (Chokron & De Agostini, Citation1995). In particular, the requirement to initially attend to the left side of a word or sentence could be a driving factor in the development of leftward biases in native left-to-right reading children (Hoyos et al., Citation2020; Girelli et al., Citation2017). Nevertheless, it is important to quantify the overall effect size that should be expected in children and identify whether biases are susceptible to the influence of moderator variables, such as the sex and hand preference of the child and the hand that was used to bisect the line. This is particularly important because typically developing children are often used as a comparison group against which the presence of spatial attention dysfunctions in neurodevelopmental conditions, such as ADHD (Boles et al., Citation2009; Rolfe et al., Citation2006, Citation2008) and dyslexia (Michel et al., Citation2011; Sireteanu et al., Citation2005), are detected.
Apart from our own recent meta-analysis of spatial attention in older adults (Learmonth & Papadatou-Pastou, Citation2021), the only other meta-analysis of pseudoneglect that has been performed to date identified that young participants generally exhibit a stronger leftward bias compared to older people (Jewell & McCourt, Citation2000). However, in this meta-analysis young people were classed as those aged <40 years old (likely due to the fact that very few studies of pseudoneglect in children were available at the time) and children or teenagers were not analysed separately. Here, we present a comprehensive, updated meta-analysis of the line bisection task in children who are under 16 years of age. We also assess the influence of key moderator variables on spatial attention bias in children and adolescents and finally, identify whether small study publication bias may be present in the wider literature.
Methods
Studies were identified from literature searches using the PsycInfo, Web of Science, and Scopus electronic databases, from database inception until 8th March 2021. The search terms (“landmark task” OR “line bisection”) AND (“children” OR “child”) were used, targeting all fields. Searches were also performed in bioRxiv, medRxiv, and PsyArXiv to identify unpublished data. After removing duplicates, the abstracts of 1626 records were screened independently by 2 researchers (DK and GL), using the inclusion and exclusion criteria listed below, and any disagreements were resolved through discussion. The full texts of 59 articles were checked for eligibility and the data were extracted independently by the same 2 researchers, again with disagreements resolved through discussion. Additional information was obtained for 2 studies via email correspondence, where there was insufficient information to extract the required data from the paper (Crollen et al., Citation2015; Waldie & Hausmann, Citation2010). A total of 31 articles (comprising 33 datasets) were included in the meta-analysis. Corresponding authors were contacted to provide data for 8 additional studies, but the data were either unavailable, the authors did not respond to requests, or the contact details were invalid (Bradshaw et al., Citation1987, Citation1988; de Hevia & Spelke, Citation2009; Fagard & Dahmen, Citation2003; Jiang et al., Citation2008; Saj et al., Citation2020; Sampaio et al., Citation1995; Vieira et al., Citation2013). The PRISMA guidelines (Page et al., Citation2021) for reporting meta-analyses and systematic reviews were followed. A schematic of the search strategy is presented in .
Figure 1. Flow diagram documenting the electronic database searches, abstract screening, and the reasons for excluding studies. Adapted from Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement (Moher et al., Citation2009).
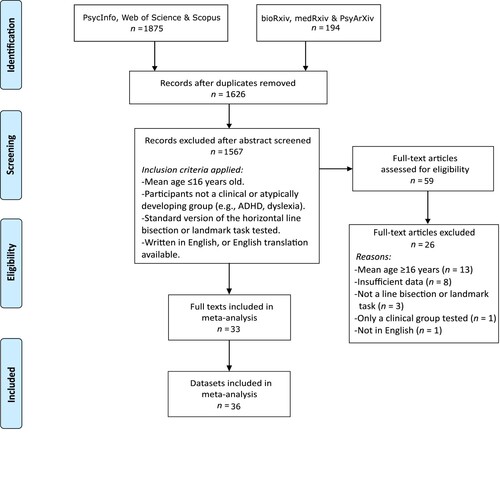
Study selection
The inclusion criteria were as follows:
1) Age: Studies were included if the participant sample consisted of children with a mean age of ≤16 years old. If no age range was reported, but the mean age was ≤16 years, the study was included. Four studies reported data on some participants whose age was >16 yrs. (maximum age = 17 years, Polikoff et al., Citation1995; maximum age = 19 years, Dennis et al., Citation2005; Ickx et al., Citation2017; Richter et al., Citation2005), but the mean age was reported as ≤16 and the studies were therefore included in the meta-analysis. Although Ickx et al. (Citation2017) included some participants aged ≥16, separate means and standard deviations were available for participants at each year of age and this information was used to calculate an effect size for the participants who were aged only ≤16.
2) Clinical status: Studies were included if the children were presumed to be typically developing and/or had not been specifically recruited as having a developmental issue. We included studies if the children formed a control group that had been recruited as a comparator for a group with developmental issues, for example ADHD (e.g., Boles et al., Citation2009) or dyslexia (e.g., Sireteanu et al., Citation2005), but only the control data were entered into the meta-analysis. Manly et al. (Citation2005) included children who had been rated as exhibiting a high or low frequency of ADHD-type behaviours by their teacher and this study was included because the children had not been formally diagnosed with ADHD.
3) Task: Studies were included when lateral visuospatial bias was assessed using standard versions of either the line bisection task or the landmark task. Thirty-three datasets (from 31 studies) reported using the line bisection task. Non-standard versions of the task, such as where the line was part of a larger shape (e.g., Portex et al., Citation2017) or where children were asked to indicate whether an object was equidistant between two other objects (e.g., Saj & Barisnikov, Citation2015), were excluded. Only horizontal, non-radially presented lines were included. Only 3 datasets (from 2 studies) used the landmark task (Hoyos et al., Citation2020 experiments 1 & 2; Liu et al., Citation2012) and this task was therefore not analysed further.
4) Language: Only studies written in English, or where an English translation was available, were included.
Moderators
1) Age: The numerical mean age of the participants in each study was included as a continuous variable. Three studies did not report the mean age and were excluded from this analysis (Dellatolas et al., Citation1996; Roeltgen & Roeltgen, Citation1989; Sireteanu et al., Citation2005).
Secondly, we identified a subgroup of 30 studies where the upper age range of the children was ≤16 years old, to assess whether the inclusion of adolescents may have influenced the results. Three studies failed to meet this criterion and were therefore excluded from this analysis (Dennis et al., Citation2005; Polikoff et al., Citation1995; Richter et al., Citation2005).
Thirdly, we compared a subset of studies that reported data for younger children (aged under 7) to a subset that included data for older children (aged over 7) to assess whether biases may emerge during mid-childhood. Nine studies reported data for at least one group of children who were all aged up to 7 years old (Asenova & Andonova-Tsvetanova, Citation2019; Chokron & De Agostini, Citation1995; De Agostini et al., Citation1999; Dellatolas et al., Citation1996; Failla et al., Citation2003; Girelli et al., Citation2017; Ickx et al., Citation2017; Patro et al., Citation2018; Roeltgen & Roeltgen, Citation1989) and 23 studies reported data where all of the children were aged 7 and over (Anelli et al., Citation2013; Boles et al., Citation2009; Chokron & De Agostini, Citation1995; Crollen et al., Citation2015; Dellatolas et al., Citation1996; Dennis et al., Citation2005; Failla et al., Citation2003; Girelli et al., Citation2017; Göksun et al., Citation2013; Hausmann et al., Citation2003; Ickx et al., Citation2017; Ninaus et al., Citation2017; Polikoff et al., Citation1995; Pulsipher et al., Citation2009; Richter et al., Citation2005; Rolfe et al., Citation2006; Sheppard et al., Citation1999 (two control groups); Sheppard et al., Citation2002; Sireteanu et al., Citation2005; van Vugt et al., Citation2000; Vollebregt et al., Citation2016; Waldie & Hausmann, Citation2010). Three datasets included children whose ages spanned both of these age subgroups and were therefore not included in this analysis (Dobler et al., Citation2001 (experiments 1 & 2 age 6.5-7.5); Manly et al., Citation2005 (age 6-11); Rolfe et al., Citation2008 (age 5.5-11.7)), as were two studies where the children’s age range was not reported (George et al., Citation2005; Michel et al., Citation2011) and two datasets where the effect size could not be extracted (Girelli et al., Citation2017 (3 year olds); Patro et al., Citation2018 (3-4 year olds)).
2) Sex: Five studies reported separate data for girls and boys (Asenova & Andonova-Tsvetanova, Citation2019; Chokron & De Agostini, Citation1995; De Agostini et al., Citation1999; Dellatolas et al., Citation1996; van Vugt et al., Citation2000). Due to the small number of studies that reported separate data for boys and girls, we also calculated the percentage of male participants in each study where the data were available (10 studies did not report this data and were excluded from this analysis: Boles et al., Citation2009; Dennis et al., Citation2005; Girelli et al., Citation2017; Hausmann et al., Citation2003; Ickx et al., Citation2017; Michel et al., Citation2011; Ninaus et al., Citation2017; Roeltgen & Roeltgen, Citation1989; Rolfe et al., Citation2008; Sheppard et al., Citation2002).
3) Hand preference: Three studies reported separate data for left- and right-handed children (Asenova & Andonova-Tsvetanova, Citation2019; Dellatolas et al., Citation1996; van Vugt et al., Citation2000). Due to the small number of studies that reported separate data for handed preference, the percentage of right-handed children was also extracted as a continuous variable. The data were unavailable for 3 studies (George et al., Citation2005; Göksun et al., Citation2013; Patro et al., Citation2018).
4) Hand used to bisect: Twelve datasets (from 11 studies) asked children to bisect the lines using their left hand and their right hand (Asenova & Andonova-Tsvetanova, Citation2019; De Agostini et al., Citation1999; Dellatolas et al., Citation1996; Dobler et al., Citation2001 (experiments 1 & 2); Failla et al., Citation2003; Hausmann et al., Citation2003; Pulsipher et al., Citation2009; Roeltgen & Roeltgen, Citation1989; Rolfe et al., Citation2006; van Vugt et al., Citation2000; Waldie & Hausmann, Citation2010).
5) Control group status: We also extracted information on the recruitment context of the participants, that is, whether the children were recruited as control participants within a clinical study or had been recruited as part of a stand-alone group of children. This was prompted by our observation in Learmonth and Papadatou-Pastou (Citation2021) of a leftward bias in older adults who had been recruited as a stand-alone older group, but no bias in older clinical control participants. Twenty datasets had recruited a stand-alone group of children and 15 datasets had recruited children as control participants (Boles et al., Citation2009; Crollen et al., Citation2015; Dennis et al., Citation2005; George et al., Citation2005; Michel et al., Citation2011; Pulsipher et al., Citation2009; Richter et al., Citation2005; Rolfe et al., Citation2006, Citation2008; Sheppard et al., Citation1999 (control groups 1 & 2), Citation2002; Sireteanu et al., Citation2005; Vollebregt et al., Citation2016; Waldie & Hausmann, Citation2010).
In addition to these 5 moderator variables, the data were also extracted for the length of the lines, the modality of presentation (i.e., paper and pencil vs. computerized), the presence of left or right lateralized cues and the spatial location of the line, but there was not enough data available to formally analyse these factors. This information is available alongside the full datasets and R scripts at https://osf.io/n68fz/.
Effect size estimates
Leftward biases were denoted by a negative value and rightward biases by a positive value. The mean spatial bias and standard deviation was extracted, where available, and used to calculate an effect size using the formula:
Some studies reported the mean and standard deviations separately across several different variables (e.g., hand preference, sex) rather than reporting the overall mean. In these cases, effect sizes were calculated separately for each variable and then averaged to create an aggregate effect size, as per Jewell and McCourt (Citation2000) and Learmonth and Papadatou-Pastou (Citation2021). In some cases, only a t-value was available from a one-sample t-test against zero (i.e., no bias or chance) and this was used to calculate the effect size using the formula:
The standard error of the mean was reported in some studies rather than the standard deviation, and this was converted to standard deviation using the formula:
The data extraction strategy employed for each study is documented in .
Statistical analysis
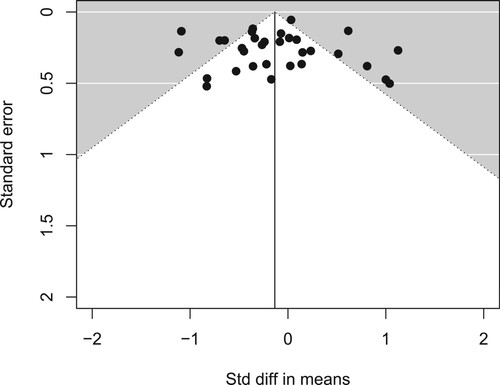
The metafor (Viechtbauer, Citation2007) and robumeta (Fisher et al., Citation2017) packages for R were used to analyse the data. An overall effect size for the line bisection task was calculated by weighting the effect size of each dataset according to the sample size using a random effects model. Heterogeneity was assessed using the Q-statistic, I2, and R2 indices. I2 was interpreted as low at 25%, moderate at 50%, and high at 75% (Higgins et al., Citation2003). Small study bias was assessed using a funnel plot and an Egger’s t-test (Egger et al., Citation1997).
Table 1. Characteristics of studies.
Meta-regression was used for continuous moderator variables (e.g., mean age, percentage of male participants) using a random effects model (restricted maximum-likelihood estimator). A non-linear cubic polynomial model was also used to assess whether a more complex relationship existed between mean age and spatial bias. The effect sizes of each level of the categorical variables (e.g., age subgroups, sex, hand preference, hand used to bisect) were compared using a random effects model. Q-statistics and p-values were used to interpret the statistical outputs. Finally, forest plots were used to visualize the effect sizes across datasets.
Results
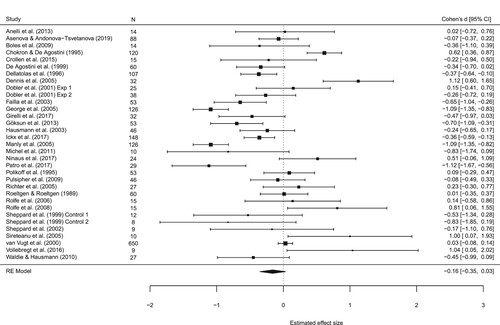
Overall estimates
A total of 33 datasets from 31 studies were included in the meta-analysis of the line bisection task, totalling n = 2101 children (). A random effects model indicated an overall weighted estimated effect size of Cohen’s d = -.16, 95% CI = -.35, .03, with no evidence of a bias to either side of space, Z = −1.62, p = .1. The Egger’s t-test and visual inspection of the funnel plot did not indicate the presence of small study bias, t (31) = -.13, p = .9 ().
Analysis of moderator variables
Due to the high levels of heterogeneity in the random effects models, we assessed the potential influence of the following moderator variables for the line bisection task: age (mean age, <7 vs >7 year olds, and studies where all participants were aged <16), sex (sex subgroups and percentage male), hand preference (right vs. left and percentage right-handed), the hand used to bisect the line, and the control group status of the participants.
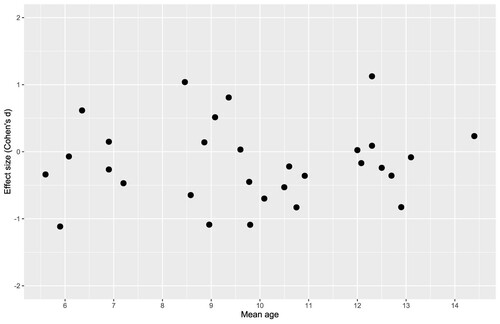
1a) Mean age: The mean age across all datasets was 9.8 years old (range = 5.6-14.4). Meta-regression of the mean age identified no linear effect, Q (1) = .22, p = .64, R2 = 0%, and no non-linear effect, Q (3) = 1.08, p = .78, R2 = 0%, of age on spatial bias.
1b) Age subgroups: A random effects model was applied to a subset of 30 studies that had reported the upper bound of the age range to be a maximum of 16 years old (i.e., this excluded the 3 studies that had included some adolescents aged >16). This identified a small bias to the left side of space, d = −.23, 95% CI = −.42, −.04, p = .02. There was no difference in spatial bias when comparing the 9 studies in which all of the children were aged under 7 years old to the 23 studies in which all of the children were aged over 7 years old, Q (1) = .6, p = .44, R2 = 0%. The younger children had a bias of d = −.21, 95% CI = −.48, .07, p = .14, and the older children a bias of d = −.06, 95% CI = −.29, .17, p = .62.
2a) Sex: No effect of sex was identified in the 5 studies that reported separate data for girls and boys, d = .04, 95% CI = −.32, .6, p = .56. Specifically, the effect size was found to be d = −.008, 95% CI = −.29, .3, p = .96 for boys and d = .14, 95% CI = −.22, .5, p = .44 for girls.
2b) Percentage male: Meta-regression of the percentage of male participants (23 datasets) identified a small effect of sex on spatial bias, Q (1) = 4.65, p = .031, R2 = 18.97%. A follow-up Pearson’s correlation between the effect size and percentage of male participants identified a small correlation of r = −.41, 95% CI = −.7, −.0005, p = .05, where spatial bias generally became more leftward when there were more male participants in the study.
3a) Hand preference: There was no evidence of a moderating effect of hand preference in the 3 studies that reported separate data for left- and right-handed children, Q (1) = .92, p = .34, R2 = 0%.
3b) Percentage right-handed: Meta-regression of the percentage of right-handed participants (30 datasets) identified no effect of hand preference on spatial bias, Q (1) = .92, p = .34, R2 = 0%.
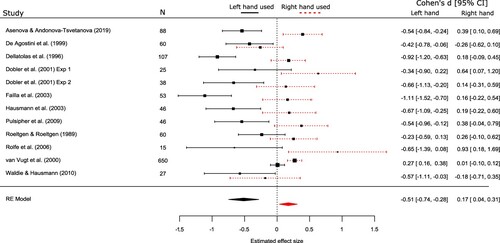
4) Hand used to bisect: There was a strong effect of the hand that was used to bisect the line, in the 12 datasets (from 11 studies) that reported separate data for bisections performed using the left and the right hand, Q (1) = 23.1, p < .0001, R2 = 55.96% (). Specifically, there was a leftward bias when children used their left hand to bisect, d = −.51, 95% CI = -.74, −.28, p < .0001, and a rightward bias when they used their right hand, d = .17, 95% CI = .037, .31, p = .013 .
5) Control group status: There was no evidence of a moderating effect of control group status on spatial bias, Q (1) = .98, p = .32, R2 = 0%. Specifically, there was a small leftward bias in the 18 studies where participants had been recruited as a stand-alone group of children, d = −.24, 95% CI = −.45, −.02, p = .03 and no bias in the 15 studies where children had been recruited as controls for a clinical or atypically developing group, d = −.03, 95% CI = −.4, .34, p = .88, however, the two groups were not statistically different to one another.
Comparison of children and older adults
The effect size obtained in children aged ≤16 was compared to the effect size obtained in older adults aged ≥50 for the line bisection task (from Learmonth & Papadatou-Pastou, Citation2021, data available at https://osf.io/d97qc/). No evidence of a difference between the two age groups was found, either when comparing children to all 63 older adult studies where the mean age of participants was ≥50 (Q (1) = 1.43, p = .23), or the subset of 34 studies where all individual participants were aged ≥50 (Q (1) = .003, p = .98).
Discussion
We performed a meta-analysis of lateralized spatial biases in typically developing children and adolescents, with a mean age of ≤16 years old, as measured by the line bisection task. The influence of age, sex, hand preference, the hand that was used to bisect the line, and the control group status of the participants were assessed as potential moderator variables. A total of 33 datasets from 31 studies, involving 2101 children, were included. Based on numerous reports of a leftward pseudoneglect bias in the adult population (see the meta-analysis of Jewell & McCourt, Citation2000) and Learmonth and Papadatou-Pastou’s (Citation2021) recent finding of a maintained leftward bias in older adults aged ≥50 when tested using the line bisection task, we also expected to observe a leftward bias in children.
Overall, we identified no evidence of bias to either side of space in children, however, there was a small leftward bias in the subset of 30 studies where all the children who had been tested were known to be aged ≤16. We found no difference between younger children (aged under 7 years old) and older children (aged over 7 years old) to indicate that spatial biases are substantially different between younger and older children. When compared to our meta-analysis in older adults (Learmonth & Papadatou-Pastou, Citation2021), the line bisection bias that we observed in children was not different to the small leftward bias that we had previously observed in our older adults aged ≥50.
The most striking finding from the analysis of moderator variables was the identification of symmetrical neglect (Bradshaw et al., Citation1987), whereby bisection biases were dependent on the hand that the children used to bisect the line. From the 12 studies that reported separate data for bisections performed using the left and the right hand, there was a clear rightward bias when the right hand was used (d = .17, 95% CI = .037, .31) and a clear leftward bias when the left hand was used (d = −.51, 95% CI = −.74, −.28). The meta-analysis of pseudoneglect in the general (predominately adult) population by Jewell and McCourt (Citation2000) concluded that visuospatial biases were enhanced leftward when bisections were actioned by the left hand, but right-handed bisections were not found to produce clear rightward bisections that crossed the midline (this was not tested within the Learmonth and Papadatou-Pastou (Citation2021) meta-analysis due to lack of sufficient data). We also did not obtain enough data to investigate further interactions of this effect, for example to identify whether symmetrical neglect is more prevalent in left- or right-handed children (Bradshaw et al., Citation1987; Dellatolas et al., Citation1996).
The comparison of left- and right-handed children did not identify any differences in spatial bias based on hand preference, however only 4 studies reported sufficient data for this analysis (again, this was also not tested in Learmonth and Papadatou-Pastou, Citation2021, due to insufficient data). In the meta-analysis of Jewell and McCourt (Citation2000), both left- and right-handers produced leftward biases, although biases were marginally more leftward in right-handers. Given the large effect of the hand used to bisect the line on spatial biases in children, our preliminary data indicates that hand preference and symmetrical neglect may be independent phenomena (the latter broadly disappearing by adulthood and the former remaining stable). However, these results should be interpreted with caution given the small number of studies included in the hand preference analysis.
In terms of a sex difference, we identified no effect of sex when girls and boys were directly compared to each other, but there was a small increase in leftward bias in studies that included a higher percentage of boys. Jewell and McCourt (Citation2000) also identified a small increase in leftward bias in males, but Learmonth and Papadatou-Pastou (Citation2021) did not identify the same effect in older adults.
One additional question that we aimed to address in this meta-analysis was: Do spatial biases change between younger and older childhood? Lateral visuospatial biases are likely to arise from the integration of perceptual and motor activity across the left and right cerebral hemispheres, with the right hemisphere eventually becoming dominant for visuospatial attention relative to the left. The development of the corpus callosum throughout childhood had been believed to be a driving force behind the emergence of spatial biases during this time (Hausmann et al., Citation2003; Roeltgen & Roeltgen, Citation1989; but see Pulsipher et al., Citation2009). On the contrary, our results showed no evidence that biases change linearly or non-linearly (using a cubic polynomial model) during childhood when the mean age of the participants was entered as a moderator variable. We also performed a secondary analysis to directly compare biases in 9 studies where the children were aged under 7 years old, to 23 studies where the children were aged over 7 years old. Again, we found no evidence of a difference in bias between these two age groups. Due to lack of available data, this meta-analysis may have failed to capture more subtle bias changes at different ages during childhood. For example, Hausmann et al. (Citation2003) reported different line bisection biases in children aged 10-12 and 13-15, and both Girelli et al. (Citation2017) and Patro et al. (Citation2018) found no bias in 3-4-year-olds but leftward biases in 5-6-year-olds. We found that there was not enough data reported for us to analyse such subgroups of different ages independently here (the majority of studies reported a single mean bias for all of their participants at the group level, together with the mean age of their participants), so it remains possible that a significant shift in spatial bias does occur within very early childhood that we were unable to capture with our methodology.
We were also unable to directly compare moderator variables across differently aged groups of children with any finer detail, such as the hand used to bisect the line. For example, the 10-12-year-olds in Hausmann et al. (Citation2003) had symmetrical neglect when bisecting using their left and right hands, but the 13-15-year-olds had an “adult-like” leftward bias for both bisection conditions. Moving forward, we would encourage researchers to provide individual participant data for children at each year of age so that a more accurate picture of developmental trajectories in early childhood can be identified in future studies. In line with this suggestion, we further recommend that further experiments are performed to examine the influence of moderator variables on pseudoneglect throughout the lifespan. In terms of hand preference, we suggest that both hand preference and hand skill are assessed and reported, as they represent different manifestations of hand preference. It would also now be prudent to perform an updated meta-analysis on healthy adults aged 16-50 to identify whether there are subtle changes in the magnitude of spatial biases in the intervening years between childhood and older adulthood.
The method of quantifying pseudoneglect in this age group is also an important consideration for researchers, as the task that was selected may have influenced the biases observed. This issue is not only pertinent to children; in adults, there is poor consistency of the direction and magnitude of biases obtained using different tasks (Learmonth et al., Citation2015; Luh et al., Citation1991; Märker et al., Citation2019; Mitchell et al., Citation2020; Nicholls et al., Citation1999). It is possible that children are less precise in their hand-eye coordination and motor movements when manually bisecting a line and that the line bisection task may underestimate the extent of visuospatial biases in this group. The reduced motor demands of the landmark task may prove to be more suitable for this age group in future research (e.g., Hoyos et al., Citation2020).
This meta-analysis may have been limited by several other factors. Firstly, the analysis of the mean age of all children per study is a “broad brush” approach to assessing spatial biases in children and may give rise to poor representativeness of the mean biases with respect to the individual children who were tested. For example, a study that tested children aged 5-15 years old, with a mean age of 10, may have included few, if any, 10-year-old children. Second, there was not enough information available to investigate whether the influence of reading direction is important for the development of spatial biases in childhood (as per Chokron & De Agostini, Citation1995). In general, meta-analysis has low power when it comes to detecting moderating variable effects, as it is a function of the number of included studies mirroring power in primary studies as a function of their sample size. The fact that not all included studies reported the moderating variables under investigation, further hindered the power of our moderating variables analysis. Moreover, we did not evaluate the moderating effects of the length of the lines, the modality of presentation (i.e., paper and pencil vs. computerized), the presence of left or right lateralized cues, and the spatial location of the line, because not enough studies reported these variables. We suggest that researchers measure and report these variables in the future and that they further break down their findings by sex and hand preference. Ideally, raw data should be provided in open-access repositories, such as the Open Science Framework (osf.io).
Conclusion
In conclusion, we identified no overall line bisection bias in children, but there was a small leftward bias in a subgroup of 30 studies where all the children were known to be aged ≤16 years old. There was no evidence that biases differed between younger children aged ≤7 and older children aged ≥7 years old. There was strong evidence of symmetrical neglect, where right-handed bisections resulted in a rightward bias, and left-handed bisections in a leftward bias. Biases were marginally more leftward in studies that included a higher percentage of male children. No linear effect of age or hand preference was found, nor an effect of the control group status of participants, and there was no evidence of small study bias in the wider literature. Taken together with our meta-analysis in older adults (Learmonth & Papadatou-Pastou, Citation2021) and the meta-analysis of Jewell and McCourt (Citation2000), we conclude that pseudoneglect is likely to be present in childhood (although its manifestation is dependent on specific task demands and characteristics of the individual) and is likely to remain present at the group level throughout the lifespan.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data and analysis scripts that support the findings of this study are openly available in the Open Science Framework at https://osf.io/u3syc/.
Additional information
Funding
References
- References marked with an asterisk (*) were included in the meta-analysis
- *Anelli, F., Ranzini, M., Nicoletti, R., & Borghi, A. M. (2013). Perceiving object dangerousness: An escape from pain? Experimental Brain Research, 228(4), 457–466. https://doi.org/10.1007/s00221-013-3577-2
- *Asenova, I. V., & Andonova-Tsvetanova, Y. R. (2019). Examining handedness and sex-related effects on line-bisection in childhood. Psychological Thought, 12(1), https://doi.org/10.5964/psyct.v12i1.311
- *Boles, D. B., Adair, L. P., & Joubert, A.-M. (2009). A preliminary study of lateralized processing in attention-deficit/hyperactivity disorder. The Journal of General Psychology, 136(3), 243–260. https://doi.org/10.3200/GENP.136.3.243-260
- Bowers, D., & Heilman, K. M. (1980). Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia, 18(4-5), 491–498. https://doi.org/10.1016/0028-3932(80)90151-7
- Bradshaw, J. L., Nettleton, N. C., Wilson, L. E., & Bradshaw, C. S. (1987). Line bisection by left-handed preschoolers: A phenomenon of symmetrical neglect. Brain and Cognition, 6(4), 377–385. https://doi.org/10.1016/0278-2626(87)90134-5
- Bradshaw, J. L., Spataro, J. A., Harris, M., Nettleton, N. C., & Bradshaw, J. (1988). Crossing the midline by four to eight year old children. Neuropsychologia, 26(2), 221–235. https://doi.org/10.1016/0028-3932(88)90076-0
- Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: The Harold model. Psychology and Aging, 17(1), 85–100. https://doi.org/10.1037/0882-7974.17.1.85
- *Chokron, S., & De Agostini, M. (1995). Reading habits and line bisection: A developmental approach. Cognitive Brain Research, 3(1), 51–58. https://doi.org/10.1016/0926-6410(95)00018-6
- Çiçek, M., Deouell, L. Y., & Knight, R. T. (2009). Brain activity during landmark and line bisection tasks. Frontiers in Human Neuroscience, 3, 1–8. https://doi.org/10.3389/neuro.09.007.2009
- *Crollen, V., Vanderclausen, C., Allaire, F., Pollaris, A., & Noël, M.-P. (2015). Spatial and numerical processing in children with non-verbal learning disabilities. Research in Developmental Disabilities, 47, 61–72. https://doi.org/10.1016/j.ridd.2015.08.013
- *De Agostini, M., Curt, F., Tzortzis, C., & Dellatolas, G. (1999). Comparing left and right hand in line bisection at different ages. Developmental Neuropsychology, 15(3), 379–394. https://doi.org/10.1080/87565649909540756
- de Hevia, M-D, & Spelke, E. S. (2009). Spontaneous mapping of number and space in adults and young children. Cognition, 110(2), 198–207. https://doi.org/10.1016/j.cognition.2008.11.003
- *Dellatolas, G., Coutin, T., & De Agostini, M. (1996). Bisection and perception of horizontal lines in normal children. Cortex, 32(4), 705–715. https://doi.org/10.1016/S0010-9452(96)80040-2
- *Dennis, M., Edelstein, K., Frederick, J., Copeland, K., Francis, D., Blaser, S. E., Kramer, L. A., Drake, J. M., Brandt, M., Hetherington, R., & Fletcher, J. M. (2005). Peripersonal spatial attention in children with spina bifida: Associations between horizontal and vertical line bisection and congenital malformations of the corpus callosum, midbrain, and posterior cortex. Neuropsychologia, 43(14), 2000–2010. https://doi.org/10.1016/j.neuropsychologia.2004.10.014
- *Dobler, V., Manly, T., Atkinson, J., Wilson, B. A., Ioannou, K., & Robertson, I. H. (2001). Interaction of hand use and spatial selective attention in children. Neuropsychologia, 39(10), 1055–1064. https://doi.org/10.1016/S0028-3932(01)00038-0
- Egger, M., Smith, G. D., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. https://doi.org/10.1136/bmj.315.7109.629
- Fagard, J., & Dahmen, R. (2003). The effects of Reading-writing direction on the asymmetry of space perception and directional tendencies: A comparison between French and Tunisian children. Laterality: Asymmetries of Body, Brain and Cognition, 8(1), 39–52. https://doi.org/10.1080/713754473
- *Failla, C. V., Sheppard, D. M., & Bradshaw, J. L. (2003). Age and responding-hand related changes in performance of neurologically normal subjects on the line-bisection and chimeric-faces tasks. Brain and Cognition, 52(3), 353–363. https://doi.org/10.1016/S0278-2626(03)00181-7
- Fink, G. R., Marshall, J. C., Shah, N. J., Weiss, P. H., Halligan, P. W., Grosse-Ruyken, M., Ziemons, K., Zilles, K., & Freund, H. J. (2000). Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology, 54(6), 1324–1331. https://doi.org/10.1212/WNL.54.6.1324
- Fisher, Z., Tipton, E., & Zhipeng, H. (2017). Robumeta: Robust variance meta-regression. R package version 2.0.
- Foxe, J. J., McCourt, M. E., & Javitt, D. C. (2003). Right hemisphere control of visuospatial attention: Line-bisection judgments evaluated with high-density electrical mapping and source analysis⋆. NeuroImage, 19(3), 710–726. https://doi.org/10.1016/S1053-8119(03)00057-0
- *George, M., Dobler, V., Nicholls, E., & Manly, T. (2005). Spatial awareness, alertness, and ADHD: The re-emergence of unilateral neglect with time-on-task. Brain and Cognition, 57(3), 264–275. https://doi.org/10.1016/j.bandc.2004.09.003
- *Girelli, L., Marinelli, C. V., Grossi, G., & Arduino, L. S. (2017). Cultural and biological factors modulate spatial biases over development. Laterality: Asymmetries of Body, Brain and Cognition, 22(6), 725–739. https://doi.org/10.1080/1357650X.2017.1279623
- *Göksun, T., Woods, A. J., Chatterjee, A., Zelonis, S., Glass, L., & Smith, S. E. (2013). Elementary school children’s attentional biases in physical and numerical space. European Journal of Developmental Psychology, 10(4), 433–448. https://doi.org/10.1080/17405629.2012.692965
- Goldstein, G., & Shelly, C. (1981). Does the right hemisphere age more rapidly than the left? Journal of Clinical Neuropsychology, 3(1), 65–78. https://doi.org/10.1080/01688638108403114
- *Hausmann, M., Waldie, K. E., & Corballis, M. C. (2003). Developmental changes in line bisection: A result of callosal maturation? Neuropsychology, 17(1), 155–160. https://doi.org/10.1037/0894-4105.17.1.155
- Higgins, J. P. T., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. https://doi.org/10.1136/bmj.327.7414.557
- *Hoyos, P. M., Kim, N. Y., Cheng, D., Finkelston, A., & Kastner, S. (2020). Development of spatial biases in school-aged children. Developmental Science, 24, https://doi.org/10.1111/desc.13053
- *Ickx, G., Bleyenheuft, Y., & Hatem, S. M. (2017). Development of visuospatial attention in typically developing children. Frontiers in Psychology, 8(DEC), 1–14. https://doi.org/10.3389/fpsyg.2017.02064
- Jewell, G., & McCourt, M. E. (2000). Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia, 38(1), 93–110. https://doi.org/10.1016/S0028-3932(99)00045-7
- Jiang, C., Shen, F., Li, G., Lin, Z., Li, W., & Jiao, Y. (2008). Unilateral spatial neglect in children with attention deficit hyperactivity disorder. Chinese Journal of Pediatrics, 46(5), 370–373.
- Learmonth, G., Gallagher, A., Gibson, J., Thut, G., & Harvey, M. (2015). Intra- and inter-task reliability of spatial attention measures in pseudoneglect. PLoS One, 10(9), e0138379. https://doi.org/10.1371/journal.pone.0138379
- Learmonth, G., & Papadatou-Pastou, M. (2021). A meta-analysis of line bisection and landmark task performance in older adults. Neuropsychology Review, 0123456789,. https://doi.org/10.1007/s11065-021-09505-4
- *Liu, L., Qian, Q.-J., Li, H.-M., Li, Z.-H., Huang, F., Yang, L., & Wang, Y.-F. (2012). Atypical brain laterality for spatial attention in children with attention-deficit/hyperactivity disorder. Chinese Mental Health Journal, 13–16.
- Luh, K. E., Rueckert, L. M., & Levy, J. (1991). Perceptual asymmetries for free viewing of several types of chimeric stimuli. Brain and Cognition, 16(1), 83–103. https://doi.org/10.1016/0278-2626(91)90087-O
- Mańkowska, A., Heilman, K. M., Williamson, J. B., Michałowski, J., & Harciarek, M. (2020). Age-related changes in the allocation of spatially directed focal attention. Aging, Neuropsychology, and Cognition, 27(5), 748–764. https://doi.org/10.1080/13825585.2019.1675581.
- *Manly, T., Cornish, K., Grant, C., Dobler, V., & Hollis, C. (2005). Examining the relationship between rightward visuo-spatial bias and poor attention within the normal child population using a brief screening task. Journal of Child Psychology and Psychiatry, 46(12), 1337–1344. https://doi.org/10.1111/j.1469-7610.2005.01432.x
- Märker, G., Learmonth, G., Thut, G., & Harvey, M. (2019). Intra- and inter-task reliability of spatial attention measures in healthy older adults. PLoS One, 14(12), e0226424. https://doi.org/10.1371/journal.pone.0226424
- *Michel, C., Bidot, S., Bonnetblanc, F., & Quercia, P. (2011). Left minineglect or inverse pseudoneglect in children withdyslexia? NeuroReport, 22(2), https://doi.org/10.1097/WNR.0b013e328342d2df
- Mitchell, A. G., Harris, J. M., Benstock, S. E., & Ales, J. M. (2020). The reliability of pseudoneglect is task dependent. Neuropsychologia, 148(August), 107618. https://doi.org/10.1016/j.neuropsychologia.2020.107618
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
- Nicholls, M. E. R., Bradshaw, J. L., & Mattingley, J. B. (1999). Free-viewing perceptual asymmetries for the judgement of brightness, numerosity and size. Neuropsychologia, 37(3), 307–314. https://doi.org/10.1016/S0028-3932(98)00074-8
- *Ninaus, M., Moeller, K., Kaufmann, L., Fischer, M. H., Nuerk, H.-C., & Wood, G. (2017). Cognitive mechanisms underlying directional and non-directional spatial-numerical associations across the lifespan. Frontiers in Psychology, 8, 1421. https://doi.org/10.3389/fpsyg.2017.01421
- Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., & Chou, R. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ, 372. https://doi.org/10.1136/bmj.n160.
- Patro, K., Nuerk, H.-C., & Brugger, P. (2018). Visuospatial biases in preschool children: Evidence from line bisection in three-dimensional space. Journal of Experimental Child Psychology, 173, 16–27. https://doi.org/10.1016/j.jecp.2018.03.002
- *Polikoff, B. R., Evans, B. J., & Legg, C. R. (1995). Is there a visual deficit in dyslexia resulting from a lesion of the right posterior parietal lobe? Ophthalmic & Physiological Optics : The Journal of the British College of Ophthalmic Opticians (Optometrists), 15(5), 513–517. https://doi.org/10.1046/j.1475-1313.1995.9500087t.x
- Portex, M., Foulin, J.-N., & Troadec, B. (2017). Cultural influence on directional tendencies in children’s drawing. Laterality: Asymmetries of Body, Brain and Cognition, 22(5), 621–640. https://doi.org/10.1080/1357650X.2016.1266363
- *Pulsipher, D. T., Seidenberg, M., & Hermann, B. P. (2009). Corpus callosum maturation and line bisection performance in healthy children. Brain Imaging and Behavior, 3(3), 307–316. https://doi.org/10.1007/s11682-009-9073-4
- *Richter, S., Schoch, B., Kaiser, O., Groetschel, H., Hein-Kropp, C., Maschke, M., Dimitrova, A., Gizewski, E., Ziegler, W., Karnath, H. O., & Timmann, D. (2005). Children and adolescents with chronic cerebellar lesions show no clinically relevant signs of aphasia or neglect. Journal of Neurophysiology, 94(6), 4108–4120. https://doi.org/10.1152/jn.00611.2005
- *Roeltgen, M. G., & Roeltgen, D. P. (1989). Development of attention in normal children: A possible corpus callosum effect. Developmental Neuropsychology, 5(2-3), 127–139. https://doi.org/10.1080/87565648909540428
- *Rolfe, M. H. S., Hamm, J. P., & Waldie, K. E. (2008). Differences in paper-and-pencil versus computerized line bisection according to ADHD subtype and hand-use. Brain and Cognition, 66(2), 188–195. https://doi.org/10.1016/j.bandc.2007.07.007
- *Rolfe, M. H. S., Hausmann, M., & Waldie, K. E. (2006). Hemispheric functioning in children with subtypes of attention-deficit/hyperactivity disorder. Journal of Attention Disorders, 10(1), 20–27. https://doi.org/10.1177/1087054705286053
- Saj, A., & Barisnikov, K. (2015). Influence of spatial perception abilities on reading in school-age children. Cogent Psychology, 2(1), 1049736. https://doi.org/10.1080/23311908.2015.1049736
- Saj, A., Heiz, J., Van Calster, L., & Barisnikov, K. (2020). Visuospatial bias in line bisection in williams syndrome. Journal of Intellectual Disability Research, 64(1), 57–61. https://doi.org/10.1111/jir.12688
- Sampaio, E., Gouarir, C., & Mvondo, D. M. (1995). Tactile and visual bisection tasks by sighted and blind children. Developmental Neuropsychology, 11(1), 109–127. https://doi.org/10.1080/87565649509540607
- *Sheppard, D. M., Bradshaw, J. L., & Mattingley, J. B. (2002). Abnormal line bisection judgements in children with Tourette’s syndrome. Neuropsychologia, 40(3), 253–259. https://doi.org/10.1016/S0028-3932(01)00109-9
- *Sheppard, D. M., Bradshaw, J. L., Mattingley, J. B., & Lee, P. (1999). Effects of stimulant medication on the lateralisation of line bisection judgements of children with attention deficit hyperactivity disorder. Journal of Neurology, Neurosurgery & Psychiatry, 66(1), 57–63. https://doi.org/10.1136/jnnp.66.1.57
- *Sireteanu, R., Goertz, R., Bachert, I., & Wandert, T. (2005). Children with developmental dyslexia show a left visual “minineglect”. Vision Research, 45(25), 3075–3082. https://doi.org/10.1016/j.visres.2005.07.030
- *van Vugt, P., Fransen, I., Creten, W., & Paquier, P. (2000). Line bisection performances of 650 normal children. Neuropsychologia, 38(6), 886–895. https://doi.org/10.1016/S0028-3932(99)00130-X
- Viechtbauer, W. (2007). Accounting for heterogeneity via random-effects models and moderator analyses in meta-analysis. Zeitschrift für Psychologie / Journal of Psychology, 215(2), 104–121. https://doi.org/10.1027/0044-3409.215.2.104
- Vieira, S., Quercia, P., Bonnetblanc, F., & Michel, C. (2013). Space representation in children with dyslexia and children without dyslexia: Contribution of line bisection and circle centering tasks. Research in Developmental Disabilities, 34(11), 3997–4008. https://doi.org/10.1016/j.ridd.2013.08.031
- *Vollebregt, M. A., Zumer, J. M., Ter Huurne, N., Buitelaar, J. K., & Jensen, O. (2016). Posterior alpha oscillations reflect attentional problems in boys with attention deficit hyperactivity disorder. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 127(5), 2182–2191. https://doi.org/10.1016/j.clinph.2016.01.021
- *Waldie, K. E., & Hausmann, M. (2010). Right fronto-parietal dysfunction in children with ADHD and developmental dyslexia as determined by line bisection judgements. Neuropsychologia, 48(12), 3650–3656. https://doi.org/10.1016/j.neuropsychologia.2010.08.023
- Yazgan, M. Y., Wexler, B. E., Kinsbourne, M., Peterson, B., & Leckman, J. F. (1995). Functional significance of individual variations in callosal area. Neuropsychologia, 33(6), 769–779. https://doi.org/10.1016/0028-3932(95)00018-X
- Zago, L., Petit, L., Jobard, G., Hay, J., Mazoyer, B., Tzourio-Mazoyer, N., Karnath, H. O., & Mellet, E. (2017). Pseudoneglect in line bisection judgement is associated with a modulation of right hemispheric spatial. Neuropsychologia, 94, 75–83. https://doi.org/10.1016/j.neuropsychologia.2016.11.024