 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Facial emotion processing (FEP) tends to be right hemisphere lateralized. This right-hemispheric bias (RHB) for FEP varies within and between individuals. The aim of the present research was to examine evidence pertaining to the prominent theories of FEP hemispheric bias as measured by a half-emotional half-neutral (no emotion) chimeric faces task. FEP hemispheric bias was indexed using laterality quotients (LQs) calculated from a Chimeric Faces Task completed by 427 adults recruited from the general population aged 18–67 years. Participants indicated which of two identical (but mirrored) emotional-neutral chimeric faces were more emotive. While all investigated emotions (fear, anger, and happiness) were right lateralized, fear was significantly more right lateralized than anger and happiness. These results provide evidence for both the right hemisphere hypothesis and the motivational hypothesis of emotion perception.
Facial emotion processing (FEP) in social contexts is a key component of daily social interactions (Frith, Citation2009; Ishii et al., Citation2018). Evidence suggests that neural processing of emotional faces is right hemisphere lateralized (Gainotti, Citation2019; Sliwinska & Pitcher, Citation2018; Stanković, Citation2021). This right-hemispheric bias (RHB) for FEP varies within and between individuals (Frith & Frith, Citation2003; Gainotti, Citation2019; Stanković, Citation2021). The strength of an individual's RHB is variable, and in some cases, people may exhibit a left hemispheric bias (Frith & Frith, Citation2003; Gainotti, Citation2019; Stanković, Citation2021).
There are several conflicting theories speculating on how the stimulus, or observed emotion, may be implicated in FEP hemispheric bias (for a review see Stanković, Citation2021). Briefly, three of the most prominent theories are the right hemisphere hypothesis (RHH), the valence-specific hypothesis (VSH), and the motivational hypothesis (MH). The RHH posits that right hemisphere brain regions are specialized for FEP of all emotions (Bourne, Citation2010; Gainotti, Citation1972, Citation2019; Wyczesany et al., Citation2018). Alternatively, the VSH proposes that right hemisphere brain regions are specialized for perception of negative emotions, and the left hemisphere is specialized for positive emotions (Fusar-Poli et al., Citation2009; Jansari et al., Citation2011; Prete et al., Citation2014). Lastly, the MH suggests the right hemisphere is specialized for withdrawal motivation emotions (e.g., sadness, fear, disgust; Carver & Harmon-Jones, Citation2009; Poole & Gable, Citation2014; Stanković, Citation2021) while the left hemisphere is specialized for approach motivation emotions (e.g., happiness, surprise, and anger). There are several other theories, such as the behavioural activation and inhibition hypothesis, and the hemispheric functional-equivalence model that consider additional behavioural and cognitive factors that may affect FEP hemispheric bias (Stanković, Citation2021). Interestingly, an investigation of the RHH and VH conducted by Bourne (Citation2010) provided evidence for the RHH, however reported that lateralization of emotions within the right hemisphere differed. Fear and surprise were the most strongly right lateralized, followed by happiness, anger, sadness and disgust (Bourne, Citation2010). Importantly, this research was limited to right-handed participants, with a mean age of 24 years (SD = 6.5).
Beyond the above discussed theories, individual factors such as handedness (David, Citation1989; Harris et al., Citation2001; Hellige et al., Citation1994) have been shown to underpin differences in hemispheric bias for FEP. While some research suggests that left-handed individuals (David, Citation1989; Hellige et al., Citation1994) tend to be less right lateralized for FEP, other research reports no such effects of handedness (Bourne, Citation2008; Vladeanu et al., Citation2012). The inconsistent findings may be due to methodological differences, including variability in measurement of hemispheric bias (e.g., some studies used limited emotions and/or actor diversity in task stimuli), and different measurement of handedness (e.g., some studies quantified handedness categorically rather than continuously). Previous research has also utilized small, and typically homogenous samples which may contribute to heterogenous findings. When employing a large and diverse sample to examine the relationship between the individual factors and FEP hemispheric bias, our recent research suggests that right-handed individuals tended to exhibit a stronger RHB for FEP than left-handed individuals (Speranza et al., Citation2024). Previous research has neglected to investigate the relationship between handedness and FEP hemispheric bias in the context of the emotion being observed, and often investigates FEP hemispheric bias only in right-handed individuals.
It is possible that individual factors known to influence FEP hemispheric bias such as handedness have not been found to influence FEP hemispheric bias in a consistent/predictable way as they may be more related to FEP hemispheric bias regarding specific emotions, rather than FEP hemispheric bias across emotions. Further insight as to how handedness may interact with hemispheric bias during FEP of specific emotions will inform the complex way humans process emotional stimuli. This knowledge may in future inform the development of clinical intervention for conditions characterized by FEP difficulties, including autism (Yeung, Citation2022). Examining the prominent theories of FEP hemispheric bias in a large and diverse sample will provide foundational knowledge for future research to examine the unique contribution of individual factors in the lateralization of individual emotion.
The present research aimed to extend on previous research conducted by Bourne (Citation2010), by examining evidence pertaining to the prominent theories of FEP hemispheric bias, as measured using a half-emotional half-neutral (i.e., no emotion) chimeric faces task, in a large and diverse sample taken from the general population. Chimeric faces tasks have been validated previously as a reliable tool for measuring hemispheric bias during FEP and can reliably measure FEP hemispheric bias in adults (Kucharska-Pietura & David, Citation2003) and children (Bava et al., Citation2005) with unilateral left and right hemispheric lesions. Specifically, those with damage to the right hemisphere exhibited reduced RHB compared to controls and those with left hemisphere damage (Bava et al., Citation2005; Kucharska-Pietura & David, Citation2003). It was hypothesized that the RHH would be the dominant theory explaining FEP hemispheric bias, however, we took an exploratory approach in investigating the VSH and MH by comparing hemispheric bias for each emotion investigated. Should the RHH be supported, we would anticipate that all emotions would be right lateralized, and there would be no difference between the lateralization of each emotion. If the VSH were to be supported, it would be expected that happiness would be right lateralized, and fear and anger would be left lateralized. Lastly, if the MH were to be supported, we would anticipate fear to be right lateralized, and anger and happiness to be left lateralized. Additionally, based on previous research concerning handedness, the present research took an exploratory approach in examining the relationship between handedness and FEP hemispheric bias.
Method
Participants
This study was approved by the human research ethics committee of Deakin University (project HEAG-H 187_2021) and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from participants prior to enrolment. Participants were reimbursed approximately GBP£3.78 for completing the study.
517 adults from the general population (n = 427 after data cleaning; 214 female, 210 male, 2 nonbinary, 1 sex not specified; note that these statistics refer to biological sex) participated in this study. Data was collected as described in Speranza et al. (Citation2024), however, different research questions are addressed in each manuscript. For more information regarding participant recruitment, and data collection and cleaning, please refer to this manuscript (Speranza et al., Citation2024). Briefly, participants were recruited via the online crowd sourcing platform Prolific (www.prolific.com, Citation2022) and were excluded if they had incomplete data, failed attention checks, failed a bot check, or demonstrated abnormal response patterns/reaction times. Due to the small number of participants who reported that their gender differed from their biological sex (n = 11), the present research lacked power to examine gender as a separate variable, and thus focused on biological sex. As only a small number of participants reported their sex as “non-binary” (n = 2) or did not report biological sex (n = 1), these participants’ data were removed from analyses that included biological sex as a factor. Additionally, eight participants’ self-reported handedness conflicted with their EHI score (n = 5 self-reported right-handedness but EHI score indicated non-right handedness; n = 3 indicated non-right handedness but EHI score indicated right-handedness). These participants were removed from analyses that included handedness as a factor. Participant characteristics are presented in .
Table 1. Participant characteristics.
Materials and procedure
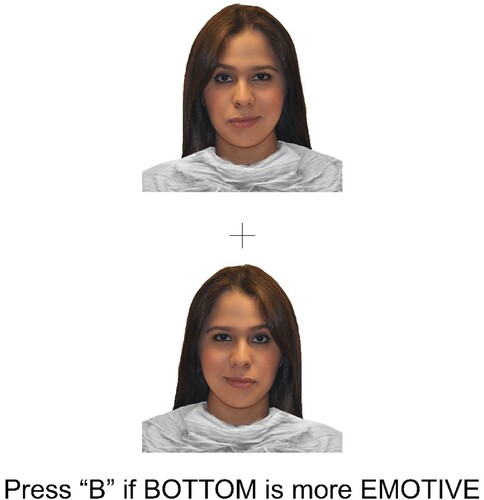
Participants first provided demographic information and completed the Edinburgh Handedness Inventory (EHI; Oldfield, Citation1971), and the Autism Spectrum Quotient (AQ; Baron-Cohen et al., Citation2001) in an online survey distribution tool (Qualtrics, Citation2020). Then, FEP hemispheric bias was measured using a chimeric faces task implemented in the online neurocognitive software suite Inquisit (Inquisit 6 [Millisecond software], Citation2022). Stimuli for this task were modified from the racially diverse affective expression (RADIATE) face stimulus set (Conley et al., Citation2018; Tottenham et al., Citation2009). One author (BS) selected eight actors (one male and one female from each race category available [Asian, Black/African American, Caucasian, and Hispanic or Latino]) from the RADIATE face stimulus set based on validity rating data (see Conley et al., Citation2018) for each emotion being investigated. During this task, participants were presented with images of chimeric faces (i.e., photographs of faces which have been split vertically down the midline, to present an emotion [happy, angry, or fearful] on one side of the photographed face, and a neutral expression [no emotion] on the other). Each chimeric face was mirrored horizontally, to create a new chimeric face that is identical to the first, but with the emotional and neutral hemifaces reversed. These images were then presented to the participant simultaneously, one above the other. This display is the trial image. Two versions of each trial image were generated and presented, one with the face depicting the emotion in the left visual field at the top, and one with this image at the bottom, to control for confounds associated with vertical image placement on the screen. Each individual trial consisted of a fixation cross presented for 1 s, followed by the trial image presented until either (a) the participant made a response, or (b) 4.5 s had passed. This was followed by a 0.5 s inter-trial interval. The task consisted of 192 trials (excluding practice and attention check trials). Participants were asked to indicate (as quickly as possible via keyboard button press) which of the two chimeric faces was more emotive. Choosing the image with the emotion in the left visual field demonstrates a RHB for FEP, while choosing the image with the emotion in the right visual field demonstrates a left hemispheric bias for FEP. Following this, participants were presented with a new fixation cross, followed by the next trial. The order of the trial images was randomized for each participant. Happiness, fear, and anger were used in this task. These emotions were deemed appropriate as they represent emotions with positive (happiness) and negative (anger, fear) valance, and emotions related to approach (happiness, anger) and avoidance (fear) motivation (Stanković, Citation2021), and thus we could expect different patterns of lateralization depending on which theory of FEP hemispheric bias is most dominant. An example of a given trial image is presented in .
Figure 1. Example trial image.
Note: Example image presented to participants. In this example, if participants chose the top image (which contains the emotion in the right visual field) this would demonstrate a left hemispheric bias for facial emotion processing. Conversely, choosing the bottom face (with the emotion in the left visual field) would indicate a right hemispheric bias for facial emotion processing.
Two indices were calculated representing hemispheric bias for FEP for each individual emotion (laterality quotients; LQs). Scores for both indices range from −1 – +1, where positive numbers indicate a RHB, and negative numbers indicate a left-hemispheric bias. LQ1 is based on participant choice alone, and was calculated using the formula:
where NL is the number of trials in which the face with the emotion in the left visual field was chosen, NR is the number of trials in which the face with the emotion in the right visual field was chosen, and Ntotal is the total number of trials. LQ2 is based on reaction time of participant choice, and was calculated using the formula:
where RTR is the average reaction time for trials in which the face with the emotion in the right visual field was chosen, RTL is the average reaction time for trials in which the face with the emotion in the left visual field was chosen, and RTTotal is the average reaction time across all valid trials. This resulted in a total of six LQs for each participant, one for each emotion (fear, anger, happiness), and one with each method (LQ1 and LQ2; LQ1happy, LQ2happy, LQ1angry, LQ2angry, LQ1fear, LQ2fear).
Statistical analysis. All data cleaning and statistical analyses were conducted in R (v4.2.2.; R Core Team, Citation2022).
First, to address our research question regarding the lateralization of individual emotion, six Wilcoxon signed-rank tests were conducted (one for each LQ). These were deemed appropriate due to the non-normal distribution of all measures of FEP hemispheric bias. These tests determine if the LQ significantly differs from zero (where in this instance zero equates to no hemispheric bias during FEP). Models one through six examined each LQ, LQ1fear, LQ1happy, LQ1angry, LQ2fear, LQ2happy, LQ2angry, respectively.
To address our hypothesis that the RHH would be the dominant theory explaining FEP hemispheric bias, four repeated measures ANOVAs were conducted. Model seven included emotion as the predictor variable, and LQ1 as the outcome variable, comparing differences in LQ1 scores for each emotion, only for right-handed participants. Model eight replicated this for non-right-handed participants. Models nine and ten replicated models seven and eight, but with LQ2 as the outcome variable. Where necessary, post hoc tests were used to examine significant ANOVAs.
For models seven and nine Shapiro-Wilks tests and Mauchly's tests of sphericity revealed that the assumptions of normality and sphericity were violated. Additionally, models seven through ten all had some extreme outliers. To account for violated assumptions and address extreme outliers, non-parametric Kruskal–Wallis tests and corresponding pairwise Wilcox post hoc tests were used when applicable.
Results
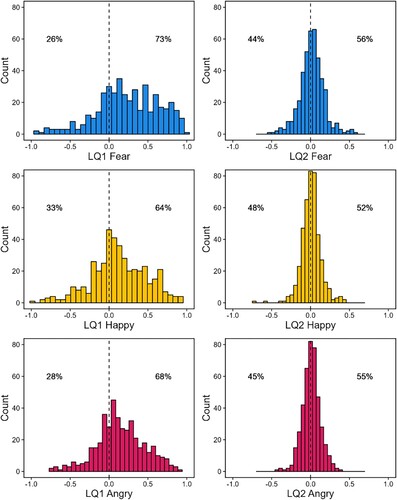
Models one to six, examining the lateralization of specific emotions, revealed that LQ1fear, LQ1happy, LQ1angry, and LQ2fear, significantly differed from zero, LQ1fear: median = .23, p < .01; LQ1happy: median = .11, p < .01; LQ1angry: median = .13, p < .01; LQ2fear: median = .02, p = .01. LQ2happy, and LQ2angry did not significantly differ from zero, LQ2happy: median = .01, p = .19; LQ1angry: median = .01, p = .07. The median and mean scores, and visual inspection revealed that LQ1fear, LQ1happy, LQ1angry, and LQ2fear were all right lateralized.
Figure 2. Median hemispheric bias scores.
Note: The distribution of hemispheric bias scores; LQ1fear: M = .24, SD = .40; LQ1happy: M = .12, SD = .35; LQ1angry: M = .15, SD = .31; LQ2fear: M = .02, SD = .16; LQ2happy: M = .01, SD = .13; LQ1angry: M = .01, SD = .12. LQ: laterality quotient; percentages indicate percent of participants above/below 0.
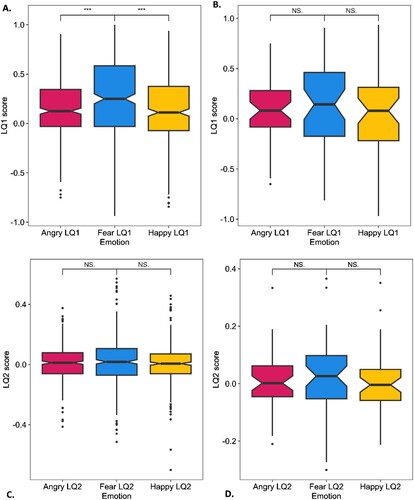
Model seven examining the difference in hemispheric bias pertaining to each emotion LQ1 (fear, anger, and, happiness) for right handed participants was significant, H(2) = 23.22,, p < .01. Post hoc analysis revealed that LQ1fear was significantly more right lateralized than LQ1happy, p < 0.01, and LQ1angry, p < 0.01. There was no significant difference between LQ1happy and LQ1angry, p = .35. There was no significant difference in LQ1 scores between emotions for non-right-handed participants, H(2) = 1.17, p = .56, and there was no significant difference in LQ2 scores between each emotion for right-, nor non-right-handed participants, right: H(2) = 1.34, p = .51; left: H(2) = 0.74, p = .69 The mean scores are presented in .
Figure 3. Differences in hemispheric bias between emotions.
Note: The difference in hemispheric bias for each emotion for (A) right-handed participants as measured by LQ1, (B) non-right-handed participants as measured by LQ1, (C) right-handed participants as measured by LQ2, and (D) non-right-handed participants as measured by LQ2 Right-handed LQ1: LQ1angry M = .16, SD = .31; LQ1fear M = .25, SD = .40; LQ1happy M = .13, SD = .34; Non-right-handed LQ1: LQ1angry M = .08, SD = .33; LQ1fear M = .14, SD = .44; LQ1happy M = .03, SD = .43; LQ: laterality quotient, right-handed LQ2: LQ2angry M = .01, SD = .12; LQ2fear M = .02, SD = .16; LQ2happy M = .01, SD = .13; Non-right-handed LQ2: LQ2angry M = .01, SD = .10; LQ2fear M = .01, SD = .14; LQ2happy M = .01, SD = .11; LQ: laterality quotient; ***significant at p < .001.
Discussion
The present study aimed to examine prominent theories of hemispheric bias during FEP in a large, diverse sample of the general population. Our suggestion that the Right Hemisphere Hypothesis (RHH) would be the dominant theory explaining FEP hemispheric bias was supported, however some evidence was provided for the Motivational Hypothesis (MH). Importantly, there was a lack of significant findings related to hemispheric bias as measured by LQ2. It is considered that measures of hemispheric bias using LQ2 (reaction time) were not sensitive enough to capture variations in hemispheric bias in the present sample, or that the measure could not differentiate between left and right hemispheric bias due to reaction time ceiling effects. Finally, our exploration of the influence of handedness on FEP hemispheric bias suggests right handed individuals exhibit stronger right lateralization for emotions than non-right-handed individuals.
The present research provides evidence for both the RHH and the MH. All three emotions investigated in this study (anger, happiness, and fear) were right lateralized (i.e., exhibit a tendency for right-hemisphere brain regions to be more involved than left-hemisphere brain regions as measured by a chimeric face task) when measured by LQ1. This aligns with the RHH (Bourne, Citation2010; Gainotti, Citation1972, Citation2019; Stanković, Citation2021; Wyczesany et al., Citation2018). Moreover, fear was more strongly right lateralized than anger and happiness when measured using LQ1. When measured using LQ2, only fear was right lateralized, whereas happiness and anger were neither right nor left lateralized. We consider these findings to align with the MH (Carver & Harmon-Jones, Citation2009; Poole & Gable, Citation2014; Stanković, Citation2021). Together this evidence suggests that while the right hemisphere may be more involved in the perception of emotional faces broadly, this is particularly true for the processing of emotions associated with withdrawal (i.e., fear, rather than approach i.e., happiness and anger) motivation. Given the indirect nature of our behavioural measure of hemispheric bias, it cannot be excluded that this measure lacked the necessary sensitivity to capture more nuanced patterns of cortical activation to truly distinguish between these two prominent theoretical explanations of FEP hemispheric bias, and thus differences in strength of right lateralization between emotions reported in the present research may reflect systematic differences in cortical activation (i.e., more left lateralization). The MH has been specifically evidenced regarding frontal and anterior temporal region activation (Poole & Gable, Citation2014). Specific cortical activation patterns could not be examined in the present research, and future research should investigate the neurobiological basis of these theories with more direct measurements of cortical activation during FEP, and to differentiate between relevant theories of FEP hemispheric bias more directly. Additionally, it is possible that our measure of FEP hemispheric bias provides a more conservative measure of hemispheric bias than other methods (Nesbit & Watling, Citation2024). For these reasons we propose that our findings provide evidence that aligns with both the RHH and MH.
In addition, the present research suggests that handedness may relate to patterns of hemispheric bias for FEP. Specifically, right-handed individuals exhibited a RHB for all emotions, while non-right-handed people exhibited a weak RHB only for fear. It is possible that handedness may reflect functional differences in cytoarchitecture and may thus provide insight into patterns of hemispheric specialization that extend beyond hand preference (Amunts et al., Citation2000; Cuzzocreo et al., Citation2009; Tuncer et al., Citation2005). Similar to our previous related research (Speranza et al., Citation2024), due to the nature of online research the present study was limited in outcome measures. Specifically, our measure of hemispheric bias during FEP is considered an indirect measurement of the construct. Although widely used, and previously validated (Ashwin et al., Citation2005; Bava et al., Citation2005; Bourne, Citation2008; Gupta & Pandey, Citation2010; Kucharska-Pietura & David, Citation2003), future research would benefit from utilizing direct measures of hemispheric bias, such as functional magnetic resonance imaging (fMRI), to more accurately investigate patterns of cortical activation. Such research may identify direct links between FEP hemispheric bias as measured by a chimeric faces task, and cortical activation patterns. Other techniques that may be beneficial in elucidating factors affecting FEP hemispheric bias include electroencephalography (EEG), and eye tracking. EEG may provide insight regarding underlying cortical mechanisms (neurophysiological components) associated with FEP hemispheric bias, while eye tracking may be used to examine associations between FEP hemispheric bias and gaze fixation patterns. Additionally, the RADIATE face stimulus set is known to have high discriminability across emotions (Conley et al., Citation2018; Tottenham et al., Citation2009). However, as the images were modified for use in the chimeric faces task for this study, these metrics are not applicable to our stimuli, and we did not collect data pertaining to the discriminability of the chimeric faces task stimuli. Some other limitations commonly associated with online research that apply to the present study include uncontrolled testing environments and data contamination due to fraudulent or fabricated responses. Despite steps being taken to identify such responses (i.e., attention and bot checks), this possibility remains. Lastly, the present study recruited significantly more right- than left-handed participants. Future research would benefit from ensuring a more balanced sample regarding handedness. Previous research suggests that handedness may be linked to patterns of hemispheric lateralization for several cognitive processes (Króliczak et al., Citation2011; Mazoyer et al., Citation2014; Speranza et al., Citation2024; Tzourio-Mazoyer & Seghier, Citation2016; Vingerhoets, Citation2019). Examining this further will provide insight into mechanisms underlying these cognitive processing, including FEP.
Implications
The present research provides insight as to how emotions are lateralized in the human brain and may inform future research seeking to investigate such constructs. Understanding patterns of hemispheric specialization for FEP may hold valuable insights for the investigation of conditions for which FEP is implicated, such as autism. Additionally, the present research suggests cognitive processes are differently lateralized for right- vs. non-right-handed individuals. Future research examining such processes ought to consider handedness as a factor.
Conclusion
The results from the present study provide evidence for both the RHH and the MH. The right hemisphere may be specialized for processing of emotional faces; however, this relationship may be affected by the motivational content of the emotion being observed. Additionally, handedness remains a predicting factor of hemispheric bias for FEP.
Acknowledgements
We would like to thank all who participated in this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, BS, upon reasonable request.
Additional information
Funding
References
- Amunts, K., Jäncke, L., Mohlberg, H., Steinmetz, H., & Zilles, K. (2000). Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia, 38(3), 304–312. https://doi.org/10.1016/S0028-3932(99)00075-5
- Ashwin, C., Wheelwright, S., & Baron-Cohen, S. (2005). Laterality biases to chimeric faces in Asperger syndrome: What is right about face-processing? Journal of Autism and Developmental Disorders, 35(2), 183–196. https://doi.org/10.1007/s10803-004-1997-3
- Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. https://doi.org/10.1023/a:1005653411471
- Bava, S., Ballantyne, A. O., May, S. J., & Trauner, D. A. (2005). Perceptual asymmetry for chimeric stimuli in children with early unilateral brain damage. Brain and Cognition, 59(1), 1–10. https://doi.org/10.1016/j.bandc.2005.03.004
- Bourne, V. J. (2008). Chimeric faces, visual field bias, and reaction time bias: Have we been missing a trick? Laterality, 13(1), 92–103. https://doi.org/10.1080/13576500701754315
- Bourne, V. J. (2010). How are emotions lateralised in the brain? Contrasting existing hypotheses using the chimeric faces test. Cognition and Emotion, 24(5), 903–911. https://doi.org/10.1080/02699930903007714
- Carver, C. S., & Harmon-Jones, E. (2009). Anger is an approach-related affect: Evidence and implications. Psychological Bulletin, 135(2), 183–204. https://doi.org/10.1037/a0013965
- Conley, M. I., Dellarco, D. V., Rubien-Thomas, E., Cohen, A. O., Cervera, A., Tottenham, N., & Casey, B. J. (2018). The racially diverse affective expression (RADIATE) face stimulus set. Psychiatry Research, 270, 1059–1067. https://doi.org/10.1016/j.psychres.2018.04.066
- Cuzzocreo, J. L., Yassa, M. A., Verduzco, G., Honeycutt, N. A., Scott, D. J., & Bassett, S. S. (2009). Effect of handedness on fMRI activation in the medial temporal lobe during an auditory verbal memory task. Human Brain Mapping, 30(4), 1271–1278. https://doi.org/10.1002/hbm.20596
- David, A. S. (1989). Perceptual asymmetry for happy-sad chimeric faces: Effects of mood. Neuropsychologia, 27(10), 1289–1300. https://doi.org/10.1016/0028-3932(89)90041-9
- Frith, C. (2009). Role of facial expressions in social interactions. Philosophical Transactions of the Royal Society Biological Sciences, 364(1535), 3453–3458. https://doi.org/10.1098/rstb.2009.0142
- Frith, U., & Frith, C. D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society Biological Sciences, 358(1431), 459–473. https://doi.org/10.1098/rstb.2002.1218
- Fusar-Poli, P., Placentino, A., Carletti, F., Allen, P., Landi, P., Abbamonte, M., Barale, F., Perez, J., McGuire, P., & Politi, P. L. (2009). Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neuroscience Letters, 452(3), 262–267. https://doi.org/10.1016/j.neulet.2009.01.065
- Gainotti, G. (1972). Emotional behavior and hemispheric side of the lesion. Cortex, 8(1), 41–55. https://doi.org/10.1016/S0010-9452(72)80026-1
- Gainotti, G. (2019). Emotions and the right hemisphere: Can new data clarify old models? The Neuroscientist, 25(3), 258–270. https://doi.org/10.1177/1073858418785342
- Gupta, G., & Pandey, R. (2010). Assessment of hemispheric asymmetry: Development and psychometric evaluation of a chimeric face test. Industrial Psychiatry Journal, 19(1), 30–36. https://doi.org/10.4103/0972-6748.77632
- Harris, L. J., Almerigi, J. B., Carbary, T. J., & Fogel, T. G. (2001). Left-side infant holding: A test of the hemispheric arousal-attentional hypothesis. Brain and Cognition, 46(1-2), 159–165. https://doi.org/10.1016/s0278-2626(01)80056-7
- Hellige, J. B., Bloch, M. I., Cowin, E. L., Eng, T. L., Eviatar, Z., & Sergent, V. (1994). Individual variation in hemispheric asymmetry: Multitask study of effects related to handedness and sex. Journal of Experimental Psychology General, 123(3), 235–256. https://doi.org/10.1037//0096-3445.123.3.235
- Inquisit 6 [Millisecond software]. (2022). https://www.millisecond.com
- Ishii, L. E., Nellis, J. C., Boahene, K. D., Byrne, P., & Ishii, M. (2018). The importance and psychology of facial expression. Otolaryngologic Clinics of North America, 51(6), 1011–1017. https://doi.org/10.1016/j.otc.2018.07.001
- Jansari, A., Rodway, P., & Goncalves, S. (2011). Identifying facial emotions: Valence specific effects and an exploration of the effects of viewer gender. Brain and Cognition, 76(3), 415–423. https://doi.org/10.1016/j.bandc.2011.03.009
- Króliczak, G., Piper, B. J., & Frey, S. H. (2011). Atypical lateralization of language predicts cerebral asymmetries in parietal gesture representations. Neuropsychologia, 49(7), 1698–1702. https://doi.org/10.1016/j.neuropsychologia.2011.02.044
- Kucharska-Pietura, K., & David, A. S. (2003). The perception of emotional chimeric faces in patients with depression, mania and unilateral brain damage. Psychological Medicine, 33(4), 739–745. https://doi.org/10.1017/s0033291702007316
- Mazoyer, B., Zago, L., Jobard, G., Crivello, F., Joliot, M., Perchey, G., Mellet, E., Petit, L., & Tzourio-Mazoyer, N. (2014). Gaussian mixture modeling of hemispheric lateralization for language in a large sample of healthy individuals balanced for handedness. Public Library of Science One, 9(6), e101165. https://doi.org/10.1371/journal.pone.0101165
- Nesbit, R. J., & Watling, D. (2024). Comparing two versions of the Chimeric Face Test: A pilot investigation. Laterality, 29(1), 19–36. https://doi.org/10.1080/1357650X.2023.2252569
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. https://doi.org/10.1016/0028-3932(71)90067-4
- Poole, B. D., & Gable, P. A. (2014). Affective motivational direction drives asymmetric frontal hemisphere activation. Experimental Brain Research, 232(7), 2121–2130. https://doi.org/10.1007/s00221-014-3902-4
- Prete, G., Laeng, B., & Tommasi, L. (2014). Lateralized hybrid faces: Evidence of a valence-specific bias in the processing of implicit emotions. Laterality, 19(4), 439–454. https://doi.org/10.1080/1357650X.2013.862255
- Qualtrics. (2020). Provo, Utah, USA, https://www.qualtrics.com
- R Core Team. (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- Sliwinska, M. W., & Pitcher, D. J. N. (2018). TMS demonstrates that both right and left superior temporal sulci are important for facial expression recognition. NeuroImage, 183(1), 394–400. https://doi.org/10.1016/j.neuroimage.2018.08.025
- Speranza, B. E., Do, M., Hill, A., Donaldson, P., Enticott, P. G., & Kirkovski, M. (2024). Do autistic traits, handedness, sex, and age predict hemispheric bias during facial emotion processing? https://doi.org/10.31219/osf.io/8dxhs
- Stanković, M. (2021). A conceptual critique of brain lateralization models in emotional face perception: Toward a hemispheric functional-equivalence (HFE) model. International Journal of Psychophysiology, 160(1), 57–70, https://doi.org/10.1016/j.ijpsycho.2020.11.001
- Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., Marcus, D. J., Westerlund, A., Casey, B., & Nelson, C. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. https://doi.org/10.1016/j.psychres.2008.05.006
- Tuncer, M. C., Hatipoğlu, E. S., & Özateş, M. (2005). Sexual dimorphism and handedness in the human corpus callosum based on magnetic resonance imaging. Surgical and Radiologic Anatomy, 27(3), 254–259. https://doi.org/10.1007/s00276-004-0308-1
- Tzourio-Mazoyer, N., & Seghier, M. L. (2016). The neural bases of hemispheric specialization. Neuropsychologia, 93, 319–324. https://doi.org/10.1016/j.neuropsychologia.2016.10.010
- Vingerhoets, G. (2019). Phenotypes in hemispheric functional segregation? Perspectives and challenges. Physics of Life Reviews, 30, 1–18. https://doi.org/10.1016/j.plrev.2019.06.002
- Vladeanu, M., Monteith-Hodge Ewa, M., & Bourne, V. J. (2012). Strength of lateralisation for processing facial emotion in relation to autistic traits in individuals without autism. Laterality: Asymmetries of Body, Brain and Cognition, 17(4), 438–452. https://doi.org/10.1080/1357650X.2010.513385
- www.prolific.com. (2022). Prolific. London, UK.
- Wyczesany, M., Capotosto, P., Zappasodi, F., & Prete, G. (2018). Hemispheric asymmetries and emotions: Evidence from effective connectivity. Neuropsychologia, 121, 98–105. https://doi.org/10.1016/j.neuropsychologia.2018.10.007
- Yeung, M. K. (2022). A systematic review and meta-analysis of facial emotion recognition in autism spectrum disorder: The specificity of deficits and the role of task characteristics. Neuroscience & Biobehavioral Reviews, 133, 104518. https://doi.org/10.1016/j.neubiorev.2021.104518
