ABSTRACT
As a part of our systematic studies on liquid crystal dimers, we present in this article the nature of phase transitions across isotropic–nematic and nematic–smectic-A exhibited by DLCs, α,ω-bis-(4-n-alkylaniline benzylidene-4ʹ-oxy) alkanes. Further, the orientational order parameter in the nematic phase of these DLCs are estimated from the molecular polarisabilities calculated using the experimental refractive indices and density results. The molecular polarisabilities αe and αo are obtained for the compounds using the above results for both Vuks and Neugebauer local field models applicable to nematic liquid crystal. αe and αo calculated in this way are used to obtain Δα. The polarisability anisotropy in the perfect order (absolute K) is calculated semi-empirically using the δ-function model developed by Lippincott et al. and molecular vibration method. The values of polarisability anisotropy for both local electric field models differ significantly. No criterion is known to decide which value is correct. To avoid the determination of uncertain α and Δα values considering different local field models, a simple procedure developed by Kuczynski et al. was used for evaluation of S, based solely on birefringence δn = (ne-no) and this value of S is compared with those obtained from field models.
1. Introduction
As a part of systematic studies [Citation1–Citation3] regarding the synthesis, characterization, phase transition and orientational order parameter, studies on symmetric dimeric liquid crystals (DLCs) are undertaken. The DLCs are also known as bimesogens or twins formed by covalently linking of two mesogenic units through a flexible spacer (alkyl or alkoxy chain).[Citation4–Citation7] The initial interest emerged in the study of these compounds because of their ability to act as model compounds for semiflexible main-chain liquid crystal polymers. Later, the study of the dimeric liquid crystalline has its own importance due to their significant behaviour over the conventional monomeric liquid crystalline compounds. The compounds studied are 3.O7O.3, 5.O7O.5 and 5.O11O.5 DLCs. The first and the last compounds exhibit only nematic liquid crystalline phase while the second material shows smectic-A in addition to nematic phase.[Citation4,Citation8,Citation9] Most of DLCs exhibit only nematic phase unlike their monomeric liquid crystals (LCs).[Citation2,Citation10,Citation11] The DLCS are two types, symmetric and non-symmetric.[Citation7] Recently, phase transition studies are carried out on a number of DLCs.[Citation2,Citation11] Further, it is also known that different workers [Citation1,Citation12–Citation15] have done birefringence investigations of different thermotropic LCs. The thermotropic LCs have special status, namely they will be used in display technology. The present manuscript deals with the synthesis, characterization, phase transition through density and order parameter studies through refractive index and density results with temperature on three symmetric DLCs, namely 3.O7O.3, 5.O7O.5 and 5.O11O.5.
2. Experimental
The compounds are synthesized according to the standard reported in the literature.[Citation4] The crude products are repeatedly recrystallized from ethyl acetate until the transition temperatures remain constant. Differential scanning calorimetry (DSC) studies are carried out using a differential scanning calorimeter (Perkin Elmer instruments, Shelton, CT, USA). Nematic and SmA phases exhibited by the compounds are identified by observing their characteristic optical textures under a polarizing microscope attached with an indigenous hot stage. The temperature resolution of the microscopic studies is ±0.1°C. The density measurements are carried out using a bicapillary pycnometer.[Citation16] The capillary diameter of the pycnometer is of the order of 3.5 × 10−4 m, and the accuracy in density measurements is ±0.1 kg m–3. The permitted cooling rate was 1°C per hour, and temperature accuracy was ±0.1°C. The transition temperatures and the enthalpy values for these compounds are given in . The molecular formula for these materials is given below.
where n = 7 and 11 and m = 3 and 5
3. Results and discussion
All the three materials except 5.O7O.5 exhibit only nematic liquid crystalline phase in between solid and isotropic phase. This particular compound exhibits SmA phase in addition to nematic phase. The methods used, the expressions employed and the procedures adopted are described below in each case for a ready reference.
3.1. Density measurements in three DLC materials
The α,ω-bis(p-n-alkylanilinebenzylidene-p′-oxy)alkanes series with the spacers n = 7, and 11 with alkyl chain m = 3, and 5, namely 3.O7O.3, 5.O7O.5 and 5.O11O.5 exhibit only nematic phase with an exception in 5.O7O.5 which exhibited SmA phase in addition to nematic phase. The synthesis and characterization of the LC phases is described in the experimental section. The phase variants exhibited by different compounds of DLCs in this series along with analogous monomers, namely nO.m compounds are given in . The nature of phase transitions studied through dilatometry in these compounds is isotropic–nematic (IN), nematic–SmA (NSmA). reveals generally that the dimerization of monomers quenches the smectic phases and the enhancement of nematic phase with long thermal ranges.
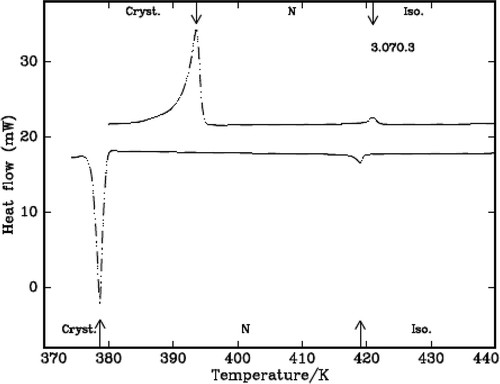
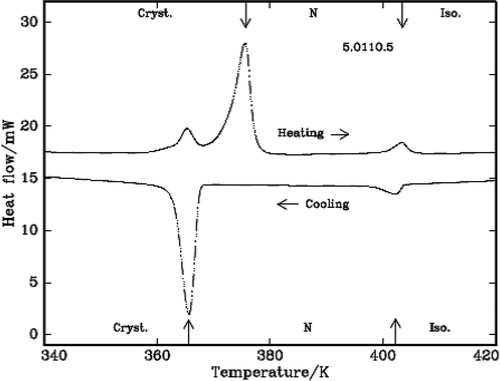
The characteristic textures are identified by the polarizing microscope attached with hot stage and the SmA texture in the case of 5.O7O.5 compound is shown in . The DSC curves are shown in and for the case of DLCs 3.O7O.3 and 5.O11O.5.
The density (ρ = m/V) of a substance is defined as the mass per volume and is generally determined by measuring the volume (V) of a known mass (m) of a substance. Very few investigators [Citation2,Citation3] have done density measurements with temperature in the case of symmetric dimeric liquid in order to obtain relations between the molecular structure and mesomorphic properties. Parameters such as specific volume (v = 1/ρ) and molar volume (Mv = molecular weight/density (ρ)), which are closely related to density are also measured in a few compounds. The thermal expansion coefficient [α = (1/Mv) (dMv/dT)] of the material can also be obtained from the dilatometric studies. The density measurement is useful in determining the order of phase transition, pre-transition behaviour and critical exponent. Fundamentally, the density is related to the derivatives of Gibb’s free energy (G). The first-order transition is characterized by a discontinuity or a steep change in specific volume associated with a thermal expansion coefficient. The density measurements with temperature will provide complementary results to calorimetric techniques such as DSC and thermal microscopy.
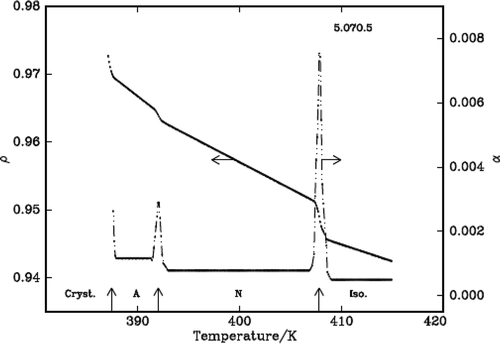
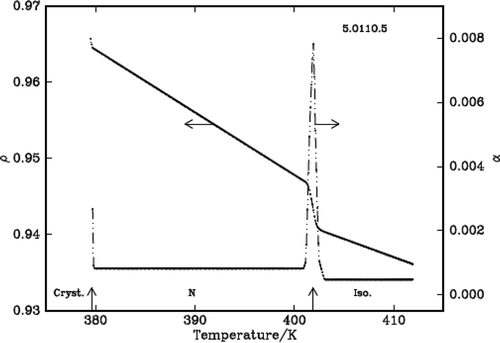
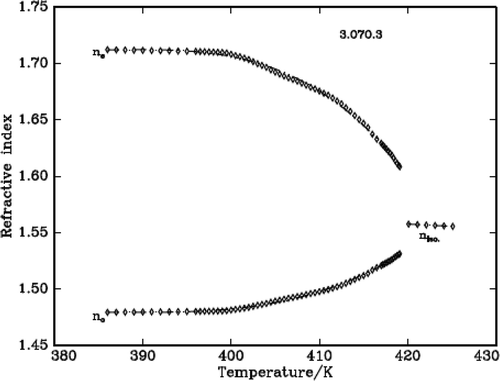
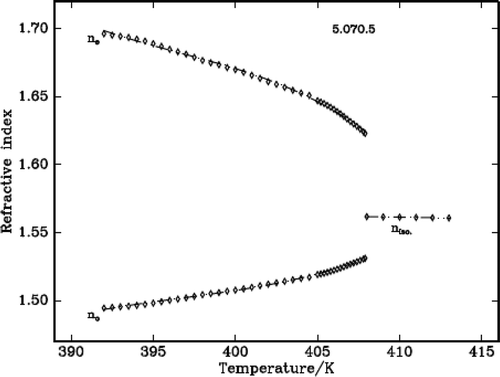
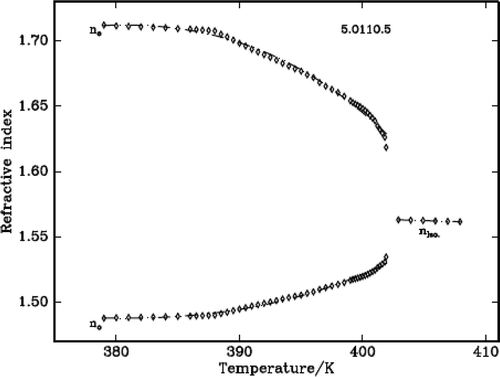
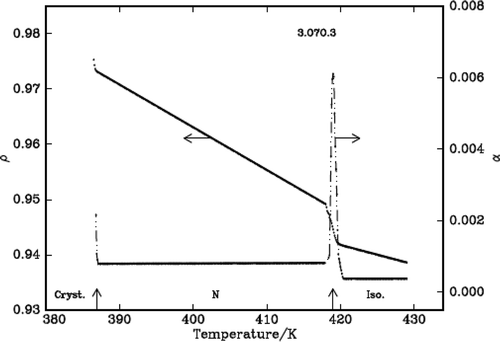
It is found that density decreases with the increase of temperature in the liquid crystalline phases except in the vicinity of phase transformations where it shows a steep increase before it attains equilibrium value of the next (lower temperature) phase. The density jump (Δρ/ρ) is calculated as the vertical distance between the density values (ρ1 and ρ2) obtained by the linear extrapolation from either sides of the transitions, which are in fact the average value of the above two extrapolated density values, i.e., [{(ρ1 – ρ2)}/{(ρ1 + ρ2)/2}]. The variation of density and the thermal expansion coefficient with temperature in all the three DLCs are given in –. The observed density jump (Δρ/ρ) and the thermal expansion coefficient maxima (αmax.) across IN and NSmA of the above three DLCs are presented in .
Table 1. The transition temperatures, the heats of transition in heating and cooling cycles from DSC and the transition temperatures from POM in cooling cycle of the compounds.
Table 2. The phase variants exhibited by different compounds of DLCs in this series along with analogous monomers, namely nO.m compounds.
Table 3. Phase variants, the percentage of density jumps, thermal expansion coefficient maxima at the phase interface and the dρ/dT values in different of dimeric liquid crystals.
Figure 3. Variation of density, ρ (g/cm3) and thermal expansion coefficient, α (10−4 °C−1) with temperature in 3.O7O.3.
3.2. IN transition
The density is found to decrease with the increase of temperature in the liquid crystalline nematic phase of all the compounds except in the vicinity of phase transition where it shows a steep increase before it attains equilibrium value of the next (lower temperature) phase. The observed percentage of density jumps (Δρ/ρ) in these compounds varies between 0.52% and 0.67%, and the values are found to be lower compared to those observed for the other dimers and however, they are higher compared to the monomers [Citation19] and of comparable magnitude in other symmetric dimeric compounds. The thermal expansion coefficient maxima observed for these compounds are given in . These values suggest the first-order nature of the transition as expected at an IN interface.
3.3. NSmA transition
Only one compound, namely 5.O7O.5 exhibits SmA phase along with the nematic phase. The order of this phase transition is found to be weak first order with a density jump of ~0.1% () at the phase transformation. This is consistent with the value of the Mc Millan parameter obtained in the case of monomers, namely nO.m compound. The reported tricritical point, where a second-order transition changes first order in the case of nO.m compounds is ≥0.959.[Citation20] The Mc Millan parameter (TNSmA/TIN) value obtained in this case is 0.961 which is slightly higher than the above reported tricritical point value and the expected weak first order is justified.
The density slopes increase with the decrease of temperature as expected and they are higher in nematic and SmA phases as the orientational order in the former case and positional order along with orientational order in the later cases develop from a disordered isotropic phase. The values are given in
4. Refractive index measurements
The refractive indices of three DLCs are measured using the modified spectrometer and a wedge-shaped cell. The temperature accuracy is ± 0.1°C. The refractive indices ne and no are measured at wavelength 589.3 nm. The refractive index in the isotropic phase (niso) shows very nominal increment with the decrease of temperature. At the IN phase transformation, the isotropic ray splits into two rays which indicates the onset of birefringence, one value higher and another lower than isotropic value corresponding to extraordinary (ne) and ordinary refractive (no) indices, respectively. This is clearly observed in the telescope of the modified spectrometer at the angle of minimum deviation. In the nematic region, the ne increases while the no decreases with the decrease of temperature. – plot the variation of refractive index with temperature in all the three DLC materials.
4.1. Estimation of orientational order parameter, S from density and refractive indices
Orientational order parameter can be expressed in terms of polarisability components as
where and
are, respectively, the molecular polarisabilities for the extraordinary and ordinary ray, and
and
are the polarisability anisotropy of the molecule when the electric vector is, respectively, parallel and perpendicular to the long axis of the molecule.
In the present work, the values of and
have been calculated from the measured refractive index and density data employing Vuks [Citation21] and Neugebauer [Citation22] models. The value of polarisability anisotropy
has been estimated separately using Haller approximation,[Citation23] Lippincott δ-function model [Citation24] and molecular vibration methods.[Citation25] From these values, S has been calculated using Equation (1). Furthermore, S has been estimated using the refractive index data employing Vuks scaling factor method. For the sake of immediate reference, a brief mention of some of these methods is made below.
4.1.1. Estimation of polarisabilities using Vuks model
Vuks [Citation21] postulated that the local field experienced by a molecule is isotropic even in an anisotropic medium. Accordingly, molecular polarisabilities are given by
Here Nlc is the number of molecules per unit volume of the liquid crystalline medium and is given by Nlc = NAρ/M, where NA is the Avogadro number, ρ is the density and M is the molecular weight, and .
4.1.2. Estimation of polarisabilities using Neugebauer model
Neugebauer assumed local field to be anisotropic and derived the following equation relating polarisabilities and refractive indices of the liquid crystalline medium:
From the Lorenz–Lorentz relation for the isotropic medium, we have
where Ni is the number of molecules per unit volume and ni is the refractive index of the sample in its isotropic phase.
On combining Equations (4) and (5), a simple quadratic equation involving can be obtained, whose solutions yield the values of
and
. Since the molecules discussed here have an elongated shape are uniaxially positive, evidently
>
. Hence, the solution which gives
>
is chosen.
4.1.3. Estimation of S using Vuks scaling factor method
The order parameter is given by
4.1.4. Neugebauer method (f(B) parameter)
In the Neugebauer method, the order parameter S is given as follows:
The scaling factors for the determination of order parameter are obtained in both the cases by plotting log–log plots between and f(B) in Vuks and Neugebauer cases, respectively, against (TC – T)/(TC – TNA/NK), that is, the reduced temperature.
The Lippincott δ-function [Citation24] and molecular vibration,[Citation25] whose particulars and the derivative particulars are given in literature, have been successfully used in many liquid crystalline compounds by Pisipati et al.[Citation1,Citation2,Citation12–Citation14] Further, the molecular anisotropy can be obtained from the Haller extrapolation technique using the experimentally evaluated molecular polarisabilities from refractive index and density data. The molecular polarisabilities αe and αo are evaluated assuming a local field that the nematic molecule experiences.
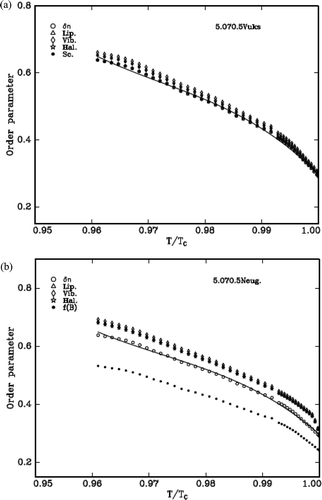
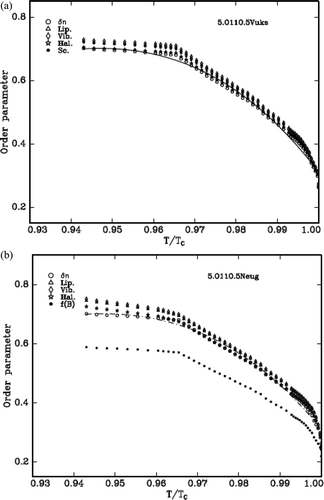
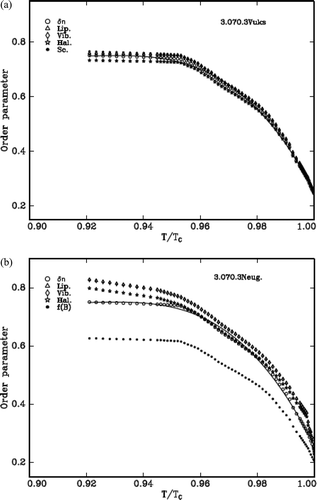
Using the Equation (4) and the values from Lippincott δ-function model and molecular vibration methods the order parameter, S is estimated. The two models, Vuks and Neugebauer, and the molecular polarisabilities, αe and αo, of all the LCs are evaluated from the refractive indices and density. The variation of S with the reduced temperature for the cases of δn, αg (which are identical to one another), Vuks model (Lippincott and vibrational) and Neugebauer model (Lippincott and vibrational) are shown in – for the three DLC materials, respectively. The values are compared with the S value evaluated from δn and αg as the S value obtained from these methods is independent of any internal field that the nematic LC molecule experiences.
Figure 9. (a) Variation of order parameter with reduced temperature (T/Tc) in 3.O7O.3 compound (Vuks). (b) Variation of order parameter with reduced temperature (T/Tc) in 3.O7O.3 compound (Neugebauer).
5. Conclusions
For the sake of comparison of S from different methods for two field models, with S from Δn, the percentage deviation of S value calculated for all methods to that calculated from Δn, the birefringence in perfect order. If the deviation is ≤15%, the values can be considered to be in agreement with the S from Δn within the experimental error. From the Tables, figures of density and order parameter, the following salient features are observed:
The isotropic to nematic transition is found to be first order as expected.
The density jumps observed across Iso–N transition in all compounds are found be on the higher side when compared with nO.m compounds.
The slopes of the density in nematic phase are found to be higher suggesting the closer packing of the molecules.
The S value obtained from the Lippincott δ-function and vibrational method in case of Vuks model agrees in all compounds.
If the values of percentage errors are compared, it is observed that all compounds exhibit more closeness with the order parameter evaluated from ∆n.
Further the figures reveal that the compounds favour Vuks model than that of Neugebauer model. The values of S are always low compared to the above that are obtained using f(B) parameter of Neugebauer method.
Finally, the overall observations from all the compounds suggest the Vuks isotropic model is favorable somewhat to that of Neugebauer model.
Acknowledgements
The authors P. Pardha Saradhi and V.G.K.M. Pisipati express their thanks to The Head, ECE Department and the Management of K. L. University, Vaddeswaram, India, for providing facilities. P.V. Datta Prasad acknowledges the S.S.D. Polymers for providing some facilities during the work. D. Venkata Rao thanks the management, S. V. Engineering College, Nellore, for giving the support.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
P. Pardhasaradhi ![]() http://orcid.org/0000-0002-6840-9254
http://orcid.org/0000-0002-6840-9254
V.G.K.M. Pisipati ![]() http://orcid.org/0000-0003-2382-4400
http://orcid.org/0000-0003-2382-4400
References
- Madhavi Latha D, Pisipatib VGKM, Pardhasaradhi P, et al. Estimation of order parameter from different models in symmetric dimeric liquid crystals. Liq Cryst Today. 2014;23:54–60. DOI:10.1080/1358314X.2014.917797.
- Pardhasaradhi P, Datta Prasad PV, Madhavi Latha D, et al. Orientational order parameter studies in two symmetric dimeric liquid crystals – an optical study. Phase Transit. 2012;85:1031–1044. DOI:10.1080/01411594.2012.671323.
- Bhuyan D, Pardhasaradhi P, Gogoi B, et al. Phase transition studies of 6.O12O.6 and 7.O6O.7 using density measurements. Mol Cryst Liq Cryst. 2011;540:205–212. DOI:10.1080/15421406.2011.568882.
- Imrie CT, Luckhurst GR, Seddon JM. Smectogenic dimeric liquid crystals. The preparation and properties of the α,ω-bis(4-n-alkylanilinebenzylidine-4′-oxy)alkanes. Liq Cryst. 1992;12:203–238. DOI:10.1080/02678299208030393.
- Heeks SK, Luckhurst GR. On the molecular organisation within the nematic phase of liquid crystal dimers. J Chem Soc Faraday Trans. 1993;89:3289–3296. DOI:10.1039/ft9938903289.
- Attard GS, Date RW, Imrie CT, et al. Non-symmetric dimeric liquid crystals The preparation and properties of the α-(4-cyanobiphenyl-4′-yloxy)-ω-(4-n-alkylanilinebenzylidene-4′-oxy)alkanes. Liq Cryst. 1994;16:529–581. DOI:10.1080/02678299408036531.
- Date RW, Luckhurst GR, Schuman M, et al. Novel modulated hexatic phases in symmetrical liquid-crystal dimers. J De Phys II. 1995;5:587–605.
- Imrie CT, Hendersona PA, Yeap G-Y. Liquid crystal oligomers: going beyond dimmers. Liq Cryst. 2009;36:755–777. DOI:10.1080/02678290903157455.
- Demus D, Goodby J, Gray GW, et al., editors. Handbook of liquid crystals. Vol. 2B, Weinheim (Germany): Wiley-VCH; 1998, p. 801–833.
- Wang H, Shao R, Zhu C, et al. Symmetric liquid crystal dimers containing hydrazide groups: parity-dependent smectic structure, hydrogen bonding and substitution effect. Liq Cryst. 2008;35:967–974. DOI:10.1080/02678290802308035.
- Pal SK, Raghunathan VA, Kumar S. Phase transitions in novel disulphide bridged alkoxycyanobiphenyl dimers. Liq Cryst. 2007;34:135–141. DOI:10.1080/02678290601061280.
- Pisipati VGKM, Madhavi Latha D, Prasada D, et al. Order parameter studies from effective order geometry in a number of liquid crystals of different homologous series. J Mol Liq. 2012;74:1–4. DOI:10.1016/j.molliq.2012.07.009.
- Rajeswari BR, Pardhasaradhi P, Ramakrishna Nanachara Rao M, et al. Optical study of orientational order parameter in (p-n-phenyl benzylidene)-p-alkyl and alkyloxy anilines. Solid State Phenom. 2011;181–182:75–78. DOI:10.4028/www.scientific.net/SSP.181-182.
- Lalitha Kumari. J, Datta Prasad. PV, Madhavi Latha D, et al. Orientational order parameter estimated from molecular polarizabilities – an optical study. Phase Transit. 2012;85:52–64. DOI:10.1080/01411594.2011.578826.
- Pardhasaradhi P, Murthy CSVS, Lalitha Kumar J, et al. Orientational order parameter-II - A birefringence study. Mol Cryst Liq Cryst. 2009;511:121–132. DOI:10.1080/15421400903053644.
- Ajeetha N, Pisipati VGKM, Nanchara Rao MR, et al. Synthesis, characterization, and dilatometric studies on N-(p-n-alkoxybenzylidene)-p-n-pentyloxyanilines compounds. Mol Cryst Liq Cryst. 2006;457:3–25. DOI:10.1080/15421400500447108.
- Pisipati VGKM. Polymesomorphism in N-(p-n-Alkoxybenzylidene)-p-n-Alkylanilines (nO.m) compounds. Z Naturforsch. 2003;58a:661.
- Gogoi B, Alapati PR, Verma AL. Phase transition studies in mesogenic dimers. Cryst Res Technol. 2002;37:1331.
- Rao NVS, Pisipati VGKM, Sankar G, et al. Phase transition studies in 70.8 and 80.8 benzylidene liquid crystals. Acustica. 1986;60:163.
- Pisipati VGKM, Rananavara SB, Freed JH. DSC characterization of nematic-smectic and smectic-smectic phase transitions in N(p-n-Alkoxy Benzilidene)-p-n-Alkylanilines (n 0.m). Mol Cryst Liq Cryst Letts. 1987;4:181–189.
- Vuks MF. Determination of optical anisotropy of aromatic molecules from double refraction in crystals. Opt Spectrosc. 1966;20:361.
- Neugebauer HEJ. Clasius-Mosotti equations for certain types of anisotropic crystals. Can J Phys. 1950;28:292.
- Haller I. Thermodynamic and static properties of liquid crystals. Prog Solid State Chem. 1975;10:103. DOI:10.1016/0079-6786(75)90008-4.
- Lippincott ER, Stutman JM. Polarizabilities from δ-function potentials. J Phys Chem. 1964;68:2926–2940. DOI:10.1021/j100792a033.
- Murthy YN, Murthy VR, Ranga Reddy RNV. Molecular vibration approach to polarizability of methyl cinnamate liquid crystal compounds. Acta Phys Pol A. 1997;91(1997):1069.