ABSTRACT
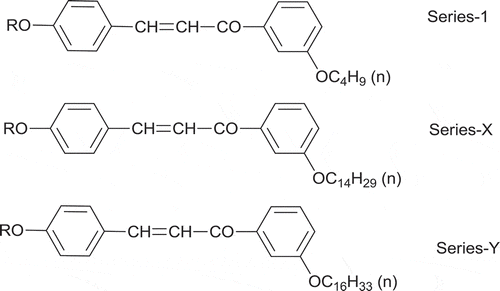
In order to investigate the influence of the central linking group and the effect of flexibility on mesomorphism, we have synthesised a newly homologous series 3-(3-butoxyphenyl)-1-(4-n-alkoxy phenyl) prop-2-en-1-one (series-l) consisting of 13 homologues C1–C8, C10, C12, C14, C16 and C18. In the present series, mesophase commences from the C6 homologue. C1–C5 homologues did not exhibit liquid crystalline (LC) property, while C6–C12 homologues exhibited an enantiotropic nematic phase and the rest of the homologues C14–C18 displayed monotropic SmC and nematic mesophase. The transition temperatures of the synthesised compounds were determined by an optical polarising microscopy equipped with a heating stage. All newly synthesised compounds were confirmed by 1H-NMR, 13C-NMR, IR and elemental analysis.
1. Introduction
Liquid crystalline (LC) [Citation1] property of a substance is a unique property, which flows on the surface like a liquid and possesses optical properties like crystals. Therefore, such substances of thermotropic or lyotropic varieties are neither fully crystalline nor fully liquid. The chalconyl derivatives, due to their geometrical shapes, may exhibit LC properties with lower thermometric transitions and bioactivity. The aims and objectives of this article are to study the effect of the molecular structure on the LC properties and behaviours [Citation2–Citation6] as a result of molecular flexibility, keeping the molecular rigidity unaltered throughout a series and changing their flexibility from series to series for the same homologue at constant rigidity. LC substances have proven their ability for LC devices to be operated at room temperature [Citation7–Citation12]. The present investigation will include synthesis and characterisation by analytical, thermal and spectral data. Thermometric data will be derived using an optical polarising microscope (POM) equipped with a heating stage and this will be discussed and interpreted in terms of molecular rigidity and flexibility in relation [Citation8,Citation13–Citation15] to molecular structure geometry, size, polarity, polarisability, etc. The LC properties of the present novel series will be compared with the structurally similar analogous series.
A wide range of chalcone derivatives has been reported to exhibit a broad spectrum of antibacterial, anti-fungal, anti-ulcer, anti-malarial, anti-tumour, anti-cancer, anti-inflammatory and anti-tubercular behaviour. The presence of the α, β-unsaturated functional group in chalcone (-CH = CH-CO-) is responsible for the antimicrobial activity, which can be altered depending on the type of substituents present on the aromatic rings [Citation16–Citation19]. Chalcone (Ar-CH = CH-CO-Ar)-based compounds are important intermediates in the synthesis of many pharmaceuticals. Some of the chalcone derivatives play a vital role in numerous fields such as crystallography [Citation20], LC polymers, dye industries and solar cells [Citation21–Citation23]. They are also utilised in material fields such as nonlinear optics, optical limiting, electrochemical sensing and Langmuir films. Π-bond conjugation with chalcones (α, β-unsaturated ketone) leads to a good charge transfer axis, and the aromatic rings act as a tool for a donor and an acceptor [Citation24]. The effect of donor–acceptor strength on the electronic properties of conjugated molecules is a major topic in the molecular design of nonlinear optical materials.
Thus, the objective of this work is to synthesise and study the effect of chalconyl-ester linkage group and geometrical shape on the mesomorphic properties of liquid crystals. Doshi et al. reported chalconyl-ester linking group-based homologues series [Citation25–Citation31]. Previously, our research group also reported homologues series based on chalconyl-ester and chalconyl-vinyl ester central group with reference to two or three phenyl rings, respectively [Citation32–Citation45].
2. Experimental
2.1. Reagents and techniques
For the synthesis of compounds of the homologous series, the following materials were used: 4-hydroxy benzaldehyde (SRL, Mumbai), alkyl bromides (SRL, Mumbai) and 3-hydroxy acetophenone (Sigma-Aldrich), which were used without purification. All the solvents were dried and distilled prior to use. Representative homologues of a series were characterised by elemental analysis, FT-IR (cm-1, KBr), 1H and 13C NMR spectra (300 MHz, CDCl3) using tetramethylsilane as an internal standard. IR spectra were recorded on Perkin-Elmer spectrum GX, and 1H NMR spectra were recorded on Bruker using CDCl3 as a solvent. Microanalysis was performed on Perkin-Elmer PE 2400 CHN analyser. Texture images of the nematic phase were determined by the miscibility method (). Transition temperature () and LC properties (textures) were determined using a POM equipped with the heating stage. The compound is sandwiched between a glass slide and a cover slip, and the heating and cooling rate is 2°C.
Table 1. Texture of the nematic phase of C14, C12, C6 and C8 by the miscibility method.
Table 2. Transition temperature by POM in °C.
2.2. Synthesis and method
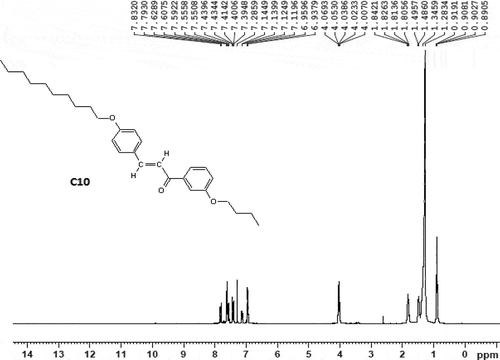
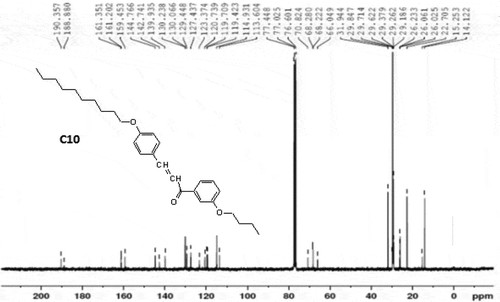
Alkylation of 4-hydroxy benzaldehyde to give 4-n-alkoxy benzaldehyde was carried out by the reported method [Citation46,Citation47]. 3-n-butoxy acetophenone was obtained by the alkylation of 3-hydroxy acetophenone by the reported method [Citation46,Citation47]. Thus, the chalconyl homologue derivatives (C) were usually prepared by the established method [Citation48]. The final products were collected and filtered, washed with ethanol solution, dried and purified until constant transition temperatures were obtained using a POM. The synthetic route to the series is mentioned below as . The 1H and 13C NMR spectra of comp.C10 is shown in and .
2.3. Reaction scheme
2.4. Analytical data
C6 (Hexyloxy): IR (KBr): vmax/cm−1 2914 (C-H str. of alkane), 2842 (C-H str. of -(CH2-)n group of -OC6H13 alkyl chain, 1640 (C = O str. of carbonyl carbon of chalconyl group), 1604 (C = C str. of alkene in chalcone), 1510, 1543 (C = C str. of aromatic ring), 999 (C-H bending of alkene), 1178 (C-O str. of ether linkage), 1288, 1246 (C-O str. of carbonyl (>CO) group, 972 (trans, -CH = CH-) group, 770 polymethylene (-CH2-) of -OC4H9, 675 polymethylene (-CH2-)n of -OC6H13, IR data confirms the molecular structure of comp.C8, 1HNMR: 0.88–0.90 (t, J = 8.0Hz, 6H, -CH3 of polymethylene -C6H13 and -C4H9), 1.76 (m, J = 7.1 Hz, 4H, -OC6H13 and -OC4H9), 1.31 (m, J = 7.1Hz, 4H, -CH2-CH2-CH2- of -OC6H13), 1.31 (q, J = 7.4, 8H, -CH2-CH3), 4.06 (t, J = 7.0 Hz, 4H, -OCH2-CH2-), 7.59–8.06 (d, J = 15.1Hz, 2H, -CH = CH-), 7.43, 7.45, 7.82 and 7.24 (4H, J = 8.1Hz, second –meta-substituted phenyl ring), 6.94, 7.56 & 7.97 (4H, J = 8.0 Hz, first phenyl ring with alkoxy chain). 13C NMR (CDCl3): 161.3, 130.2, 129.3, 120.7, 114.9, 114.1, (Ar-C), 14.1 (-CH3), 15.25, 31.9, 68.2, 29.6, 25.9, 29.6, 29.3, 22.7 (-CH2), 145.1, 121.3 (-CH of olefin). elemental analysis: calculated for C25H32O3: C, 78.94; H, 8.42; O, 12.63%; found: C, 78.88; H, 8.38; O, 12.57%.
C8 (Octyloxy): IR (KBr): vmax/cm−1 2914 (C-H str. of alkane), 2842 (C-H str. of -(CH2-)n group of -OC8H17 alkyl chain, 1660 (C = O str. of carbonyl carbon of chalconyl group), 1604 (C = C str. of alkene in chalcone), 1510, 1543 (C = C str. of aromatic ring), 996 (C-H bending of alkene), 1178 (C-O str. of ether linkage), 1286, 1240 (C-O str. of carbonyl (>CO) group, 972 (trans, -CH = CH-) group, 771 polymethylene (-CH2-) of – OC4H9, 782 polymethylene (-CH2-)n of -OC8H17, IR data confirms the molecular structure of comp.C8, 1HNMR: 0.89–0.90 (t, J = 8.0 Hz, 6H, -CH3 of polymethylene -C8H17 and -C4H 9), 1.76 (m, 4H, J = 7.1 Hz, CH3-CH2-CH2-CH2-CH2- of -OC8H17, 1.28 (m, J = 7.1 Hz, 8H -CH2-CH2-CH2- of -OC8H17), 1.31 (q, J = 7.1 Hz, 8H, -CH2-CH3 of -OC8H17), 4.06 (t, J = 7.1Hz, 4H, -OCH2-CH2- of -OC8H17, -OC4H9), 7.59–8.06 (d, J = 15.1, 2H, -CH = CH-), 7.43, 7.28 & 7.83 (4H, J = 8.0Hz, -meta- substituted phenyl ring), 6.94 and 7.62 (4H, J = 7.5 Hz, phenyl ring with alkoxy chain), 13C NMR (CDCl3): 161.3, 130.2, 129.3, 120.7, 114.9, 114.1, (Ar-C), 14.1 (-CH3), 15.25, 31.9, 68.7 (-CH2- bridge), 29.6, 25.9, 29.6, 29.3, 22.7 (-CH2), 145.1, 121.3 (-CH of olefin) elemental analysis: calculated for C27H36O3: C, 79.41; H, 8.82; O, 11.76%; found: C, 79.36; H, 8.78; O, 11.74%.
C10 (Decyloxy): IR (KBr): vmax/cm−1 2914 (C-H str. of alkane), 2842 (C-H str. of -(CH2-)n group of -OC10H21 alkyl chain, 1640 (C = O str. of carbonyl carbon of chalconyl group), 1604 (C = C str. of alkene in chalcone), 1510, 1543 (C = C str. of aromatic ring), 999 (C-H bending of alkene), 1178 (C-O str. of ether linkage), 1288, 1246 (C-O str. of carbonyl (>CO) group, 964 (trans, -CH = CH-) group, 771 polymethylene (-CH2-) of -OC4H9, 792 polymethylene (-CH2-)n of -OC10H21, IR data confirms the molecular structure of comp. C10, 1HNMR (CDCl3): 0.89 to 0.90 (t, J = 8.0 Hz, 6H, -CH3 of polymethylene -C10H21 and – C4H9), 1.80 (m, J = 7.1Hz, 4H, CH3-CH2-CH2-CH2-CH2- of -OC10H21), 1.28 (m, 6H -CH2-CH2-CH2- of -OC10H21), 1.31 (q, 4H, -CH2-CH3 of -OC10H21), 4.06 (t, J = 7.1Hz, 4H, -O-CH2-CH2- of -OC10H21 and -OC4H9), 8.06 (d, J = 15.1Hz, 2H, -CH = CH-), 7.43, 7.28 and 7.79 (4H, J = 8.1 Hz, -meta-substituted second phenyl ring), 7.62 and 7.83 (4H, J = 8.0 Hz, first phenyl ring with alkoxy chain), 13C NMR (CDCl3): 161.3, 158.6, 130.2, 129.3, 120.7, 114.9, 114.1, (Ar-C), 14.1 (-CH3), 15.25, 31.9, 68.7 (-CH2- bridge), 68.4 (29.6, 25.9, 29.6, 29.3, 22.7 (-CH2), 145.1, 121.3 (-CH of olefin) elemental analysis: calculated for C29H40O3: C, 79.81; H, 9.17; O, 11.00%; found: C, 79.76; H, 9.11; O, 10.62%.
C12 (Dodecyloxy): IR (KBr): vmax/cm−1 2992 (C-H str. of alkane), 2880 (C-H str. of -(CH2-)n group of -OC12H25 alkyl chain, 1660 (C = O str. of carbonyl carbon of chalconyl group), 1604 (C = C str. of alkene in chalcone), 1510, 1543 (C = C str. of aromatic ring), 999 (C-H bending of alkene), 1178 (C-O str. of ether linkage), 1288, 1242 (C-O str. of carbonyl (>CO) group, 972 (trans, -CH = CH-) group, 771 polymethylene (-CH2-) of – OC4H9, 804 polymethylene (-CH2-)n of -OC12H25, IR data confirms the molecular structure of comp.C12, 1HNMR: 0.88 (t, 6H, -CH3 of polymethylene -C12H25 and -C4H9), 1.79 (p, 10H, CH3-CH2-CH2-CH2-CH2- of -OC12H25 and -OC4H9), 1.28 (m, 8H – CH2-CH2-CH2- of -OC12H25), 1.29–1.31 (q, 8H, -CH2-CH3 of -OC12H25 and -OC4H9), 4.06 (t, 4H, -OCH2-CH2-), 7.59 (d, J = 15.2 Hz, 2H, -CH = CH-), 7.43, 7.28 and 7.83 (4H, J = 8.0Hz, -meta-substituted second phenyl ring), 7.62 and 6.94, 6.45 (m, 4H, J = 8.1Hz, first phenyl ring with alkoxy chain), 13C NMR (CDCl3): 164.6, 158.6, 130.2, 129.3, 126.8, 120.2, 114.6, 114.3, (Ar-C), 14.1 (-CH3), 15.25, 31.9, 68.2, 29.6, 25.9, 29.6, 29.3, 22.7 (-CH2), 145.1, 121.3 (-CH of olefin) elemental analysis: calculated for C31H44O3: C, 80.17; H, 9.48; O, 10.34%; found: C, 79.11; H, 9.43; O, 10.28%.
3. Result and discussion
3.1. POM investigation
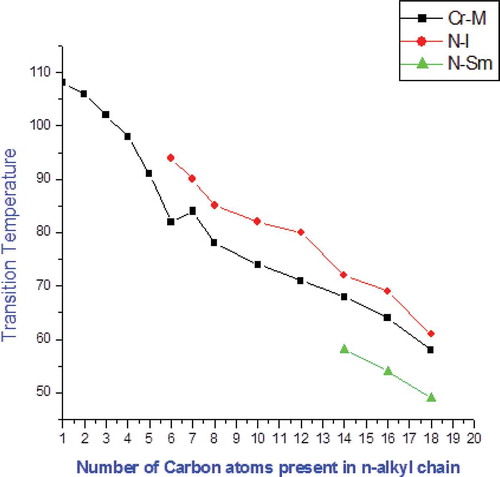
The target chalconyl base compounds and their intermediates were synthesised as outlined in . 4-n-alkoxy benzaldehydes condensation with 3-butoxy acetophenone yielded 13 chalconyl derivatives. The common structural features of the compounds are that they consist of the same linkage group and the left n-alkoxy terminal side chain at one end. Compounds C6–C18 show LC property in an enantiotropical manner, whereas compounds C14–C18 exhibit monotropically nematogenic and smectogenic phases. C1–C5 homologues are non-mesogenic in nature due to the presence of short alkyl spacer in the -meta group. The phase diagram of the present series is shown in . The transition temperatures () of the present newly homologous series-1 are determined by POM, and the phase diagram is plotted against the transition temperature versus the number of carbon atoms present in the n-alkyl chain ‘R’ of the-OR terminal group and subsequently on linking like or related points, the transition curves Cr–I/N, I–N or N–I, N–Sm are obtained. The Cr–I/N transition curve falling up to the C6 homologue increases at the C7 homologue because of the odd–even effect and then continues to descend up to the last C18 homologue. The N–I or I–N transition curve falls with descending tendency up to the last homologue of the present series. An odd–even effect is absent in the N–I or I–N transition curve. Thus, it behaved in a normal manner neglecting minor deviating effect from the C8 homologue beyond merging of the N–I/I–N curves for the higher homologue of longer n-alkyl chain ‘R’ of the -OR group. The monotropic N–Sm transition curve starts descending with increasing right-meta-substituted alkyl spacer from the C14 to C18 homologue to induce a smectic mesophase. The mesophase length for N–Sm transition is very short of the fraction for 4–5°C. The textures of the nematic phase are threaded or rod type and those of the smectic C phase are needle type as judged directly from the heating top of the POM. Analytical, thermal and spectral data supported the molecular structures of the newly synthesised compounds. Thermal stability of nematic to isotropic phase is 64.3°C. Thus, the temperature ranges of the mesophase are very small or shorter. The LC properties of thermotropic novel LC chalcones from homologue to homologue in the same series undergo variation with changing length of the molecules or permanent dipole moment across the longer molecular axis.
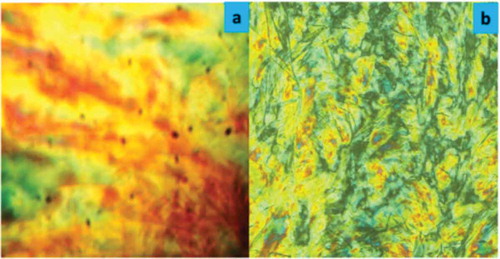
The sequential order of mesophase appearance for monotropy and enantiotropy is relapsed compared with the normal order of phase sequence observed in other homologous series. The variations in the mesogenic behaviours from homologue to homologue in the same series are observed throughout a novel series with increasing number of carbon atoms in the n-alkyl chain ‘R’, keeping the rest of the molecular part unchanged. Exhibition of mesomorphism either in monotropic or in enantiotropic manner by the homologues of the present newly synthesised series, except for five non-mesomorphic compounds, is attributed to the increase in molecular length, molecular polarity and polarisability, molecular rigidity and flexibility, permanent dipole moment across the long molecular axis, suitable magnitudes of dispersion forces and dipole–dipole interactions, which under exposed thermal vibrations lead to facilitating the molecular arrangement required to induce the smectic and/or nematic phase reversibly or irreversibly above or below the isotropic temperature from the C6 homologue under floating condition. The texture images of compounds C10 and C14 are shown in . The nematic texture image of compound C10 is exhibited at 74°C in the heating condition, while the typical texture image of the SmC phase for compound C14 is observed at 58°C on cooled condition.
Figure 4. Textures of compound of series-1 observed between cross-polarisers: (a) nematic texture image of C10 at 74°C; (b) SmC texture image of C14 at 58°C.
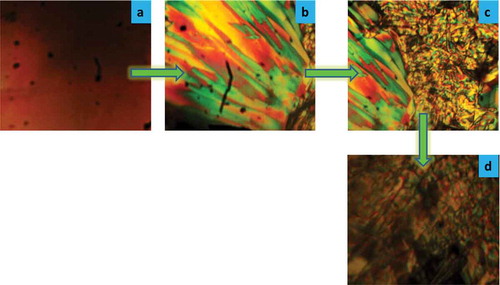
The phase sequence of compound C16 on the cooling condition is shown in . The compound C16 homologue is cooled from its isotropic temperature; the nematic phase is formed at 64°C; on further cooling it is transformed into a needle-type SmC phase at 54°C. Finally, a solid crystal is obtained at lower temperature.
Figure 5. Phase sequence of the C16 homologue on cooling condition by POM: (a) isotropic at 69°C; (b) nematic texture image appeared at 64°C; (c) needle-type SmC texture image observed at 54°C; (d) solid crystal is formed below 54°C.
In the present synthesised series, an attempt is made to synthesise LC material based on two phenyl rings and a single chalconyl linking group. We observed that the stability of the mesophase is lower due to one central linkage group and increasing the flexibility by two side chains to induce mesophase at lower temperature. As a result, lowering of the transition temperatures of homologues is attributed to the predominance of intermolecular distances due to widening of the molecule, which causes a reduction in the cohesive forces in competition with the cohesive forces generated by polarisability occurring by the laterally –meta-substituted –OC4H9 (n) flexible group and changing of the left side chain from the C6 and C18 homologues to exhibit nematic and smectic phase, respectively. The odd-even effect observed for the N–I transition curve is due to the sequentially added methylene unit. The non-mesomorphic behaviours of the C1–C5 homologues are due to the low magnitudes of dispersion forces and the low magnitudes of the dipole–dipole interactions, leading to high crystallising tendency and inducing unsuitable magnitudes of anisotropic forces of end-to-end and lateral attractions. Diminishing of the odd-even effect for higher homologues beyond the C7 homologue of longer n-alkyl chain ‘R’ of – OR and – OC4H9 (n) lateral flexible groups is attributed to coupling with the major axes of the core structure of a molecule. Therefore, the reversal of monotropy and enantiotropy in the present novel series may be due to the unexpected nature and uncertainty arising from the unpredictable status of the n-alkyl chain ‘R’ of – OR and – C4H9(n) of the -meta-substituted lateral group.
3.2. Comparative parameter
The variations in the LC property and the degree of mesomorphism from homologue to homologue in the same series or from series to series for the same homologue are attributed to the changing magnitudes of molecular rigidity and flexibility due to changing molecular polarity and polarisability and other related parameters concerning the suitable magnitudes of intermolecular cohesions and closeness. The mesogenic properties of the presently investigated homologous series-1 are compared with structurally similar series-X [Citation43] and -Y [Citation49], as shown in .
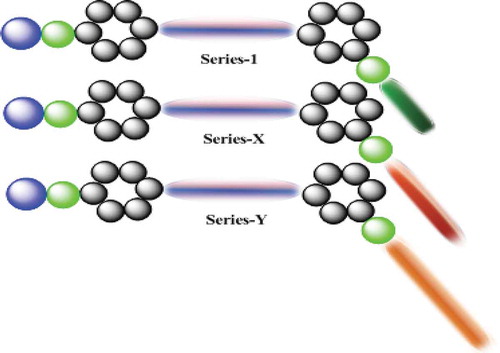
Homologous series-1, -X and -Y under a comparative study consisted of two phenyl rings and one central chalcone bridge (–CH = CH-CO-) in the present series-1 and also series-X and -Y. The left terminal alkoxy group (–OR), in which the n-alkyl chain ‘R’ varies with the number of methylene units from homologue to homologue in the same series but the number of methylene units of ‘R’ on the left remains unaltered for the same homologue from series-1 to series-Y, contributes partly towards the sharing of the total molecular flexibility. For the laterally substituted tail groups – OC4H9(n), -OC14H29(n) and – OC16H33(n), the molecular flexibility, group polarities and polarisability vary from series to series with the broadening of the alkyl spacer but remain unaltered from homologue to homologue in the same series, respectively, due to their respective lateral groups being linked to the -meta position of the second phenyl ring but vary with changing – R of the alkoxy terminal group. Thus, variations in the LC properties among the series-1, -X and -Y can be attributed to the varying features related to mesomorphism because of even a minute changing feature or the total changing flexibility of the terminal and/or lateral groups for the same homologue from series to series.
3.3. Thermal stability and temperature range of the mesophase
represents some thermometric data evaluated from the present investigation of series-1 compared with series-X and -Y.
Table 3. Thermal stability in °C.
The homologous series-1, -X and -Y are identical with respect to the exhibition of smectogenic and nematogenic properties. Exhibition of the smectogenic mesophase commences from C14 (series-1), C6 (series-X) and C7 (series-Y). The nematic mesomorphism commences from C6 (series-X) and C2 (series-X, -Y). Thermal stability for the nematic phase increases from series-X to -1 to -Y, respectively. However, the stabilisation of the smectogenic character is very poor in series-1 compared with series-2 and -3.
The geometrical shapes of the presently synthesised series and other comparative series are shown in . The aromaticity, permanent dipole moment and molecular rigidity of lower member (C1) to higher member (C18) became unaltered at right terminal side with differs in right side substituted tail ended group under comparative study. The length of the laterally -meta-substituted groups increases from series-1, -X and -Y in order of – OC4H9 (n) < -OC14H29 (n) < – OC16H33 (n), which differs by increasing the – CH2- unit from series-1 to series-X to series-Y. However, the longer n-alkyl -meta-substituted chain bonded through the oxygen atom of the lateral group also may cause the difference in polarity and polarisability due to the gradually added – CH2-CH2- unit and the same longer n-alkyl chains may couple with the major axis of the core structure of the molecule, which may modify the magnitudes of flexibility and the magnitudes of intermolecular cohesions and closeness for the same homologue from series to series under the influence of the exposed thermal vibrations when floated on the surface. However, the experimentally evaluated thermometric data suggest that there is very little difference in the thermal stabilities, commencements of nematic phase, degrees of mesomorphism or facilitations of the upper and lower total temperature range of the mesophase as well as the types of mesophases for the same homologue from series to series.
4. Conclusions
In this article, we have described the synthesis and thermotropic properties of liquid crystal based on the chalcone linkage group. A novel chalconyl homologous series is predominantly nematogenic and partly smectogenic with lower transition temperatures. C1–C5 display non-LC property whereas C6–C18 show an enantiotropic nematic phase. Compounds C14, C16 and C18 show a smectic mesophase on cooling. The thermal stability of the nematic phase in series-1 is higher than that of series-X and -Y due to the short alkyl chain substituted at the second phenyl ring while in series-X tetradecyloxy and in series-Y hexadecyloxy alkyl chain is present, which increases the flexibility of the molecule and decreases its thermal stability. The group efficiency order is derived on the basis of thermal stabilities, commencement of the mesophase and the upper and lower temperature ranges for the smectic and nematic mesophases.
Acknowledgements
The authors acknowledge Dr R. R. Shah, principal and management of K. K. Shah Jarodwala Maninagar Science College, Ahmedabad. The authors are also thankful to NFDD Centre for providing analytical and spectral services.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hird M, Raoul Y, Goodby JW, et al. Chirality issues and interesting properties of achiral and racemic materials with bent molecular structures. Ferroelectrics. 2004;309:95–100.
- Glendenning ME, Goodby GW, Hird M, et al. The synthesis and properties of fluoroterphenyls for high dielectric biaxiality ferroelectric liquid crystal mixtures. Mol Cryst Liq Cryst. 1999;332:321–328.
- Demus D. 100 years liquid crystals chemistry. Mol Cryst Liq Cryst. 1988;165:45.
- Hird M. Banana-shaped and other bent-core liquid crystals. Liq Cryst Today. 2005;14:9–21.
- Seed AJ, Hird M, Styring P, et al. Heterocyclic esters exhibiting frustrated liquid crystal phases. Mol Cryst Liq Cryst. 1997;299:19–25.
- Kuang GC, Jia XR, Teng MJ, et al. Organogels and liquid crystalline properties of amino acid-based dendrons: a systematic study on structure–property relationship. Chem Mater. 2012;24:71–80.
- Singh B, Pandey A, Singh SK. Liquid crystalline properties of 3-[4-(4′-Alkoxybenzoyloxybenzylidene) amino]-1,2,4-triazines: synthesis and characterization. Mol Cryst Liq Cryst. 2010;517:127–137.
- Kim WS, Elston SJ, Raynes FP. Hybrid method for modelling light leakage by a spherical object in a liquid crystal layer. Displays. 2008;29:458–463.
- Hird M, Toyne KJ, Gray GW. Palladium-catalysed cross-coupling reactions in the synthesis of some high polarizability materials. Liq Cryst. 1993;14:741–761.
- Goodby JD. A pictorial approach to helical macrostructures in smectic liquid crystals. Mol Cryst Liq Cryst. 1997;292:245–263.
- Marcos M, Omenat A, Serrano JL, et al. A ferroelectric liquid crystal dimer: synthesis and properties. Adv Mater. 1992;4:285–287.
- Klein C, Baranoff E, Nazeeruddin MK, et al. Convenient synthesis of functionalized 4,4′-disubstituted-2,2′-bipyridine with extended π-system for dye-sensitized solar cell applications. Tetrahedron Lett. 2010;51:6161–6165.
- Thaker BT, Patel PH, Vansadiya AD, et al. Substitution effects on the liquid crystalline properties of thermotropic liquid crystals containing schiff base chalcone linkages. Mol Cryst Liq Cryst. 2009;515:135–147.
- Yelamaggad CV, Bonde NL, Achalkumar AS, et al. Frustrated liquid crystals: synthesis and mesomorphic behavior of unsymmetrical dimers possessing chiral and fluorescent entities. Chem Mater. 2007;19:2463–2472.
- Thaker BT, Kanojiya JB. Synthesis, characterization and mesophase behavior of new liquid crystalline compounds having chalcone as a central linkage. Mol Cryst Liq Cryst. 2011;542:84.
- Gaikwad PP, Desai MT. Liquid crystalline phase and its pharma application. Int J Pharma Res Rev. 2013;2(12):40–52.
- Domínguez JN, León C, Rodrigues J, et al. Synthesis and evaluation of new antimalarial phenylurenyl chalcone derivatives. J Med Chem. 2005;48:3654–3658.
- Nielsen SF, Larsen M, Boesen T, et al. Cationic chalcone antibiotics. design, synthesis, and mechanism of action. J Med Chem. 2005;48:2667–2677.
- Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42:125–137.
- Rao YK, Fang SH, Tzeng YM. Differential effects of synthesized 2′-oxygenated chalcone derivatives: modulation of human cell cycle phase distribution. Bioorg Med Chem. 2004;12:2679–2686.
- Asiri AM, Khan SA. Synthesis, characterization and optical properties of mono- and bis-chalcone. Mater Lett. 2011;65:1749–1752.
- Rajakumar P, Thirunarayanan A, Raja S, et al. Photophysical properties and dye-sensitized solar cell studies on thiadiazole–triazole–chalcone dendrimers. Tetrahedron Lett. 2012;53:1139–1143.
- Modzelewska A, Pettit C, Achanta G, et al. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg Med Chem. 2006;14:3491–3495.
- Shuai Z, Ramasesha S, Bredas JL. Nonlinear optical properties of nitro-aniline and methyl-aniline compounds — an exact correction vector INDO-SDCI study. Chem Phys Lett. 1996;250:14–18.
- Patel RB, Patel VR, Doshi AV. Synthesis and mesomorphism of novel liquid-crystalline Isobutyl-p-(p′-n-Alkoxy Cinnamoyloxy) cinnamates. Mol Cryst Liq Cryst. 2012;552:3–9.
- Chaudhary RP, Patel RB, Doshi AV. Synthesis and mesomorphic properties of novel ethylene derivatives. Mol Cryst Liq Cryst. 2013;577:95–102.
- Suthar. DM, Doshi AA, Doshi AV. Synthesis and evaluation of liquid crystalline properties of a novel homologous series: α-3-(4′-n-alkoxy benzoyloxy] phenyl-β-4″-nitro benzoyl ethylenes. Mol Cryst Liq Cryst. 2013;574:75–83.
- Chauhan HN, Doshi AV. The synthesis and mesomorphic properties of a novel homologous series: α-4-[4′-n-Alkoxy benzoyloxy] Benzoyl -β-2″-nitro Phenyl Ethylenes. Mol Cryst Liq Cryst. 2013;570:92–100.
- Chaudhari RP, Chauhan ML, Doshi AV. Synthesis and mesogenic behavior of novel liquid crystals with a –CH˭CH–CO– central bridge. Mol Cryst Liq Cryst. 2013;575:88–95.
- Bhoya UC, Vyas NN, Doshi AV. Determination of latent mesogenic behavior in nonmesogenic compounds by extrapolation method. Mol Cryst Liq Cryst. 2012;552:104–110.
- Patel RB, Doshi AV. Synthesis and study of mesogenic material through homologous series: n-pentyl-p-(p′-n-alkoxy cinnamoyloxy) cinnamates. Der Pharma Chemica. 2011;3(1):72–82.
- Sharma VS, Patel RB. Study of mesomorphism in isomeric and nonisomeric linear and nonlinear chalconyl series. Ilcpa. 2015;60:182–191. Scipress.
- Sharma VS, Patel RB. Molecular structure and mesomorphism: effect of tail/lateral group. Mol Cryst Liq Cryst. 2016;630:58–68.
- Sharma VS, Patel RB. Molecular flexibility dependence mesomorphism. Mol Cryst Liq Cryst. 2016;630:162–171.
- Sharma VS, Jain BB, Chauhan HN, et al. Study of the mesomorphism of benzoates and cinnamates. Mol Cryst Liq Cryst. 2016;630:79–86.
- Solanki RB, Sharma VS, Patel RB. Dependence of thermotropic mesomorphism on molecular flexibility of changing tail group. Mol Cryst Liq Cryst. 2016;631:107–115.
- Sharma VS, Patel RB. Synthesis and study of mesomorphic properties in rod-like chalconyl compounds. Mol Cryst Liq Cryst. 2017;643:52–61.
- Sharma VS, Patel RB. Tuning the mesomorphic properties of nonisomeric chalconyl-ester bases homologous series: the effect of tail group. Mol Cryst Liq Cryst. 2017;643:40–51.
- Sharma VS, Solanki RB, Patel PK, et al. Study of mesomorphism and its relation to molecular structure with special reference to central bridges viz. -COO- and -CH=CH-COO- of the homologous series. Mol Cryst Liq Cryst. 2016;625:137–145.
- Jain BB, Jadeja UH, Sharma VS, et al. Study of a new homologous series of liquid crystals: isopropyl-p-(p/-n-alkoxy cinnamoyloxy) cinnamates. Mol Cryst Liq Cryst. 2016;625:146–153.
- Sharma VS, Patel RB. Molecular flexibility of longer n-alkoxy tail group operated mesomorphism. Ilcpa. 2015;59:115–123. Sci.Press.
- Sharma VS, Patel RB. Synthesis of liquid crystals with substituents in terminal benzene cores and their mesomorphic behaviour. Ilcpa. 2015;58:144–153. Scipress.
- Sharma VS, Jain BB, Patel RB. Study of the effects of decreasing molecular rigidity on mesomorphism. Ilcpa. 2016;64:69–76. Scipress.
- Sharma VS, Patel. RB. Mesomorphic study of novel chalconyl-ester-based nonisomeric series: synthesis and characterization. Mol Cryst Liq Cryst. 2017;643:13–27.
- Sharma VS, Patel RB. Study the effects of terminal side chain and –nitro group on mesomorphic behaviour of cinnamate-chalconyl based liquid crystal. Mol Cryst Liq Cryst. 2017;643:1–12.
- Greene TW, Wuts PGM. Protective groups in organic synthesis. 2nd ed. New York: John Wiley and Sons; 1991.
- Kocienski PJ. Protecting groups. Stuttgart: Georg Thieme; 1994.
- Furniss BS, Hannford AI, Smith PWG, et al., Revisors. Vogels textbook 245 of practical organic chemistry. 4th ed. Singapore: Longmann; 1989.
- Sharma VS, Patel RB. Dependence of mesomorphism on molecular rigidity of chalconyl liquid crystals with two phenyl rings. Mol Cryst Liq Cryst. 2016;637:19–27.