ABSTRACT
Objectives: The need for assessment of possible drug-related signs and symptoms in older people with severe cognitive impairment has increased. In 2009, the PHASE-20 rating scale for identifying symptoms possibly related to medication was the first such scale to be found valid and reliable for use with elderly people. In this project, the aim was to develop and examine the psychometric properties and clinical utility of PHASE-Proxy, a similar scale for proxy use in assessing elderly people with cognitive impairment.
Methods: Three expert groups revised PHASE-20 into a preliminary proxy version, which was then tested for inter-rater reliability, internal consistency, and content validity. Its clinical usefulness was investigated by pharmacist-led medication reviews. Group interviews and a study-specific questionnaire with nursing home staff were used to investigate the feasibility of use.
Results: The PHASE-Proxy scale had satisfactory levels of inter-rater reliability (Spearman's rank correlation coefficient; rs = 0.8), and acceptable internal consistency (Cronbach's alpha coefficient; α = 0.73). The factor analysis resulted in a logical solution with seven factors, grouped into two dimensions: signs of emotional distress and signs of physical discomfort. The medication reviews, interviews, and questionnaires also found the proxy scale to be clinically useful, and feasible to use.
Conclusion: The PHASE-Proxy scale appears to be a valid instrument that enables proxies to reliably assess nursing home residents who cannot participate in the assessment, to identify possible drug-related signs and symptoms. It also appears to be clinically useful and feasible for use in this population.
Introduction
Older people are especially prone to developing adverse drug effects as a result of, for example, impaired organ function and polypharmacy. Such adverse side effects may be difficult to distinguish from symptoms and signs of disease, and there is an obvious risk of cascade prescribing, the process in which adverse drug effects are treated by the addition of new drugs (Rochon & Gurwitz, Citation1997). In Sweden, residents in nursing homes are prescribed on average 8–10 medications (Swedish National Board of Health and Welfare, Citation2010). The extensive use increases the risk of drug-related problems. For people with dementia and other cognitive disorders, appropriate prescribing is especially challenging, as the sensitivity to drug effects in this population is increased (Olsson et al., Citation2010). For example, people affected by dementia are known to be more sensitive to anticholinergic side effects in the central nervous system such as cognitive decline (Cancelli, Massililiano, Gigli & Valente, Citation2009; Fox et al., Citation2011) and peripheral symptoms such as dry mouth and constipation (Lieberman, Citation2004).
Medication reviews have become a prioritized area in Sweden as a result of legislation from the Swedish National Board of Health and Welfare (Citation2012). Since 2012, health authorities in Sweden have been obliged to offer people aged 75 years or older who are prescribed five or more drugs a medical review at least once a year. The medication reviews have to be performed, if possible, in consultation with the patient.
PHASE-20 (PHArmacotherapeutical Symptom Evaluation, 20 questions) is a rating scale used to identify symptoms that may be related to medication in older people. The Swedish instrument PHASE-20 was, to our knowledge, the first such scale to be found both valid and reliable for use with elderly people (Hedström, Lidström, & Hulter Åsberg, Citation2009). The symptoms included in PHASE-20 are all common side effects of drug treatment. The presence of a symptom can thus reveal the presence of drug-related problems such as inappropriate doses, drug interactions, or an inappropriate choice of drugs. In short, the development process of PHASE-20 included:
A literature search for common symptoms related to drug treatment in elderly people and clinical input by experienced geriatricians and clinical pharmacists for identifying questionnaire items for a preliminary scale consisting of 39 symptoms and one open response item to identify any other common signs or symptoms related to medication. The 39 symptoms were grouped in 10 problem areas; general physical discomfort, pain, sleeping, emotional, skin, gastrointestinal, heart-lung, neuromuscular, urination, and eye/sight.
This scale was tested at two nursing homes where 47 cognitively clear residents completed the scale with assistance from the member of staff who knew the resident the best. Residents and staff experienced the completed task as important and meaningful although the questionnaire was perceived as too extensive and thus exhausting to the residents. To reduce the number of items and improve the sensitivity of the questionnaire, the following measures were taken:
A clinical pharmacist performed a medication review for all residents. The percentage ratio for the relation between a perceived presence of a symptom and the pharmacists’ assessment of the likeliness that this symptom was drug-related for the specific individual was calculated for all symptoms. If the ratio exceeded 40%, the symptom was preliminary considered relevant in relation to drug treatment. Of the original 39 symptoms, 22 reached the 40% limit.
The clinical pharmacist and a geriatrician, both with extensive experience of drug reviews in elderly patients, reviewed the results and assessed three symptoms below the 40% limit as important although not that common, i.e. palpitations, rash, and nightmares. One symptom assessed as important in relation to medication was identified in the open response items, frequent urination. After this revision, 26 items remained, grouped in eight problem areas, the areas neuromuscular and eyes/sight being omitted in the process.
The correlation between the items in each problem area was calculated using Spearman's correlation coefficient. Items with significant correlations were merged into one symptom group, e.g. poor sleep pattern/nightmares or nausea/vomiting.
After revision, the scale consisted of 19 symptoms or symptom groups and one open response item. This instrument, PHASE-20, was tested in a randomized controlled trial with elderly persons living in two nursing homes before and after evaluation and correction of their therapeutic drugs. PHASE-20 was found to possess an acceptable consistency (Cronbach's alpha = 0.8), test–retest reliability (r = 0.71), and internal as well as face validity. Construct validity was not supported in this study, as there were no significant differences between groups after the intervention (Hedström et al., Citation2009).
In recent studies, it has been shown that pharmacist-led medication reviews based on medication lists and PHASE-20 reduce potentially inappropriate medications in people aged 75 years or older in primary health care (Milos et al., Citation2013) and among residents in nursing homes (Gustafsson et al., Citation2015). PHASE-20 is recommended in the Quality indicators for good drug therapy in the elderly, which was developed by the Swedish National Board of Health and Welfare (Citation2010), and is currently used in all counties in Sweden.
For people with cognitive disorders, a proxy rating scale for assessing drug-related signs and symptoms would be of great value. Unfortunately, such an instrument has been lacking, which means that persons with the greatest need of safety in drug medication must do without such a rating scale. Instead, PHASE-20 has often been used for these patients. There is an ethical dilemma when instruments intended for self-assessment of subjective states are used by proxy, as individuals are assessed on aspects only known to the individual herself. Thus, staff is asked to perform impossible assessments. Consequently, the results may be unreliable and could constitute a risk for patient safety. For example, proxies tend to underestimate patients’ quality of life (Arons, Krabbe, Shölzel-Dorenbos, van der Vilt, & Rikkert, Citation2013; Sheehan et al., Citation2012) and overestimate their pain (Jensen-Dahm, Vogel, Waldorff, & Waldemar, Citation2012). However, proxies have been shown to be fairly accurate about objective, observable phenomena such as physical mobility and functioning (Boyer, Novella, Morrone, Jolly, & Blanchard, Citation2004; Novella et al., Citation2006). Therefore, it is recommended that instruments intended for proxy assessments should focus on directly observable assessment of functions rather than subjective assessment.
When developing a new scale, it is vital to test its psychometric properties, i.e. its reliability and validity. The reliability of an instrument refers to the adequacy and reproducibility of the assessments, while the validity deals with whether the instrument measures what is intended (Streiner & Norman, Citation2008). It is also essential to establish the clinical value of the instrument. According to Glad, Jergeby, Gustafsson, and Sonnander (Citation2012), most researchers seem to agree that clinical utility is an important quality concept in the assessment of instruments such as these, although there is no consensus regarding the definition of this concept. From a clinical perspective, the information derived from an instrument must be relevant and appropriate, and the instrument must be practical to use and possible to complete within a reasonable time frame (Bowyer, Lee, Kramer, Taylor, & Kielhofner, Citation2012). These aspects of clinical utility are here referred to as usefulness and feasibility (Glad et al. Citation2012).
This paper describes the quality improvement project of developing and examining the psychometric properties and clinical utility of PHASE-Proxy, a scale for identifying symptoms and signs that may be related to medication, for use with elderly people with cooperation difficulties due to advanced stages of dementia or other communication disabilities.
Methods
Revising PHASE-20 to form PHASE-Proxy
Three groups of experts were appointed to revise PHASE-20 to form a proxy version. Their task was to identify subjective items, suggest objective, and observable alternatives for these, and identify other possibly relevant signs or symptoms from drug reviews in dementia care. These groups consisted of:
Dementia experts (MDs) and municipality officials involved with counseling for the dementia population in the region (n = 7). The group discussion generated ideas for exclusion of irrelevant items and inclusion of relevant ones.
Registered nurses (RNs) working in dementia care (RNs) (n = 16). With access to both PHASE-20 and the ideas generated by group 1, discussions were first held in three smaller groups, in which the ideas from group 1 were accepted, and new ideas were generated for both signs and symptoms and response items. Finally, in a joint discussion by all 16 RNs, consensus was reached.
Geriatricians and pharmacists with extensive experience of drug reviews in elderly patients (n = 4) reviewed the results from both groups, and agreed with the consensus decision of group 2.
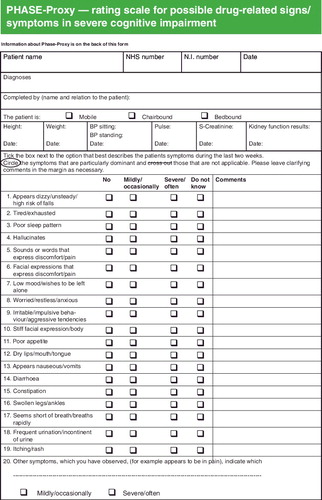
Items from PHASE-20 that were excluded from PHASE-Proxy included: nightmares, abdominal pain/chest pain, headache, forgetfulness, and palpitations. Additional items that were included were: sounds or words that express discomfort/pain, facial expressions that express discomfort/pain, and hallucinations. Some items were clarified in the proxy version, e.g. ‘appears dizzy’ was used instead of ‘dizzy’, and observable behavior patterns were added for others, e.g. ‘wishes to be left alone’ was added to ‘low mood’. When completed, PHASE-Proxy consisted of 19 symptoms or groups of symptoms and one open response item. The response alternatives in PHASE-20, which graded intensity only (no problem; minor problem; moderate problem; severe problem), were also changed to alternatives suitable for proxy rating, i.e. frequency (no; mild/occasionally; severe/often; do not know).
Participants and data collection
Data for assessing psychometric properties
Seven nursing homes in the county of Uppsala, Sweden, participated in the quality improvement project. All of the nursing homes used PHASE-20 on a routine basis for the identification and assessment of possible drug-related symptoms. All nursing home managers agreed to take part in the project of revising PHASE-20 into a proxy version. The RN in charge decided for which of the residents PHASE-Proxy should be used instead of PHASE-20; the proxy version was used for those who were not at all able to participate in the assessment because of severe cognitive impairment, e.g. advanced dementia. As in standard procedures for PHASE-20 assessments, the PHASE-Proxy assessments were performed by the contact person or by another carer who knew the resident the best, independently or in consultation with a colleague or an RN. The RN in charge chose the raters for the inter-rater reliability tests. The instructions were to choose the two carers that best knew the resident and for them to make the assessments independently and on the same day. For confidentiality reasons, all background data that could contribute to identification of the residents were removed before the researchers received the completed documents. In total, PHASE-Proxy assessments were performed instead of PHASE-20 for 158 residents, comprising 106 women and 52 men, aged 52–103 (mean 84; median 86) years. The data were collected over a period of one year, beginning in June 2012 and ending in June 2013.
Data for assessing clinical utility –usefulness
As part of the standardized care of nursing home residents in Uppsala County, clinical pharmacists are sometimes involved in supporting general practitioners (GPs) in performing medication reviews, either on site at the nursing home or at a distance by sending written recommendations to the GP. In the context of the clinical pharmacist's normal work, medication reviews at a distance would be performed based on information taken from the patient's medication list, medical record notes, laboratory values, and results from PHASE-20. As a part of the quality improvement project, PHASE-20 assessments were replaced by PHASE-Proxy assessments for 16 individuals with advanced dementia. Clinical pharmacists who were not part of the research group performed these medication reviews in two steps. In the first phase, the reviews were based on information available in the electronic medical notes and current list of medications for each patient. The pharmacists identified potential drug-related problems and listed all the recommendations for drug therapy changes that were generated. In the next step, the medication reviews were performed again, this time with access to information from the completed PHASE-Proxy forms, and again the recommendations were listed. The two sets of recommendations were then compared to investigate the effect of the added information. All data from the medication reviews available to the authors were completely de-identified.
Data for assessing clinical utility – feasibility
The perceived ease and feasibility of using PHASE-Proxy was evaluated at group interviews of staff at three nursing homes (at two nursing homes, the interviews comprised five carers and three RNs, respectively, and five carers and one RN at the third) and by a study-specific questionnaire that was distributed to staff (comprising the carers who did the assessments and the RNs in charge) at two other nursing homes. In the interviews, questions were posed regarding the practitioners’ experiences of using PHASE-Proxy (with respect to the 19 items, the response alternatives and the user instructions) and the perceived clinical value of the instrument.
The questionnaire contained questions concerning opinions of clinical value and the acceptability of the format, for which a four-point Likert scale (0 = not at all, 3 = yes, definitely) was used. In addition, the respondents were asked to estimate how much time they needed to complete the PHASE-Proxy assessment and whether they had completed it alone or in consultation with a colleague. Thirty-three carers and four RNs responded to the questionnaire.
Statistical analysis
Statistical analyses were conducted using SPSS version 21. Criterion validity was tested using Spearman's correlations to compare PHASE-Proxy with the only available comparable instrument, PHASE-20; although this scale was not intended for proxy assessment. It was hypothesized, however, that a statistically significant correlation would be obtained, as the two scales both aim to measure the same phenomena, i.e. possible drug-related signs and symptoms in the elderly.
Reliability was assessed between raters (inter-rater reliability) and for internal consistency. Inter-rater reliability, measured for a subset of the residents who were assessed by two different informants on the same occasion, was assessed using the Spearman's rank correlation coefficient (rs) for the total scale and the intra-class correlation coefficient (ICC) for the separate items. Internal consistency, measured as the average of the correlations of all the items in the construct, was assessed using Cronbach's alpha coefficient. The acceptable level for internal consistency was set at α ≥ 0.7 (Nunnally, Citation1978).
The content validity was assessed by orthogonal factor analysis, a principal component analysis with varimax rotation, in order to delineate the underlying factor structure of the instrument. This was carried out in the final population sample for the PHASE-Proxy instrument (n = 110) (see ). The 19 items of the PHASE-Proxy instrument were used as data in the analysis. The sample size calculation was based on the assumption that the number of subjects should be larger than five times the number of variables (Nunnally, Citation1978). Items with loadings above 0.4 were included in the factors. The Bartlett test of sphericity gave Chi-square = 338.934, df = 171, p = 0.00, and KMO = 0.629, indicating that it was psychometrically relevant to perform the analysis. A solution with eight factors with eigenvalues over 1.0 explained 69% of the variance, but a seven-factor solution explaining 64% was chosen since that solution was more logically interpretable.
Table 1. The time-line and population sizes for the various procedures used to evaluate the PHASE-Proxy psychometric properties.
Data regarding clinical utility are presented descriptively. Whether or not the feasibility was perceived differently between the carers who had completed the assessment independently and those who had consulted a colleague was analyzed using the Mann-Whitney U-test (two-tailed). Results were considered statistically significant if p ≤ 0.05.
Results
Psychometric properties
The process of developing the PHASE-Proxy rating scale was evaluated by measuring various psychometric properties. The time-line and populations tested in the evaluation are presented in
Criterion validity
The correlation between PHASE-20 and PHASE-Proxy (n = 30) was rs = 0.65 (p < 0.01), indicating criterion validity as the two scales appear to measure the same construct.
Inter-rater reliability
The first inter-rater reliability test (n = 18) resulted in a correlation of rs = 0.68 (p < 0.01). The ICC values for the separate items revealed four items with no or negative correlations: dry mouth = 0; vomiting/retching = 0; short of breath during effort = −0.14; itching/rash = −0.45. The wordings for these items were then modified and instructions on how to assess them were added to the form. The next inter-rater reliability test (n = 20) of the revised instrument resulted in a correlation of rs = 0.8 (p < 0.01); the correlations for the revised items were 0.57, 0.97, 0.93, and 0.97, respectively.
Content validity and internal consistency
The results of the factor analysis (n = 110) are presented in . The seven factors were: Factor I, signs of emotional distress; Factor II, expressing signs of discomfort; Factor III, reduced energy level; Factor IV, tendency for edema; Factor V, neurodegenerative signs; Factor VI, signs of anticholinergic side effects; and Factor VII, signs of irritability.
Table 2. The factor loadings of the seven-factor solution, and the Cronbach's alpha-coefficients for the seven factors.
The seven factors were classified into two main dimensions: signs of emotional distress and signs of physical discomfort. The emotional distress dimension was the same as Factor I while the physical discomfort dimension contained Factors II–VII. The relevant Cronbach's alpha coefficients (n = 110) were α = 0.73 for the complete instrument, α = 0.70 for signs of emotional distress, and α = 0.68 for signs of physical discomfort, indicating an acceptable level of internal consistency. The complete PHASE-Proxy scale is presented in
Clinical utility
Usefulness
Of the 16 residents for whom medication reviews were performed were 11 women; the average age of the group was 83 years, ranging from 68 to 100 years. They were prescribed a mean of 8.3 (range 2–13) drugs per person, and all had a diagnosis of dementia. Other common diagnoses were hypertension (six residents) and depression (six residents). The 16 reviews generated 48 recommendations (mean 3.0, range 0–7, per resident) for the first step and 54 (mean 3.4, range 0–9) after access to the information from the PHASE-Proxy assessment. For all but two residents, the added information resulted in modifications of the clinical recommendations. Nine new clinical recommendations were added to, and three were omitted from the original list. In 13 cases, the information from PHASE-Proxy strengthened the original clinical recommendations. One example of a changed recommendation was for an 83-year-old man who, according to PHASE-Proxy, often had a poor appetite and sleeping problems, while also suffering from depression. As a consequence, the pharmacist recommended mirtazapine, an anti-depressant drug that also increases the appetite and promotes sleep when taken at bedtime, in place of the commonly used citalopram, which does not have those properties. The absence of edema in two residents led to the recommendation to discontinue the daily use of furosemide, thereby reducing the risk of electrolyte disturbances, frequent urination, and other symptoms associated with diuretics.
Feasibility
Generally, there was a high acceptance of the PHASE-Proxy instrument. All participants in the group discussions preferred PHASE-Proxy to PHASE-20 when performing proxy assessments. Participants found that both the inclusion of more observable symptoms and signs in the items and the revised response alternatives for the items were better adapted to the target group. ‘The response alternatives are better in this one. The former (PHASE-20) was hard to assess for another person.’ Many respondents emphasized the importance of only assessing residents they knew very well. ‘For proxy assessments, one needs to know a lot about the person, to be able to interpret their gestures and their gaze.’ It was also suggested by all groups that in order to achieve the most reliable results, assessments should be done in cooperation with a colleague, as ‘two heads are better than one.’ ‘We discuss with each other if there is something of which we are not quite certain.’ Some suggested that the ideal team would be a carer and an RN doing the assessment together.
The questionnaire results (n = 33) indicated that the estimated time spent on a PHASE-Proxy assessment ranged between 5 and 60 (mean 15; median 10) minutes. The opinions of staff on the feasibility of using the PHASE-Proxy instrument are presented in
Table 3. Assessment of the feasibility of the PHASE-Proxy instrument; opinions from carers (n = 33) and registered nurses (n = 4).
The carers who made the assessment in consultation with a colleague (n = 20) were more likely to think that PHASE-Proxy should be used in their clinical setting compared to those who made the assessment independently (n = 13) (z = −1,93, p = 0.05). There were no other statistically significant differences between these groups.
Discussion
There is an increased need for a common terminology for signs and symptoms for medication reviews used by the RN, the caring staff, the clinical pharmacist, and the consultant GP for elderly patients who cannot participate in their reviews due to cognitive or communication deficits. The GP's consulting time in nursing homes is limited and the doctor is in need of continuous information from the nursing staff in order to make appropriate medication changes. PHASE-20, which is extensively used in Sweden, is based on self-assessments, which are not feasible for these patients. When first hand reports are not feasible, proxy assessments from people who know the person well are the second best, as Bergland and co-workers (Citation2014) pointed out. PHASE-Proxy, which is a new rating scale presented in this article, is based on objectively recognizable symptoms and signs which may be related to medication problems. Our results indicate that PHASE-Proxy has satisfactory reliability, validity, and clinical utility for the assessment of possible drug-related signs and symptoms among residents in nursing homes that cannot participate in their own assessment.
When reviewing the 158 completed questionnaires, we found that there were almost no responses to the open question asking the responder to name ‘other symptoms which you have observed’, indicating that the most common and observable symptoms were already present in PHASE-Proxy. In the initial phase, three expert groups with vast knowledge of dementia were appointed to revise PHASE-20 into a proxy version. The importance of this phase cannot be overlooked, and is in itself an indication of the internal validity of the instrument. In addition, the correlation between PHASE-20 and PHASE-Proxy had a coefficient of rs = 0.65, indicating criterion validity, as the two scales appear to measure the same construct (Streiner & Norman, Citation2008).
The factor analysis gave a logical solution with seven factors, all of them relevant when considering optimal drug therapy among older people with cognitive impairment. These seven factors were classified into two dimensions: signs of emotional distress and signs of physical discomfort. The reliability was relatively low for five of the factors. This finding is not surprising given that the construct of interest, i.e. symptoms in older people that may be related to medication, is a heterogeneous concept, related to various medications and drug-related problems. In addition, because most of the factors included only two or three items, and because the Cronbach's alpha formula is dependent on the number of items, fewer items will likely lead to a low alpha result (Streiner & Norman, Citation2008). For the two dimensions representing emotional or physical problems, however, the alpha coefficients were satisfactory. These two dimensions fit well with the aims of the instrument. Thus, both the content and the face value of the instrument can be considered valid. Taken together, we conclude that the results of the validation processes indicate that PHASE-Proxy successfully measures what it is intended to measure.
According to Streiner and Norman (Citation2008), inter-rater reliability coefficients should exceed 0.5, although higher values might be required depending on the use of the test. The inter-rater reliability for the final version of PHASE-Proxy was high, rs = 0.8, and no staff training was required. The carers were instructed to only assess residents they worked close with on a daily basis. Gräske, Fisher, Kuhlmey, and Wolf-Osterman (Citation2012) found that the quality-of-life ratings for persons with dementia that were made by the primary nurses in charge of those patients were significantly more in agreement with the residents’ ratings than those made by other staff. These findings are in accordance with data from our interviews, in which respondents emphasized the importance of only assessing residents known very well by the assessor. It was also suggested by all groups that assessments should be performed in cooperation with other co-workers in order to produce more reliable reports. In this project, the carers who made the assessment in consultation with a colleague were more likely to think that PHASE-Proxy should be used in their clinical setting than those who made the assessment independently. The cooperation between workers may have contributed to valuable exchanges of views and knowledge.
There is evidence that medication reviews based on medication lists and PHASE-20 can reduce the number of inappropriate medications (Milos et al., Citation2013; Gustafsson et al., Citation2015). The rapid spread and extensive use of PHASE-20 in Sweden also indicate that clinicians find it a useful and worthwhile tool. In this project, information provided by the PHASE-Proxy resulted in modifications to the pharmacists’ recommendations for 14 of 16 residents tested, as compared to the recommendations made with no information from a symptom rating scale. This indicates that the information was useful in relation to medication reviews in the sense that it allowed actual rather than potential drug-related problems to be identified.
According to Bowyer and co-workers (Citation2012), the clinical usefulness of tools such as this can sometimes be inadequately considered by researchers. The usefulness perceived by those who were intended to perform the assessments was investigated using both qualitative and quantitative approaches that employed group discussions and questionnaires (Bowyer et al., Citation2012). As Mariann Hedström performed the group interviews, there is a risk of discussions being biased by social desirability. Therefore, the questionnaires were answered anonymously. According to both sources, the instrument was found to be relevant and its use feasible.
Data were collected at seven nursing homes in various parts of Uppsala County. The non-random selection of residents, proxy informants, and nursing homes limit the generalizability of the results. Another limitation involves the small sample sizes for the inter-rater reliability measures, imposing caution on interpretation of the results. In addition, further research into the sensitivity of PHASE-Proxy to changes over time and after pharmaceutical interventions is needed. Such studies could benefit from taking into account the residents’ functional levels and cognitive abilities.
Conclusion
PHASE-Proxy appears to be a valid instrument that enables reliable proxy assessments for identification of possible drug-related signs and symptoms in nursing home residents who cannot participate in their assessment. It has also been shown to be a clinically useful tool that is feasible to use. Further studies are warranted to ascertain if its use will lead to optimization of medical treatment for this population.
Acknowledgments
We especially want to thank Dr Lena Kilander for her expert input on the early version of PHASE-Proxy, and the pharmacists Anncharlotte Grudén and Catherine Duarte-Martins for performing the medication reviews. Thanks are also due to the staff at the nursing homes Ebbagården, Eriksdalsgården, Olandsgården, Parkvägen 7-9, Sandelska huset, Wesslandia, and Årstagården for their help and their willingness to share their experiences with us.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arons, A.M., Krabbe, P.F., Schölzel-Dorenbos, C.J., van der Wilt, G.J., & Rikkert, M.G. (2013). Quality of life in dementia: A study on proxy bias. BMC Medical Research Methodology, 13, 110–119. doi:10.1186/1471-2288-13-110
- Bergland, Å., Kirkevold, M., Sandman, P.-O., Hofoss, D., Vassbø, T., & Edvardsson, D. (2014). Thriving in long-term care facilities: Instrument development, correspondence between proxy and residents' self-ratings and internal consistency in the Norwegian version. Journal of Advanced Nursing, 70, 1672–1681. doi:10.1111/jan.12332
- Bowyer, P., Lee, J., Kramer, J., Taylor, R.R., & Kielhofner, G. (2012). Determining the clinical utility of the Short Child Occupational Profile (SCOPE). British Journal of Occupational Therapy, 1, 19–26. doi:10.4276/030802212X13261082051373
- Boyer, F., Novella, J.L., Morrone, I., Jolly, D., & Blanchard, F. (2004). Agreement between dementia patient report and proxy reports using the Nottingham Health Profile. International Journal of Geriatric Psychiatry, 19, 1026–1034. doi:10.1002/gps.1191
- Cancelli, J., Massililiano, B., Gigli, G.L., & Valente, M. (2009). Drugs with anticholinergic properties: Cognitive and neuropsychiatric side-effects in elderly patients. Neurological Sciences, 30, 87–92. doi:10.1007/s10072-009-0033-y
- Fox, C., Richardson, K., Maidment, I.D., Savva, G.M., Matthews, F.E., Smithard, D., … Brayne, C. (2011). Anticholinergic medication use and cognitive impairment in the older population: The Medical Research Council Cognitive Function and Ageing Study (CFAS). Journal of the American Geriatric Society, 59, 1477–1483. doi:10.1111/j.1532-5415.2011.03491.x
- Glad, J., Jergeby, U., Gustafsson, C., & Sonnander, K. (2012). Social work practitioners’ experience of the clinical utility of the Home Observation for Measurement of the Environment (HOME) Inventory. Child and Family Social Work, 17, 23–33. doi:10.1111/j.1365-2206.2011.00769.x
- Gräske, J., Fisher, T., Kuhlmey, A., & Wolf-Osterman, K. (2012). Quality of life in dementia care – differences in quality of life measurements performed by residents with dementia and by nursing staff. Aging & Mental Health, 7, 819–827. doi:10.1080/13607863.2012.667782
- Gustafsson, M., Sandman, P.-O., Karlsson, S., Isaksson, U., Schneede, J., Sjölander, M., & Lövheim, H. (2015). Reduction in the use of potentially inappropriate drugs among old people living in geriatric care units between 2007 and 2013. European Journal of Clinical Pharmacology, 71, 507–515. doi:10.1007/s00228-015-1825-z
- Hedström, M., Lidström, B., & Hulter Åsberg, K. (2009). PHASE-20: ett nytt instrument för skattning av möjliga läkemedelsrelaterade symptom hos äldre personer i äldreboende [PHASE-20: A new instrument for assessment of possible therapeutic drug-related symptoms among elderly in nursing homes]. Vård i Norden, 29, 9–14.
- Jensen-Dahm, C., Vogel, A., Waldorff, F.B., & Waldemar, G. (2012). Discrepancy between self- and proxy-rated pain in Alzheimer's disease: Results from the Danish Alzheimer Intervention Study. Journal of the American Geriatrics Society, 60, 1274–1278. doi:10.1111/j.1532-5415.2012.04036.x
- Lieberman, J.A., (2004). Managing anticholinergic side effects. The Primary Care Companion to the Journal of Clinical Psychiatry, 6(Suppl. 2), 20–23.
- Milos, V., Rekman, E., Bondesson, Å., Eriksson, T., Jakobsson, U., Westerlund, T., & Midlöv, P. (2013). Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: A randomized controlled study. Drugs Aging, 30, 235–246. doi:10.1007/s40266-013-0057-0
- Novella, J.L., Boyer, F., Jochum, C., Jovenin, N., Morrone, I., Jolly, D., … Blanchard, F. (2006). Health status in patients with Alzheimer's disease: An investigation of inter-rater agreement. Quality of Life Research, 15, 811–819. doi:10.1007/sl1136-005-5434-7
- Nunnally, J.C. (1978). Psychometric theory. New York, NY: McGraw-Hill.
- Olsson, J., Bergman, Å., Carlsten, A., Oké, T., Bernsten, C., Schmidt, I., & Fastbom, J. (2010). Quality of drug prescribing in elderly people in nursing homes and special care units for dementia. A cross-sectional computerized pharmacy register analysis. Clinical Drug Investigation, 30, 289–300. doi:10.2165/11534320-000000000-00000
- Rochon, P.A., & Gurwitz, J.H. (1997). Optimising drug treatment for elderly people: The prescribing cascade. British Medical Journal, 315, 1096–1099.
- Sheehan, B.D., Lall, R., Stinton, C., Mitchell, K., Gage, H., & Katz, J. (2012). Patient and proxy measurement of quality of life among general hospital in-patients with dementia. Aging & Mental Health, 5, 603–607. doi:10.1080/13607863.2011.653955
- Streiner, D.L., & Norman, G.R. (2008). Health measurement scales: A practical guide to their development and use (4th ed.). Oxford: Oxford University Press.
- The Swedish National Board of Health and Welfare. (2010). Indikatorer för god läkemedelsterapi hos äldre [Indicators for evaluating the quality of older people's drug therapy] (publication no. 2010-6-29). Retrieved from http://www.socialstyrelsen.se
- The Swedish National Board of Health and Welfare. (2012). SOSFS Statues 2012:9. [in Swedish]. Retrieved from http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18766/2012-6-43.pdf