ABSTRACT
Background: Promoting adaptation, improving well-being and maintaining an optimal quality of life (QOL) is an important aspect in dementia care. The purpose of this study was to identify determinants of QOL in young onset dementia, and to assess differences in QoL domains between people with Alzheimer's disease (AD) and frontotemporal dementia (FTD).
Methods: In total 135 persons with AD and 58 persons with FTD were included from two prospective cohort studies. QOL was assessed with the proxy reported quality of life in Alzheimer's disease questionnaire (QoL-AD). Possible determinants were explored using multiple linear regression and included sociodemographic variables, diagnosis, dementia severity, disease awareness, neuropsychiatric symptoms, met and unmet needs and hours of personal and instrumental care. Differences between QOL domains in people with AD and FTD were calculated using Mann-Whitney U tests.
Results: Lower QOL was associated with more depressive symptoms, lower disease awareness, and a higher amount of needs, both met and unmet. People with AD scored lower on the memory and higher on the friends' subscale. No differences were found for the other items.
Conclusion: This study demonstrates a unique set of determinants of QOL in AD and FTD. Interventions directed towards these specific factors may improve QOL.
Introduction
Dementia is a syndrome caused by a number of neurodegenerative disorders affecting cognitive abilities such as memory and behavior, resulting in progressive loss of independence in daily activities and the ability to participate in social activities (World Health Organisation, Citation2012). When dementia starts before the age of 65, it is commonly referred to as young onset dementia (YOD). The prevalence of YOD is estimated between 67 and 81 per 100,000 in the age group of 45–65 years (Harvey, Skelton-Robinson, & Rossor, Citation2003; Ratnavalli, Brayne, Dawson, & Hodges, Citation2002). However, the number of people with YOD is most likely to be underestimated, as there are insufficient studies on the epidemiology of YOD and most prevalence studies are registry based (Alzheimer's Disease International, Citation2015). In contrast to late onset dementia (LOD), YOD is more frequently genetic or metabolic due to rare causes, or secondary to potentially treatable conditions (Rossor, Fox, Mummery, Schott, & Warren, Citation2010; Sampson, Warren, & Rossor, Citation2004). The subtypes in YOD appear to be more heterogeneous than in LOD. AD is, however, still the most common cause of dementia also in young adults (Vieira et al., Citation2013). Frontotemporal dementia (FTD) accounts for a larger proportion of the cases in YOD compared to LOD (Harvey et al., Citation2003). The clinical phenotypes of AD and FTD differ (Sampson et al., Citation2004). Due to the early involvement of medial temporal lobe structures, the majority of people with AD initially have more apparent memory loss. Language and social functioning are generally preserved until later on (Sampson et al., Citation2004). However, when AD starts at a younger age there are more phenotypic variants, including a rather big group of cases with non-amnestic deficits, compared to late onset AD (Koedam et al., Citation2010). FTD is characterized by behavioral disturbances, personality changes, social comportment, loss of empathy and motivation and a decrease in cognition (Rossor et al., Citation2010).
People with YOD also differ from people with LOD due to age-specific needs with family responsibilities, employment issues and different societal roles and obligations (Baptista et al., Citation2016; van Vliet, de Vugt, Bakker, Koopmans, & Verhey, Citation2010). These various responsibilities and roles contribute to a sense of meaning in life and being an engaged individual in daily living (Harris & Keady, Citation2009). Difficulties in these areas may affect integral parts of a person's sense of selfhood (Harris & Keady, Citation2009) and can lead to a considerable decrease in his or her quality of life (QOL). Furthermore, cognitive, behavioral and functional symptoms of dementia can significantly impact people's general well-being and QOL (Teng, Tassniyom, & Lu, Citation2012).
QOL is initially defined by the World Health Organization as the ‘individuals' perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.’ This concept is affected by physical, psychological and social well-being (World Health Organisation Group, Citation1995). When the person is affected by a disability or chronic disease, such as dementia, these goals and expectations often have to be adjusted. In order to be able to continue to function with fulfilment, people with dementia have to adapt to these changes and cope with the physical, emotional and social challenges they face (Huber et al., Citation2011). Therefore, it is important that there is a shift in focus from symptoms and disability towards the capacity and potential of the person with dementia. This has become a major topic of interest within dementia research (Vernooij-Dassen & Jeon, Citation2016). Not everyone is able to adapt to the consequences of the dementia, likely adversely affecting QOL. This will jeopardize the capacity of the person with dementia to fulfil their potential as well as their ability to participate in their environment. In order to improve their QOL, more knowledge about the factors that influence QOL is necessary. This will also allow for identifying potential targets for early intervention.
There has been extensive research on determinants of QOL in LOD. No clear or consistent associations between QOL and socio-demographic variables including age, education and gender were found (Banerjee et al., Citation2009). A consistent pattern is found in the literature for lower QOL and high levels of depressive symptoms (Bosboom, Alfonso, Eaton & Almeida, Citation2012; Logsdon, Gibbons, McCurry, & Teri, Citation2002) and more dependence in activities of daily living (Black et al., Citation2012; Conde-Sala, Garre‐Olmo, Turró‐Garriga, López‐Pousa, & Vilalta‐Franch, Citation2009). In general, high levels of behavioral problems are associated with decreased QOL (Banerjee et al., Citation2009). However, there seems to be a difference in the relative importance of the specific behavioral symptoms on QOL ratings (Gómez-Gallego, Gómez-Amor, & Gómez-García, Citation2012; Hoe, Katona, Roch, & Livingston, Citation2005). The relationship between QOL and cognition as well as dementia severity is less clear. Correlations are generally low (Banerjee et al., Citation2009) but some studies link better cognitive functioning to higher QOL (Beer et al., Citation2010; Crespo, Hornillos, & de Quirós, Citation2013). QOL has also been associated with an increased amount of unmet needs in the study of Hoe et al. (Citation2005). However, this effect was not shown in another study (Bakker et al., Citation2014).
To our knowledge only one study specifically investigated determinants of QOL in persons with YOD (Bakker et al., Citation2014). Only depression seemed to be a significant determinant of QOL. In addition, no studies compared QOL in persons with YOD and LOD, raising the question whether there are different determinants involved. Furthermore, it is not clear how QOL differs for the various subtypes of YOD.
Especially in people with FTD, initial symptoms predominantly affect personality and behavior, as opposed to memory and attention in AD, this could impact their social health in various ways, affecting their QOL in different areas. The aim of the current study is to identify and explore determinants of QOL in people with YOD, and to assess differences in specific domains between people with FTD and AD, using data from two European multicenter studies.
Methods
Study design
This study used baseline data of two prospective cohort studies; the Dutch NeedYD (Needs in Young onset Dementia) study (van Vliet, Bakker, et al., Citation2010) and the Nordic multicenter study of QOL and needs for health care services in YOD (Hvidsten et al., Citation2015). The study protocols were approved by the local ethics committees and informed consent from all participants or their legal representatives was obtained prior to the study. Approval was granted by the Regional Committees for Medical and Health Research Ethics in Norway for the exchange of de-identified information from the Nordic YOD-study.
Participants
Two hundred and nine participants were recruited from university medical centers, regional hospitals, regional community mental health services and from YOD specialized day care facilities in the Netherlands. Only participants with AD and FTD were selected for the present study. The dementia diagnosis was established according to the criteria from the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, Citation2000) on the basis of clinical, neuropsychological and neuroimaging data and the Dutch consensus guidelines (Consultatiebureau voor Ouderen (CBO), Citation2005). In the Nordic cohort, 88 participants were recruited from memory clinics in Norway, Denmark and Iceland. The diagnosis was established according to the consensus criteria for behavioral variant-FTD and the language variants of FTD (Mesulam, Citation2003; Neary et al., Citation1998), and the ICD-10 criteria for AD. Inclusion criteria were symptom onset before the age of 65, community-dwelling at the time of inclusion and the availability of an informant with regular contact. Exclusion criteria were people with FTD related to motor neuron disease or other dementias with frontal lobe affection, current alcohol or substance abuse and mental retardation.
Primary outcome
The Quality of Life-Alzheimer's Disease scale (QOL-AD) was used to assess QOL (Logsdon, Gibbons, McCurry, & Teri, Citation1999). The scale consists of 13 items including physical health, energy, mood, living situation, memory, family, marriage, friends, chores, fun, money, self and life as a whole. Each item is scored on a 4-point Likert scale, ranging from poor to excellent. Scores on the individual items are summed to obtain a total QOL score, with a higher score indicating a better QOL with a maximum score of 52. If the person with dementia did not have a spouse, the scores on the marriage item were weighted according to the total scale score and imputed (Conde-Sala et al., Citation2009). Proxy ratings were used since the study included people in the advanced stages of dementia as well. The QOL-AD is reported to have good inter-rater reliability, fair to good test–retest reliability and excellent internal consistency (Thorgrimsen et al., Citation2003).
Independent variables
The independent explanatory variables were selected based on previous findings on determinants of QOL in dementia. Dementia severity was assessed with the Global Deterioration Scale (Reisberg, Ferris, de Leon, & Crook, Citation1982) in the Dutch cohort study and with the clinical dementia rating (Hughes, Berg, Danziger, Coben, & Martin, Citation1982) questionnaire in the Nordic study. Severity of dementia was reclassified into mild, moderate and severe dementia according to the guidelines of the dementia performance measurement set of the American Medical Association with higher scores indicating more severe dementia (American Medical Association, Citation2011). In addition, the Mini Mental State Examination (MMSE) (M. Folstein, S. Folstein, & McHugh, Citation1975) was administered to assess cognitive functioning. The MMSE is a 30 points scale and a higher score denotes better cognitive functioning. Depressive symptoms were assessed with the Cornell Scale for Depression in Dementia and a higher score indicates more depressive symptoms (Alexopoulos, Abrams, Young, & Shamoian, Citation1988). The Camberwell Assessment of Needs in the Elderly (CANE) was used to explore and quantify the number of met and unmet needs (Reynolds et al., Citation2000). The CANE investigates 24 domains that cover social, physical, psychological and environmental needs. For each item it assesses whether there is an existing need and if this need is met. For this study, the number of existing needs was used for both met and unmet needs with proxy ratings. To assess a wide range of behavioral symptoms, the Neuropsychiatric Inventory (NPI) (Cummings et al., Citation1994) was used. The symptoms that are included in the questionnaire are delusions, hallucinations, agitation, depression, anxiety, apathy, irritability, euphoria, disinhibition, aberrant motor behavior, night-time behavior disturbances and appetite and eating abnormalities. The severity scores (range 1--3) of the observed individual neuropsychiatric symptoms were summed to generate a global score with a higher score for more severe behavioral symptoms. The degree of disease awareness was measured with the Guidelines for the Rating of Awareness Deficits (Verhey, Rozendaal, Ponds, & Jolles, Citation1993) in the Dutch cohort and according to the Reed anosognosia scale (Reed, Jagust, & Coulter, Citation1993) in the Nordic cohort. Both scales define awareness as the presence of explicit knowledge or recognition of own cognitive deficits according to a four-point scale. A higher score denotes better awareness. The time the caregiver spent caring for the person with dementia was obtained through specific items of the Resource Utilization Scale (Wimo, Jonsson, & Zbrozek, Citation2010) and measured in hours per month spent on instrumental as well as personal care. Furthermore, demographic information was collected including age, gender and level of education.
Data analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0. Prior to the analysis, all data were checked for missing values, outliers and normality to ensure there were no violation of assumptions. Differences between the two diagnostic groups were tested with χ2-tests for categorical and t-tests for continuous variables for both the independent variables as well as the scores on the different items of the QOL-AD. In case of non-normality, the non-parametric Mann–Whitney U-test was conducted. Tests of significance were performed two-tailed, with a significance level of 0.05. Hierarchical multiple regression was used to assess the ability of the explanatory variables to determine QOL. The blocks of variables were composed as related sets. We started with the socio-demographic variables (age, gender, education) we wanted to control for before testing the predictive value of the disease-related factors such as diagnosis, severity and awareness. The consecutive order of the remaining blocks was based on previously reported determinants in LOD. In the first step, demographic variables were entered (age, gender, education), then the dementia-related variables (diagnosis, severity, awareness) were entered in the second step. In the third step depressive symptoms were added, in the fourth step the total severity scores of the neuropsychiatric symptoms were added, met and unmet needs were entered in the fifth step, and finally in the sixth step, hours of instrumental and personal care were added. The R square change value for each step was used to evaluate the additive explanatory power of each model after adding the next set of variables, while the beta coefficients were used to evaluate the contribution of each variable to the final model. The final model was selected based on the highest predictive value, denoted by the R2.
Results
Participants
Eighty eight participants were recruited from the Nordic YOD-study, and 160 from the NeedYD-study. Of the 248 individuals with YOD, 169 had AD and 79 FTD.From the total study population, 193 completed all assessments that were necessary for the regression analysis. There were no significant differences between the participants in the analyses and those that were excluded regarding age, gender, education or diagnosis, nor concerning their median scores on the MMSE, Cornell, NPI or QOL-AD.
Mean age was 62 years (IQR 8) with no significant age difference between the two diagnostic groups. Moreover, the groups did not differ in gender, level of education, disease awareness or depression scores. However, individuals with FTD had significantly higher MMSE scores, less advanced dementia and experienced more behavioral symptoms ().
Table 1. Participant characteristics.
QOL
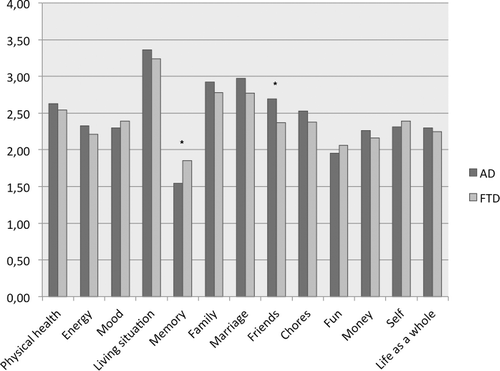
The median total QOL score was 31 (IQR 10) in the AD group and 32 (9.3) in the FTD group. Analysis of the different sub items of the QOL-AD scale showed that people with AD scored lower on the memory subscale (p = .003), and higher on the friends subscale (p = .010) compared to people with FTD. No differences were found for the other items ().
The first model in the hierarchical regression analyses showed that the demographic variables accounted for 4% of the variance in the total QOL score. Entry of the disease-related variables accounted for a significant 24% increase in explanatory value of the model (p < .001), the addition of depressive symptoms further improved the model (9% increase, p < .001). However, addition of the global NPI severity score did not yield a significant increase in predictive value (p = .75). Adding the number of met and unmet needs did improve the model further with a 7% increase (p < .001). The sixth and final model with the hours of instrumental and personal care added did not improve the predictive value of the model (1% increase, p = .28) (). The final model accounted for 45% of the variance in the total QOL score.
Table 2. Results from the regression analysis.
The predictive values of the explanatory variables are also shown in . Lower QOL was significantly and independently associated with more severe depressive symptoms, lower disease awareness and a higher amount of needs, whether they were met or unmet. There were no significant differences related to dementia severity, behavioral symptoms or personal or instrumental care.
Discussion
We studied the influence of several variables on QOL of people with YOD in a unique sample from two large-scale cohort studies. Depressive symptoms and both met and unmet needs were negatively related to QOL. Better disease awareness was, on the other hand, related to a higher QOL score. A comparison of the two diagnostic groups showed no difference in global QOL, but individuals with AD scored lower on the memory subscale and higher on the friends’ subscales than people with FTD.
The results implicate that both individuals with FTD and AD face challenges in daily living that negatively impact their QOL to a similar degree. However, for people with AD, difficulties are more apparent in the memory domain. This is in line with previous research findings emphasizing the early involvement of medial temporal lobe structures in AD initially leading to forgetfulness (Sampson et al., Citation2004). The clinical presentation of FTD is on the other hand to a large degree characterized by behavioral disturbances and personality changes (Rossor et al., Citation2010) which might explain the lower scores on the friends subscale. Remarkably, this was not shown in the more intimate social circles reflected by the items marriage and family, suggesting that within the home environment the distinct characteristics of FTD impact QOL to a lesser extent. This might partly be explained by the feelings of stigma and taboo many YOD caregivers experience. Dementia in the young is rare and not always understood by the environment. As a consequence, the caregivers may not share information on what is going on with others. This might, in turn, cause a desire to avoid social situations (Ducharme, Kergoat, Antoine, Pasquier, & Coulombe, Citation2013). This might be even more evident in FTD because of behavioral difficulties such as disinhibition, inappropriate social behavior and impulsivity. In addition, the people close to the person with dementia might be more willing to adjust to the changes in their significant other.
We found that several factors were significantly related to QOL, including depression, needs and awareness. Depression showed the strongest negative relation with QOL, indicating that more depressive symptoms are related to lower QOL. This finding has been a consistent factor in past research as well (Banerjee et al., Citation2009). The total number of needs was also a determinant of reduced QOL. This could be expected, as different subscales of the CANE are closely related to domains of the QOL-AD, including accommodation, chores, daytime activities, memory, physical health, company and money. Furthermore, both the CANE and the QOL-AD were assessed by caregiver proxies. It did not make a difference whether these needs were met or unmet as they both showed a negative relationship with QOL. So even if the individual with YOD received enough help when there was an existing need, it was associated with lower QOL. This might be explained by being confronted with experienced limitations and an experienced loss of autonomy as more help is needed to fulfill existing care needs. A lack of autonomy and dependency on others for daily activities is an important aspect in QOL (Andersen, Wittrup-Jensen, Lolk, Andersen, & Kragh-Sorensen, Citation2004; Ettema, Dröes, de Lange, Mellenbergh, & Ribbe, Citation2005), and is also one of the domains identified in social health (Huber et al., Citation2011).
Another important variable was disease awareness, which was positively related to QOL, indicating higher QOL with better awareness. According to the model of Clare (Citation2004), several domains are relevant in awareness in Alzheimer's including experiencing the impact of changes and adjusting to changes. Insight into one's limitations is an important prerequisite for making necessary adaptations in life and to keep participating in valued and essential aspects of daily living. This shows that awareness is closely related to acceptance of these changes and has important implication for coping styles and strategies (Clare, Citation2004). Coping with the disease and successfully adapting allows people to feel healthy despite the experienced limitations (Hubert et al., Citation2011). In addition, higher awareness provides the opportunity for the person with dementia to take an active part in his or her own care planning and future. This involvement in daily decision-making can also have a positive effect on reported QOL in the person with dementia (Menne, Judge, & Whitlatch, Citation2009). The opportunity for informed decision-making requires full disclosure about the diagnosis and its implications from the health care professionals. The use of euphemisms or non-disclosure does not only contribute to uphold the stigma associated with dementia (Gove, Downs, Vernooij-Dassen, & Small, Citation2016), but also limits the empowerment of the person involved.
Previous research has also linked better awareness in the person with YOD to higher QOL in the caregiver (Rosness, Mjørud, & Engedal, Citation2011). Since the proxy ratings of QOL in people with dementia may be influenced by the well-being of the caregivers themselves (Logsdon et al., Citation1999), the relationship of awareness and QOL might be partly mediated by the positive effect of awareness on caregivers. This finding raises the question of the suitability of the proxy ratings to evaluate QOL in people with dementia, and is a limitation of our study. A large majority of people with mild to moderate dementia is perfectly capable of reporting their own QOL, and proxy ratings are often lower than those made by persons themselves (Banerjee et al., Citation2009; Vogel, Mortensen, Hasselbalch, Andersen, & Waldemar, Citation2006). These different ratings might be caused by an overcritical attitude of the caregivers or, on the other hand, to an adaptive shift of the person with dementia to their limitations and the resulting adjustment of expectations (Banerjee et al., Citation2009). Furthermore, QOL in people with dementia is underestimated by caregivers with increased burden (Gómez-Gallego et al., Citation2012) and evaluations of the QOL by proxy seem to be influenced by the caregivers own emotional state and inner experience (Gomez-Gallego, Gomez-Garcia & Ato-Lozano, Citation2015). However, we were unable to include self-report ratings, since both the NeedYD and the Nordic YOD-study included people with YOD in all stages of the disease, including individuals with severe cognitive impairment. Therefore, the decision was made to use the caregiver perspective.
The present study is one of the few studies assessing QOL specifically in people with YOD. The strength of the study is that combining two data sets gave information from a large sample of people with YOD including a substantial amount of people with FTD. However, this also comes with limitations as the pooling of the data generated some challenges and some variables were assessed a bit differently. Furthermore, no self-reported measures were available for all participants preventing the possibility to use both self-rated and by proxy reports. The sample characteristics also limit generalizability to the entire YOD population, as we only included people with AD and FTD thereby excluding a considerable proportion of people with YOD with other etiologies.
For a better understanding of factors that might improve QOL in the different stages of the disease, longitudinal research is needed. A combined use of quantitative and qualitative methods will further enable the understanding of the concept of QOL, and highlight which domains are particularly important in YOD. Self-report measures of QOL in people in all stages of the disease in future research will contribute valuable complementary information to caregiver reports. How determinants of QOL differ between by proxy and self-reports should be investigated more in depth, also to clarify how these proxy ratings might be biased. Furthermore, it would be interesting to study variables that are more directly related to caregiver characteristics, such as quality of the relationship, caregiver burden, coping and caring strategies. Our results indicate that merely the quantity of care measured in hours was not a significant determinant for QOL, indicating that other factors related to the caregiving situation might be more important. Especially since caregivers who care for someone with YOD, frequently experience difficulties balancing their multiple roles and responsibilities with the care situation (Millenaar et al., Citation2016) and report low QOL (Bakker et al. Citation2014) and psychological complaints (Riedijk, Duivenvoorden, Van Swieten, Niermeijer, & Tibben, Citation2009). Other variables that might be important contributors of the experienced QOL in people with YOD are the availability of stimulating activities and social contact, as they often feel a lack of meaningful activity and a loss of sense of purpose in their daily lives as they are still in an active life phase (Harris & Keady, Citation2004; Roach & Drummond, Citation2014).
Taken together, we have identified a unique set of determinants for QOL. These have implications for efforts to enhance QOL in people with YOD. First of all, it is important to detect and treat depressive symptoms. These symptoms are often misdiagnosed or misattributed, and left untreated and significantly decrease QOL (Menne et al., Citation2009). Second, interventions should acknowledge the importance of awareness in the person with dementia and the effect it has on their QOL. This allows them to take an active part in the decision-making process concerning their own care and future. Third, it is often assumed that QOL is lower with more severe dementia, however, this is neither supported by our findings nor by previous studies as dementia severity was not a significant determinant of QOL (Banerjee et al., Citation2009). Therefore, a shift in focus from disability and loss towards the possibilities and positive aspects in the daily lives of people with dementia, also in the more advanced stages, should become an important topic in dementia research (Huber et al., Citation2011). This might be especially relevant for people with YOD, as they are in a more active life phase and want to fulfill a meaningful role and feel useful (Roach, Keady, Bee, & Hope, Citation2008). Finally, it is important to target care needs, because they negatively impact QOL and furthermore have been identified as determinants of early nursing home placement (Bakker et al., Citation2013). However, the challenge is to find the right balance between providing sufficient help and respecting the autonomy of the person with dementia.
Acknowledgments
We would like to thank all the research assistants who assisted in collecting the data and Y. Pijnenburg for her contributions to the NeedYD study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alexopoulos, G.S., Abrams, R.C., Young, R.C., & Shamoian, C.A. (1988). Cornell scale for depression in dementia. Biological Psychiatry, 23(3), 271–284. doi:10.1016/0006-3223(88)90038-8
- Alzheimer's Disease International. (2015). World Alzheimer Report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends. London: Author. Retrieved from: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf
- American Medical Association. (2011). Dementia performance measurement set. Retrieved from https://www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3.Practice_Management/2.Quality_Improvement/1.Quality_Measures/1.All_Measures/dementia.pdf
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders DSM-IV-TR (text revision). Washington, DC: Author.
- Andersen, C.K., Wittrup-Jensen, K.U., Lolk, A., Andersen, K., & Kragh-Sorensen, P. (2004). Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health and Quality of Life Outcomes, 2, 52. doi:10.1186/1477-7525-2-52
- Bakker, C., de Vugt, M.E., van Vliet, D., Verhey, F.R., Pijnenburg, Y.A., Vernooij-Dassen, M.J., & Koopmans, R.T. (2013). Predictors of the time to institutionalization in young-versus late-onset dementia: Results from the Needs in Young Onset Dementia (NeedYD) study. Journal of the American Medical Directors Association, 14(4), 248–253. doi:10.1016/j.jamda.2012.09.011
- Bakker, C., de Vugt, M.E., van Vliet, D., Verhey, F., Pijnenburg, Y.A., Vernooij-Dassen, M.J., & Koopmans, R.T. (2014). Unmet needs and health-related quality of life in young-onset dementia. The American Journal of Geriatric Psychiatry, 22(11), 1121–1130.
- Banerjee, S., Samsi, K., Petrie, C.D., Alvir, J., Treglia, M., Schwam, E.M., & del Valle, M. (2009). What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. International Journal of Geriatric Psychiatry, 24(1), 15–24. doi:10.1002/gps.2090
- Baptista, M.A., Santos, R.L., Kimura, N., Lacerda, I.B., Johannenssen, A., Barca, M.L., … Dourado, M.C. (2016). Quality of life in young onset dementia: An updated systematic review. Trends in Psychiatry and Psychotherapy, 38(1), 6–13. doi:10.1590/2237-6089-2015-0049
- Beer, C., Flicker, L., Horner, B., Bretland, N., Scherer, S., Lautenschlager, N.T., … Almeida, O.P. (2010). Factors associated with self and informant ratings of the quality of life of people with dementia living in care facilities: A cross sectional study. PLoS One, 5(12), e15621.
- Black, B.S., Johnston, D., Morrison, A., Rabins, P.V., Lyketsos, C.G., & Samus, Q.M. (2012). Quality of life of community-residing persons with dementia based on self-rated and caregiver-rated measures. Quality of Life Research, 21(8), 1379–1389.
- Bosboom, P.R., Alfonso, H., Eaton, J., & Almeida, O.P. (2012). Quality of life in Alzheimer's disease: Different factors associated with complementary ratings by patients and family carers. International Psychogeriatrics, 24(5), 708–721.
- CBO, Consultatiebureau voor Ouderen. (2005). Guideline diagnosis and pharmacological treatment of dementia. Alphen aan den Rijn: Van Zuiden Communications B.V.
- Clare, L. (2004). The construction of awareness in early stage Alzheimer's disease: A review of concepts and models. British Journal of Clinical Psychology, 43(2), 155–175.
- Conde‐Sala, J.L., Garre‐Olmo, J., Turró‐Garriga, O., López‐Pousa, S., & Vilalta‐Franch, J. (2009). Factors related to perceived quality of life in patients with Alzheimer's disease: The patient's perception compared with that of caregivers. International Journal of Geriatric Psychiatry, 24(6), 585–594.
- Crespo, M., Hornillos, C., & de Quirós, M.B. (2013). Factors associated with quality of life in dementia patients in long-term care. International Psychogeriatrics, 25(4), 577–585.
- Cummings, J.L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D.A., & Gornbein, J. (1994). The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44(12), 2308–2314.
- Ducharme, F., Kergoat, M.-J., Antoine, P., Pasquier, F., & Coulombe, R. (2013). The unique experience of spouses in early-onset dementia. American Journal of Alzheimer's Disease and Other Dementias, doi: 1533317513494443.
- Ettema, T.P., Dröes, R.-M., de Lange, J., Mellenbergh, G., & Ribbe, M.W. (2005). A review of quality of life instruments used in dementia. Quality of Life Research, 14, 675–686.
- Folstein, M.F., Folstein, S.E., & McHugh, P.R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. doi:10.1016/0022-3956(75)90026-6
- Gómez-Gallego, M., Gómez-Amor, J., & Gómez-García, J. (2012). Determinants of quality of life in Alzheimer's disease: Perspective of patients, informal caregivers, and professional caregivers. International Psychogeriatrics, 24(11), 1805–1815.
- Gomez-Gallego, M., Gomez-Garcia, J., & Ato-Lozano, E. (2015). Addressing the bias problem in the assessment of the quality of life of patients with dementia: Determinants of the accuracy and precision of the proxy ratings. The Journal of Nutrition, Health & Aging, 19(3), 365–372.
- Gove, D., Downs, M., Vernooij-Dassen, M., & Small, N. (2016). Stigma and GPs' perceptions of dementia. Aging & Mental Health, 20(4), 391–400. doi:10.1080/13607863.2015.1015962
- Harris, P.B., & Keady, J. (2004). Living with early onset dementia: Exploring the experience and developing evidence-based guidelines for practice. Alzheimer's Care Today, 5, 111–122.
- Harris, P.B., & Keady, J. (2009). Selfhood in younger onset dementia: Transitions and testimonies. Aging & Mental Health, 13(3), 437–444. doi:10.1080/13607860802534609
- Harvey, R.J., Skelton-Robinson, M., & Rossor, M.N. (2003). The prevalence and causes of dementia in people under the age of 65 years. Journal of Neurology, Neurosurgery & Psychiatry, 74(9), 1206–1209.
- Hoe, J., Katona, C., Roch, B., & Livingston, G. (2005). Use of the QOL-AD for measuring quality of life in people with severe dementia—the LASER-AD study. Age and Ageing, 34(2), 130–135.
- Huber, M., Knottnerus, J.A., Green, L., van der Horst, H., Jadad, A.R., Kromhout, D., … Smid, H. (2011). How should we define health? BMJ, 343, d4163. doi:10.1136/bmj.d4163
- Hughes, C.P., Berg, L., Danziger, W.L., Coben, L.A., & Martin, R.L. (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry, 140, 566–572.
- Hvidsten, L., Engedal, K., Selbaek, G., Wyller, T.B., Høgh, P., Snaedal, J., … Kersten, H. (2015). Young onset dementia study – A prospective cohort study of quality of life and specific needs in persons with young onset dementia and their families. Journal of Clinical Trials, 5, 204. doi:10.4172/2167-0870.1000204
- Koedam, E.L., Lauffer, V., van der Vlies, A.E., van der Flier, W.M., Scheltens, P., & Pijnenburg, Y.A. (2010). Early-versus late-onset Alzheimer's disease: More than age alone. Journal of Alzheimer's Disease, 19(4), 1401–1408.
- Logsdon, R.G., Gibbons, L.E., McCurry, S.M., & Teri, L. (1999). Quality of life in Alzheimer's disease: Patient and caregiver reports. Journal of Mental Health and Aging, 5(1), 21–32.
- Logsdon, R.G., Gibbons, L.E., McCurry, S.M., & Teri, L. (2002). Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine, 64(3), 510–519. doi:0033-3174/02/6403-0510
- Menne, H.L., Judge, K.S., & Whitlatch, C.J. (2009). Predictors of quality of life for individuals with dementia: Implications for intervention. Dementia, 8(4), 543–560. doi:10.1177/1471301209350288
- Millenaar, J.K., Bakker, C., Koopmans, R.T., Verhey, F.R., Kurz, A., & Vugt, M.E. (2016). The care needs and experiences with the use of services of people with young‐onset dementia and their caregivers: A systematic review. International Journal of Geriatric Psychiatry. doi:10.1002/gps.4502
- Mesulam, M.M. (2003). Primary progressive aphasia–A language-based dementia. The New England Journal of Medicine, 349(16), 1535–1542. doi:10.1056/NEJMra022435
- Neary, D., Snowden, J.S., Gustafson, L., Passant, U., Stuss, D., Black, S., … Benson, D.F. (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554.
- Ratnavalli, E., Brayne, C., Dawson, K., & Hodges, J.R. (2002). The prevalence of frontotemporal dementia. Neurology, 58(11), 1615–1621.
- Reed, B.R., Jagust, W.J., & Coulter, L. (1993). Anosognosia in Alzheimer's disease: Relationships to depression, cognitive function, and cerebral perfusion. Journal of Clinical and Experimental Neuropsychology, 15(2), 231–244. doi:10.1080/01688639308402560
- Reisberg, B., Ferris, S.H., de Leon, M.J., & Crook, T. (1982). The global deterioration scale for assessment of primary degenerative dementia. The American Journal of Psychiatry 139(9), 1136–1139.
- Reynolds, T., Thornicroft, G., Abas, M., Woods, B., Hoe, J., Leese, M., & Orrell, M. (2000). Camberwell Assessment of Need for the Elderly (CANE) Development, validity and reliability. The British Journal of Psychiatry, 176(5), 444–452.
- Riedijk, S., Duivenvoorden, H., Van Swieten, J., Niermeijer, M., & Tibben, A. (2009). Sense of competence in a Dutch sample of informal caregivers of frontotemporal dementia patients. Dementia and Geriatric Cognitive Disorders, 27(4), 337–343.
- Roach, P., & Drummond, N. 2014. ‘It's nice to have something to do’: Early-onset dementia and maintaining purposeful activity. Journal of Psychiatric Mental Health Nursing, 21, 889–895.
- Roach, P., Keady, J., Bee, P., & Hope, K. (2008). Subjective experiences of younger people with dementia and their families: Implications for UK research, policy and practice. Reviews in Clinical Gerontology, 18(2), 165–174.
- Rosness, T.A., Mjørud, M., & Engedal, K. (2011). Quality of life and depression in carers of patients with early onset dementia. Aging & Mental Health, 15(3), 299–306.
- Rossor, M.N., Fox, N.C., Mummery, C.J., Schott, J.M., & Warren, J.D. (2010). The diagnosis of young-onset dementia. Lancet Neurology, 9(8), 793–806. doi:10.1016/s1474-4422(10)70159-9
- Sampson, E.L., Warren, J.D., & Rossor, M.N. (2004). Young onset dementia. Postgraduate Medical Journal, 80(941), 125–139.
- Teng, E., Tassniyom, K., & Lu, P.H. (2012). Reduced quality-of-life ratings in mild cognitive impairment: Analyses of subject and informant responses. The American Journal of Geriatric Psychiatry, 20(12), 1016–1025. doi:10.1097/JGP.0b013e31826ce640
- Thorgrimsen, L., Selwood, A., Spector, A., Royan, L., de Madariaga Lopez, M., Woods, R.T., & Orrell, M. (2003). Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer's Disease (QoL-AD) scale. Alzheimer Disease & Associated Disorders, 17(4), 201–208.
- van Vliet, D., Bakker, C., Koopmans, R.T., Vernooij-Dassen, M.J., Verhey, F.R., & de Vugt, M.E. (2010). Research protocol of the NeedYD-study (Needs in Young onset Dementia): A prospective cohort study on the needs and course of early onset dementia. BMC Geriatrics, 10, 13. doi:10.1186/1471-2318-10-13
- van Vliet, D., de Vugt, M.E., Bakker, C., Koopmans, R.T., & Verhey, F.R. (2010). Impact of early onset dementia on caregivers: A review. International Journal of Geriatric Psychiatry, 25(11), 1091–1100. doi:10.1002/gps.2439
- Verhey, F.R.J., Rozendaal, N., Ponds, R.W.H.M., & Jolles, J. (1993). Dementia, awareness and depression. International Journal of Geriatric Psychiatry, 8(10), 851–856. doi:10.1002/gps.930081008
- Vernooij-Dassen, M., & Jeon, Y.H. (2016). Social health and dementia: The power of human capabilities. International Psychogeriatrics, 28(5), 701–703. doi:10.1017/s1041610216000260
- Vieira, R.T., Caixeta, L., Machado, S., Silva, A.C., Nardi, A.E., Arias-Carrión, O., & Carta, M.G. (2013). Epidemiology of early-onset dementia: A review of the literature. Clinical Practice & Epidemiology in Mental Health, 9, 88–95.
- Vogel, A., Mortensen, E.L., Hasselbalch, S.G., Andersen, B.B., & Waldemar, G. (2006). Patient versus informant reported quality of life in the earliest phases of Alzheimer's disease. International Journal of Geriatric Psychiatry, 21(12), 1132–1138. doi:10.1002/gps.1619
- Wimo, A., Jonsson, L., & Zbrozek, A. (2010). The Resource Utilization in Dementia (RUD) instrument is valid for assessing informal care time in community-living patients with dementia. The Journal of Nutrition, Health & Aging, 14(8), 685–690.
- World Health Organisation. (2012). Dementia: A public health priority. Retrieved from http://www.who.int/mental_health/publications/dementia_report_2012/en/
- World Health Organisation Group. (1995). The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Social Science & Medicine, 41(10), 1403–1409.