ABSTRACT
Objectives: To investigate the association between modifiable risk and protective factors and severe cognitive impairment and dementia in the very old. Additionally, the present study tests the predictive validity of the ‘LIfestyle for BRAin health’ (LIBRA) score, an index developed to assess an individual's dementia prevention potential.
Method: Two hundred seventy-eight individuals aged 85 years or older from the Cambridge City over-75s cohort study were followed-up until death. Included risk and protective factors were: diabetes, heart disease, hypertension, depression, smoking, low-to-moderate alcohol use, high cognitive activity, and physical inactivity. Incident severe cognitive impairment was based on the Mini-Mental State Examination (score: 0-17) and incident dementia was based on either post-mortem consensus clinical diagnostic assessments or death certificate data. Logistic regressions were used to test whether individual risk and protective factors and the LIBRA score were associated with severe cognitive impairment or dementia after 18 years follow-up.
Results: None of the risk and protective factors or the LIBRA score was significantly associated with increased risk of severe cognitive impairment or dementia. Sensitivity analyses using a larger sample, longer follow-up period, and stricter cut-offs for prevalent cognitive impairment showed similar results.
Conclusion: Associations between well-known midlife risk and protective factors and risk for severe cognitive impairment or dementia might not persist into very old age, in line with suggestions that targeting these factors through lifestyle interventions should start earlier in life.
Introduction
In 2012, dementia was proclaimed a public health priority by the World Health Organization (World Health Organization and Alzheimer's Disease International, Citation2012). Given the current lack of available treatments for dementia, research focus has shifted to prevention strategies (Baumgart et al., Citation2015). Even if a future cure for dementia becomes available, primary prevention should arguably remain one of the pillars of public health campaigns to reduce the number of affected individuals or to delay symptom onset. Several studies suggest that targeting modifiable risk factors is essential to reduce dementia risk (Barnes & Yaffe, Citation2011; Deckers et al., Citation2015; Norton, Matthews, Barnes, Yaffe, & Brayne, Citation2014). Questions still remain as to which factors should be targeted and which period during a person's lifespan would be the window of opportunity for most effective and efficient prevention. Since only a few randomized controlled trials have investigated the effects of single or multivariate risk factor reduction on cognitive decline or dementia incidence (Ngandu et al., Citation2016; van Charante et al., Citation2016), the evidence for most factors comes from observational studies (Barnes & Yaffe, Citation2011; Deckers et al., Citation2015; Plassman, Williams, Burke, Holsinger, & Benjamin, Citation2010). Recent systematic literature reviews and meta-analyses showed consistent support for a wide range of modifiable risk and protective factors associated with dementia, including cardiovascular and metabolic factors (e.g. hypertension, obesity, hypercholesterolemia, diabetes, coronary heart disease), lifestyle factors (e.g. diet, smoking, physical activity, alcohol consumption, cognitive activity) and psycho-social factors (e.g. depression) (Barnes & Yaffe, Citation2011; Baumgart et al., Citation2015; Deckers et al., Citation2015; Norton et al., Citation2014; Plassman et al., Citation2010). These factors could be targeted by tailored lifestyle interventions, preferably in midlife, when lifestyle adjustments are more feasible and probably most effective by reducing brain damage accumulated during long-term exposure to these factors (Lafortune et al., Citation2016). Indeed, previous studies have shown that the effects of certain risk factors vary across the life course. For instance, obesity and hypertension in midlife have more pronounced effects on dementia risk rather than in late life (Anstey, Cherbuin, Budge, & Young, Citation2011; Barnes & Yaffe, Citation2011; Deckers et al., Citation2015). Additionally, late life studies showed inconsistent results regarding the predictive ability of health and lifestyle factors (Anstey et al., Citation2011; Anstey, Lipnicki, & Low, Citation2008; Barnes & Yaffe, Citation2011; Power et al., Citation2011). There are a few studies that looked at the effects of metabolic syndrome (including obesity, hypertension, diabetes and hypercholesterolemia) towards cognitive decline. The association between metabolic syndrome and cognitive decline, which is found to be positive in younger populations, was not significant in the very old (Harrison et al., Citation2015; Katsumata et al., Citation2012). However, studies investigating the (combined) effects of modifiable risk and protective factors on dementia risk in a very old population (i.e. 85+ years) are particularly rare.
Therefore, the overall aim of the current study is to investigate the association between known modifiable risk and protective factors and severe cognitive impairment and dementia in individuals aged 85 years and older, as part of the Cambridge City over-75s cohort (CC75C) study, a longitudinal study of ageing in the very old (Brayne, Huppert, Paykel, & Gill, Citation1992; Fleming, Zhao, O'Connor, Pollitt, & Brayne, Citation2007). In addition, the study tests the predictive validity of the recently developed ‘LIfestyle for BRAin health’ (LIBRA) score (Deckers et al., Citation2015), a simple summary index that assesses an individual's dementia prevention potential by combining information on major modifiable health and lifestyle factors.
Methods
Study population
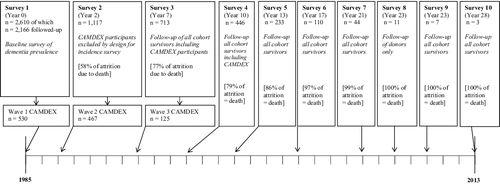
The CC75C study is a population-based study originally started in 1985 to measure the prevalence of dementia in people aged 75 years and over from a selection of geographically and socially representative general practices in the city of Cambridge, United Kingdom (UK) (Brayne et al., Citation1992; Fleming et al., Citation2007). From the original sample of 2610, a total of 2166 participants from all but one general practice formed the sample that was followed-up every two to four years until the last participant's death after the final survey in 2013 (year 28). Each survey used a structured interviewer-administered schedule to collect information on socio-demographic variables, activities of daily living, cognitive functioning (Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, Citation1975), health problems, medication and use of health and social services. After the first survey, proxy informant interviews were sought as needed to minimize loss to follow-up of the frailest individuals. From Survey 2 onwards, participants were asked whether they were willing to participate in the brain donation program. Each study phase was approved by the Cambridge Research Ethics Committee. At Survey 4 (year 10), the most comprehensive assessment of modifiable risk and protective factors took place and was hence considered the baseline assessment for the present study (n = 446; age range = 84–102), yielding a maximum follow-up period of 18 years (see ).
Dementia and severe cognitive impairment diagnosis
Participants with dementia were identified using three CC75C study data sources: (1) psychiatrist-administered assessments: the Cambridge Diagnostic Examination for the Elderly (CAMDEX) (Roth et al., Citation1986). In Survey 1, cognitively impaired participants (MMSE scores ≤23) and one in three participants with milder cognitive impairment (MMSE scores 24–25) underwent CAMDEX assessments. CAMDEX assessments following Surveys 2 and 3 also included participants with high cognitive scores (MMSE 26–30); (2) post-mortem consensus clinical diagnostic assessments were conducted to confirm dementia diagnosis or absence of dementia by the time of death for the sub-sample who had donated to the study's brain donation programme; (3) the cohort was flagged for mortality with the Office of National Statistics so the study resource includes cause of death data from death certificates. Prevalent dementia at Survey 4 was defined as dementia diagnosed by latest CAMDEX assessment (all prior to Survey 4) and incident dementia was based on either post-mortem consensus diagnosis or death certificate data, excluding prevalent CAMDEX dementia cases. More than half of Survey 4 participants had undergone at least one CAMDEX assessment (231/446), of whom 94/231 were diagnosed with prevalent dementia. More than a quarter of Survey 4 participants had post-mortem consensus clinical diagnostic assessment (121/446). Of these, 70/121 were diagnosed with dementia, of whom the majority (51/70) had incident dementia, i.e. no prior CAMDEX dementia diagnosis. Death certificate data were available for all participants, for whom 66 death certificates mentioned dementia. For 37 of these 66 there had been neither a CAMDEX dementia diagnosis nor any post-mortem clinical consensus diagnostic assessment. This totals 88 cases of incident dementia after Survey 4.
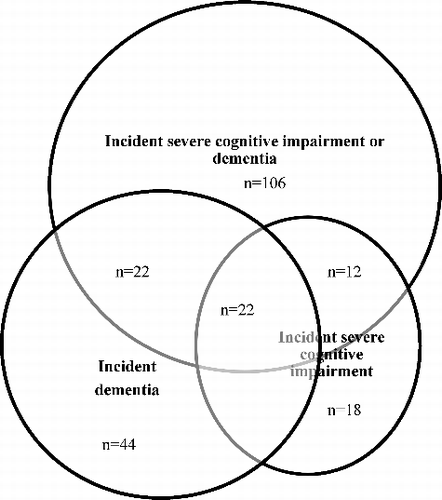
As the CC75C data sources may not capture all prevalent and incident dementia, we also included severe cognitive impairment in our overall outcome. Individuals with MMSE scores 0–17 were categorized as severely cognitively impaired (Tombaugh & McIntyre, Citation1992). Eighty-five cases of prevalent severe cognitive impairment could be identified based on their MMSE score at Survey 4 and 52 individuals developed incident severe cognitive impairment after Survey 4. Combining these two outcomes resulted in 140 prevalent and 84 incident cases of severe cognitive impairment or dementia (see ).
Demographics
Age and sex were confirmed from general practice lists when originally enrolled in the study and educational level was self-reported at baseline through questions on what age the participant left school and years of education after school leaving age.
Modifiable risk and protective factors
For the present study, data were available on 8 out of 12 modifiable risk and protective factors identified in a recent review: diabetes, depression, coronary heart disease, hypertension, alcohol consumption, smoking status, physical activity and cognitive activity (Deckers et al., Citation2015). Risk factors were based on either self-reported or proxy-reported information and were either dichotomized according to cut-offs as described below or rated present or absent. Information from proxy informants was used when participants’ cognitive abilities interfered with accurate reporting. The presence of coronary heart disease (angina pectoris or heart attack), diabetes and hypertension were based on self- or proxy-report of a doctor's diagnosis. For smoking, participants were divided into current smokers and non-smokers. Alcohol consumption was based on the reported frequency of current alcohol use, with 1–14 glasses per week considered low-to-moderate, according to recent UK alcohol guidelines (Department of Health, Citation2016). Individuals were considered depressed if they scored 6 or higher on the 10-item CAMDEX Depressive Symptoms Scale (range 0–11) (Girling et al., Citation1995). The physical activity measure categorized participants as active individuals who engaged in one or more forms of physical activity or exercise (e.g. walking, cycling, do-it-yourself, gardening, etc.) during the last week and the inactive who did none of these. For cognitive activity (intellectual engagement), participants were categorized as active or inactive based on reported activities undertaken in the last fortnight (e.g. visits to places of interest, hobbies, reading) and having taken part in education or training in recent years. Engagement in three or more of these activities was considered as cognitively active.
LIBRA-index
The LIBRA score was developed after triangulation of results from a systematic literature review on risk and protective factors for dementia and an expert consensus study (Deckers et al., Citation2015), as part of the European (FP7) INnovative, Midlife INtervention for Dementia Deterrence (In-MINDD) project (O'Donnell et al., Citation2015). It consists of 12 modifiable risk and protective factors that can be targeted by tailored lifestyle interventions and primary prevention: physical inactivity, smoking, (low-to-moderate) alcohol use, (high) cognitive activity, healthy diet, depression, hypertension, obesity, diabetes, hypercholesterolemia, coronary heart disease and renal disease. A weight is assigned to each factor, based on the factor's relative risk (Deckers et al., Citation2015). Weights are then standardized and summed to yield the final LIBRA score (range from −5.9 to +12.7), with higher scores indicating greater risk. In contrast to other risk indices, LIBRA is based on modifiable risk and protective factors only, and hence assesses an individual's potential for dementia prevention. A modified version of the LIBRA score was developed for the purpose of validation in older cohorts. It consists of 10 factors, excluding the risk factors obesity and hypertension, since these are considered to be major risk factors in midlife only. In CC75C, status information was available for 7 of the 10 factors from the modified LIBRA score (range from −4.2 to + 7.0). No information was available for diet, renal dysfunction and hypercholesterolemia.
Statistical analysis
Independent samples t-tests and χ2-tests were used to examine differences in risk factors and demographic variables between participants with incident severe cognitive impairment or dementia and non-affected individuals. Multiple imputation was used to impute missing values for the eight risk and protective factors, but only for participants with less than three missing factors (others were listwise-deleted). Multivariate imputation by chained equations was carried out using all non-missing data on risk and protective factors and socio-demographic covariates (age, sex and educational level) (White, Royston, & Wood, Citation2011). Ten imputed datasets were created and the results combined using Rubin's rules (Rubin, Citation1996). Separate logistic regressions tested whether individual risk and protective factors and the continuous LIBRA score were associated with odds for severe cognitive impairment or dementia in crude analyses (Model 1) or after adjustment for age, sex and educational level (Model 2). All analyses were done in Stata 13.1 (StataCorp, College Station, TX, USA), and the level of statistical significance used was p < 0.05 in two-sided tests.
Results
Sample characteristics
After exclusion of 140 cases of prevalent severe cognitive impairment or dementia and 10 individuals with missing MMSE data after Survey 4, the total outcome-free sample at Survey 4 consisted of 296 participants. The mean age was 87.9 (SD 3.2, 84–102) years, and 201 (68%) were female. During the 18-year follow-up, 84 individuals (28%) developed severe cognitive impairment or dementia.
Risk and protective factors
Status information for all eight risk and protective factors was available in 226 participants (76%). As described above, data were imputed for those participants with one (n = 38) or two (n = 14) missing factors. From the total of 2368 values for the 8 factors, 66 values were imputed (2.8%). We excluded 18 participants with more than two missing factors from the analyses, of whom 9 (50%) individuals developed severe cognitive impairment or dementia. This resulted in a total sample of 278 participants with a mean age of 87.8 (SD 3.1, 84–102) years, of whom 189 (68%) were female. Of these, 75 individuals (27%) developed severe cognitive impairment or dementia. The characteristics of this sample are illustrated in . None of the risk or protective factors was significantly associated with severe cognitive impairment or dementia in crude analyses or after adjustment for age, sex and educational level. Similar results were found in separate analyses for dementia and severe cognitive impairment (see ).
Table 1. Characteristics of Survey 4 participants (excluding prevalent cases and participants with data missing for more than two risk/protective factors) by outcome status.
Table 2. Prediction of outcome by risk factors and LIBRA score.
LIBRA-index
Seven of the eight risk factors from imputed datasets were used to calculate individuals’ modified LIBRA scores (hypertension omitted as explained above; range from −0.8 to 2.2). Lower LIBRA scores were significantly associated with increased odds of severe cognitive impairment or dementia, but this association was no longer significant when age, sex and educational level were added as covariates (see ). Separate analyses for dementia showed no significant results, but lower LIBRA scores were significantly associated with higher odds of severe cognitive impairment even after adjustment for socio-demographic covariates.
Sensitivity analyses
We performed two sensitivity analyses. First, to further investigate the direction of effects, the same analysis procedure for incident dementia as outcome was repeated with participants in Survey 3 (year 7; n = 560 after exclusion of 153 cases of prevalent dementia), a larger sample with longer follow-up but with information available on only six risk factors: diabetes, heart disease, hypertension, depression, high cognitive activity and physical inactivity. Again, data were imputed for those participants with one (n = 78) or two (n = 29) missing factors (imputation of 4.2%). As a result, the sample for these sensitivity analyses totalled 546 participants (mean age = 85.5 (SD 3.6, 81–103) years; 69% females; 17% developed dementia). Individuals with dementia were on average younger and more often female, and they died at a younger age in comparison with participants without dementia. Physical inactivity showed a protective effect and high cognitive activity was associated with an increased risk of incident dementia, but these effects were no longer significant or of only borderline significance when adjusted for age, sex and educational level (data not shown). Second, we studied whether results were due to inclusion of participants with mild or moderate cognitive impairment at Survey 4. For this, we restricted the sample to those with an MMSE score >21 and those with an MMSE score >25. Results were similar to the full sample analyses (data not shown).
Discussion
Our study shows that modifiable risk and protective factors did not predict odds of severe cognitive impairment or dementia in the very old, even after adjustment for age, sex and educational level. Similar results were found when severe cognitive impairment and dementia were taken as separate outcomes, when using a larger sample and longer follow-up period (up to 21 years; Survey 3; incident dementia as outcome) and when using stricter cut-offs for prevalent cognitive impairment (MMSE score >21 or >25), though results for physical inactivity and high cognitive activity were less clear and in an unexpected direction. Additionally, higher LIBRA scores did not increase the odds for dementia. It seems that the predictive value of modifiable risk and protective factors for dementia in the very old is poor. Therefore, dementia risk prediction models focusing on very old populations (i.e. 85+ years) developed to date have included other factors such as age, cognitive test performance, brain imaging measures or apolipoprotein E genotype (Barnes et al., Citation2009; Pekkala et al., Citation2017; Tang et al., Citation2015). It is important to note that inclusion of these non-modifiable factors will only increase the predictive accuracy of the risk prediction model, but will not provide information regarding an individual's potential for dementia prevention.
These findings suggest that targeting common modifiable risk factors in the very old might not have a serious impact on future dementia risk, and hence a different approach might be more appropriate. Indeed, previous randomized trials focusing on non-midlife populations generally produced negative findings (Peters et al., Citation2008; van Charante et al., Citation2016). The HYVET-COG trial showed that antihypertensive treatment did not reduce the incidence of dementia in participants aged 80 years or older (Peters et al., Citation2008). The multidomain pre-DIVA trial focusing on vascular care in persons aged 70–78 years showed non-significant results, although dementia risk could be reduced by antihypertensive treatment in those not previously treated (van Charante et al., Citation2016). Therefore, the Lancet Neurology Commission recently advised that dementia prevention studies should start in midlife (Winblad et al., Citation2016).
It is possible that other risk or protective factors may play a role in the aetiology of dementia in the very old. This group of older people has probably survived several morbidities earlier in life and managed to live with chronic conditions like diabetes or coronary heart disease until advanced age. They may possess longevity genes or other resilience factors, which protect them from getting dementia at a higher rate than those unexposed to such risk factors. On the other hand, it is also possible that dementia is an irreversible process in very old adults. In other words, the CC75C study participants who developed incident dementia may have had fairly advanced underlying pathology at baseline already (neurodegenerative, vascular or mixed) but of a slowly progressive nature that had not been diagnosed at an earlier stage. We hypothesize that this degenerative process cannot be reversed by lifestyle adaptations. It is also notable that the direction of most of the effect estimates was counter-intuitive (even in the sensitivity analysis). Inverse associations were found for diabetes, heart disease, hypertension, depression, low-to-moderate alcohol use, high cognitive activity, physical inactivity and the LIBRA score. Based on the above, it could be hypothesized that individuals at high (polygenetic) risk for dementia but with a healthy lifestyle survive longer and therefore have higher odds of developing dementia in late life in comparison with the rest of the survivors. Taken together, these results indicate that more studies are needed that investigate the effects of modifiable risk and protective factors on dementia risk in the very old, including studies aimed at detection of novel candidate risk factors.
The strengths of our study include the prospective study design focused on a representative population of the very old, the long follow-up period (up to 18 years), and the use of interviews with proxy informants to minimize lost to follow-up that could under-represent the frailest elderly and to replace missing data. However, our study has several limitations. First, the sample size was relatively small and, although sizeable for research with this very old age group, has limited power to detect significant associations. Therefore, a sensitivity analysis with a larger sample and longer follow-up period was conducted to further investigate the direction of effects.
Second, some selection bias may have occurred since individuals who were too ill or refused to participate due to medical reasons were more likely to drop out of the study. Third, the ascertainment of exposures was based on self-reported or proxy-reported information which could have led to response bias and non-differential exposure misclassification. Given the old age and physical condition of the study participants and the absence of sufficient resources to conduct neuropsychological and neurological examinations, full examinations of medical records, laboratory test and brain scans at each survey and for each participant, the structured survey interview was the best option. Fourth, the diagnosis of dementia was partly based on death certificate records. A drawback of such data is the lack of information on how any dementia reported was diagnosed. Moreover, absence of death certificate recorded dementia cannot be taken as confirmation that dementia was absent, with the possibility of non-differential outcome misclassification (e.g. under-reporting). The discrepancy between the prevalence of incident dementia and incident severe cognitive impairment in Survey 4 may reflect inadequate identification of dementia after death and may also reflect non-random missing cognitive data. Participants whose cognitive abilities declined rapidly may not have been interviewed after this downfall, either having died or having only proxy interviews by the next survey, and are therefore missing from the severe cognitive impairment category. Fifth, death before follow-up and non-response are likely to be associated with both the outcomes and factors investigated. Additionally, participants with more than two missing factors were excluded from the analyses. Higher proportion of this sub-sample (n = 18) than of the included participants lived in a long-term care institute, were disabled, had dementia and had missing MMSE data. These issues are likely to influence the investigated associations.
In sum, our results indicate that in the very old the associations between well-known risk and protective factors and subsequent development of dementia are not well established, but further research is required. It supports the idea that the effects of these factors are more pronounced at other life stages such as midlife. Future campaigns should focus their preventive message on these younger age groups in anticipation of long-term health benefit.
Acknowledgments
The authors wish to thank particularly the Cambridge City over-75s cohort study respondents, their families, friends and the staff in many care homes and collaborating general practices without whose help none of this research would be possible. The current research team gratefully acknowledges the contributions of CC75C study previous investigators and past research team members (see http://www.cc75c.group.cam.ac.uk/contacts/study-personnel/ for full list).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anstey, K.J., Cherbuin, N., Budge, M., & Young, J. (2011). Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obesity Reviews, 12(5), e426–437. doi:10.1111/j.1467-789X.2010.00825.x
- Anstey, K.J., Lipnicki, D.M., & Low, L.F. (2008). Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. American Journal of Geriatric Psychiatry, 16(5), 343–354. doi:10.1097/JGP.0b013e31816b72d4
- Barnes, D.E., Covinsky, K.E., Whitmer, R.A., Kuller, L.H., Lopez, O.L., & Yaffe, K. (2009). Predicting risk of dementia in older adults: The late-life dementia risk index. Neurology, 73(3), 173–179. doi:10.1212/WNL.0b013e3181a81636
- Barnes, D.E., & Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurology, 10(9), 819–828. doi:10.1016/S1474-4422(11)70072-2
- Baumgart, M., Snyder, H.M., Carrillo, M.C., Fazio, S., Kim, H., & Johns, H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer's & Dementia, 11(6), 718–726. doi:10.1016/j.jalz.2015.05.016
- Brayne, C., Huppert, F., Paykel, E., & Gill, C. (1992). The Cambridge project for later life: Design and preliminary results. Neuroepidemiology, 11(Suppl. 1), 71–75.
- Deckers, K., van Boxtel, M.P., Schiepers, O.J., de Vugt, M., Munoz Sanchez, J.L., Anstey, K.J., … Kohler, S. (2015). Target risk factors for dementia prevention: A systematic review and Delphi consensus study on the evidence from observational studies. International Journal of Geriatric Psychiatry, 30(3), 234–246. doi:10.1002/gps.4245
- Department of Health. (2016). Alcohol guidelines review — report from the guidelines development group to the UK Chief Medical Officers. Retrieved from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/545739/GDG_report-Jan2016.pdf
- Fleming, J., Zhao, E., O'Connor, D.W., Pollitt, P.A., & Brayne, C. (2007). Cohort profile: The Cambridge City over-75s Cohort (CC75C). International Journal of Epidemiology, 36(1), 40–46. doi:10.1093/ije/dyl293
- Folstein, M.F., Folstein, S.E., & McHugh, P.R. (1975). "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198.
- Girling, D.M., Huppert, F.A., Brayne, C., Paykel, E.S., Gill, C., & Mathewson, D. (1995). Depressive symptoms in the very elderly—their prevalence and significance. International Journal of Geriatric Psychiatry, 10(6), 497–504. doi:10.1002/gps.930100609
- Harrison, S.L., Stephan, B.C., Siervo, M., Granic, A., Davies, K., Wesnes, K.A., … Jagger, C. (2015). Is there an association between metabolic syndrome and cognitive function in very old adults? The Newcastle 85+ Study. Journal of the American Geriatrics Society, 63(4), 667–675. doi:10.1111/jgs.13358
- Katsumata, Y., Todoriki, H., Higashiuesato, Y., Yasura, S., Willcox, D.C., Ohya, Y., … Dodge, H.H. (2012). Metabolic syndrome and cognitive decline among the oldest old in Okinawa: In search of a mechanism. ( The KOCOA Project). The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 67A(2), 126–134. doi:10.1093/gerona/glr189
- Lafortune, L., Martin, S., Kelly, S., Kuhn, I., Remes, O., Cowan, A., & Brayne, C. (2016). Behavioural risk factors in mid-life associated with successful ageing, disability, dementia and frailty in later life: A rapid systematic review. PLoS One, 11(2), e0144405. doi:10.1371/journal.pone.0144405
- Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., … Kivipelto, M. (2016). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet, 385(9984), 2255–2263. doi:10.1016/S0140-6736(15)60461-5
- Norton, S., Matthews, F.E., Barnes, D.E., Yaffe, K., & Brayne, C. (2014). Potential for primary prevention of Alzheimer's disease: An analysis of population-based data. Lancet Neurology, 13(8), 788–794. doi:10.1016/S1474-4422(14)70136-X
- O'Donnell, C.A., Browne, S., Pierce, M., McConnachie, A., Deckers, K., van Boxtel, M.P., … Verhey, F.R. (2015). Reducing dementia risk by targeting modifiable risk factors in mid-life: Study protocol for the Innovative Midlife Intervention for Dementia Deterrence (In-MINDD) randomised controlled feasibility trial. Pilot and Feasibility Studies, 1(40), 1–12. doi:10.1186/s40814-015-0035-x
- Pekkala, T., Hall, A., Lotjonen, J., Mattila, J., Soininen, H., Ngandu, T., … Solomon, A. (2017). Development of a late-life dementia prediction index with supervised machine learning in the population-based CAIDE study. Journal of Alzheimer's Disease, 55(3), 1055–1067. doi:10.3233/jad-160560
- Peters, R., Beckett, N., Forette, F., Tuomilehto, J., Clarke, R., Ritchie, C., … Bulpitt, C. (2008). Incident dementia and blood pressure lowering in the hypertension in the very elderly trial cognitive function assessment (HYVET-COG): A double-blind, placebo controlled trial. Lancet Neurology, 7(8), 683–689. doi:10.1016/s1474-4422(08)70143-1
- Plassman, B.L., Williams, J.W., Jr., Burke, J.R., Holsinger, T., & Benjamin, S. (2010). Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine, 153(3), 182–193. doi:10.7326/0003-4819-153-3-201008030-00258
- Power, M.C., Weuve, J., Gagne, J.J., McQueen, M.B., Viswanathan, A., & Blacker, D. (2011). The association between blood pressure and incident Alzheimer disease: A systematic review and meta-analysis. Epidemiology (Cambridge, Mass.), 22(5), 646–659. doi:10.1097/EDE.0b013e31822708b5
- Roth, M., Tym, E., Mountjoy, C.Q., Huppert, F.A., Hendrie, H., Verma, S., & Goddard, R. (1986). CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. British Journal of Psychiatry, 149, 698–709.
- Rubin, D.B. (1996). Multiple imputation after 18+ years. Journal of the American Statistical Association, 91(434), 473–489. doi:10.2307/2291635
- Tang, E.Y.H., Harrison, S.L., Errington, L., Gordon, M.F., Visser, P.J., Novak, G., … Stephan, B.C.M. (2015). Current developments in dementia risk prediction modelling: An updated systematic review. PLoS One, 10(9), e0136181. doi:10.1371/journal.pone.0136181
- Tombaugh, T.N., & McIntyre, N.J. (1992). The mini-mental state examination: A comprehensive review. Journal of The American Geriatrics Society, 40(9), 922–935.
- van Charante, E.P., Richard, E., Eurelings, L.S., van Dalen, J.W., Ligthart, S.A., van Bussel, E.F., … van Gool, W.A. (2016). Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): A cluster-randomised controlled trial. Lancet, 388(10046), 797–805. doi:10.1016/s0140-6736(16)30950-3
- White, I.R., Royston, P., & Wood, A.M. (2011). Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine, 30(4), 377–399. doi:10.1002/sim.4067
- Winblad, B., Amouyel, P., Andrieu, S., Ballard, C., Brayne, C., Brodaty, H., … Zetterberg, H. (2016). Defeating Alzheimer's disease and other dementias: A priority for European science and society. Lancet Neurology, 15(5), 455–532. doi:10.1016/s1474-4422(16)00062-4
- World Health Organization and Alzheimer's Disease International. (2012). Dementia: A public health priority. Geneva: World Health Organization.