ABSTRACT
Objectives: The main objective of the current study is to compare chronic pain characteristics of older patients with Medically Unexplained Symptoms (MUS), to those of patients with Medically Explained Symptoms (MES), and to those of patients with Major Depressive Disorder (MDD).
Method: By combining data from the OPUS and NESDO study, we compared pain characteristics of 102 older (>60 years) MUS-patients to 145 older MES-patients and 275 older MDD-patients in a case-control study design. Group differences were analyzed using ANCOVA, adjusted for demographic and physical characteristics. Linear regression was applied to examine the association between pain characteristics and somatization (BSI-53 somatization scale) and health anxiety (Whitely Index).
Results: Older MUS-patients have approximately two times more chance of having chronic pain when compared to older MES-patients (OR = 2.01; p = .013) but equal chances as opposed to MDD-patients. After adjustments, MUS-patients report higher pain intensity and disability scores and more pain locations when compared to MES-patients, but equal values as MDD-patients. Health anxiety and somatization levels were positively associated with the number of pain sites in MUS-patients, but not with pain severity or disability.
Conclusion: Older MUS-patients did not differ from MDD-patients with respect to any of the chronic pain characteristics, but had more intense and disabling pain, and more pain locations when compared to older MES-patients.
Introduction
Chronic pain belongs to the most common, costly, and disabling conditions in later life (Institute of Medicine, Citation2011). Although a recent meta-analysis showed that anxiety, somatization and –to a lesser extent- depressive symptoms are common in chronic pain patients (Burke, Mathias, & Denson, Citation2015), only a minority of patients in pain clinics seem to have been diagnosed with a psychiatric condition (Merskey et al., Citation1987; Tyrer, Capon, Peterson, Charlton, & Thompson, Citation1989). When a medical explanation for the pain is lacking, as is most likely the case in patients with comorbid pain and depression (Katona et al., Citation2005), it is often assumed that the pain has a psychological origin.
In older age groups, research on pain in somatoform and depressive disorders is complicated by high levels of physical comorbidity that also result in pain symptoms (Blazer, Citation2003; Wijeratne, Brodaty, & Hickie, Citation2003). Nonetheless, pain has been extensively examined in late-life depression. Firstly, most studies show that pain precedes depression (Arola, Nicholls, Mallen, & Tomas, Citation2010; Fishbain, Cutler, Rosomoff, & Rosomoff, Citation1997; Hilderink, Burger, Deeg, Beekman, & Oude Voshaar, Citation2012), although some studies also demonstrated a reciprocal association (Chou, Citation2007; Geerlings, Twisk, Beekman, Deeg, & van Tilburg, Citation2002). Secondly, these studies point out the importance of psychological determinants like anxiety (Casten, Parmelee, Kleban, Lawton, & Katz, Citation1995; Hanssen, Naarding, Collard, Comijs, & Oude Voshaar, Citation2014) and self-efficacy (Turner, Ersek, & Kemp, Citation2005) in experiencing and managing pain in depressed older adults. Although we assume that somatization and hypochondriac cognitions might affect pain experience in older pain patients that are primarily diagnosed with a psychiatric condition, pain characteristics of older patients that have medically unexplained symptoms (MUS), somatoform disorders or depression have never been directly compared to those of older patients with pain due to an identified, physical disease.
Pain presentation and determinants of chronic pain could differ between these patient groups and such knowledge might be of help to refine treatment programs for chronic pain in later life. Therefore, we have combined data from both the Older Persons with medically Unexplained Symptoms (OPUS) Study (Hanssen, Lucassen, Hilderink, Naarding, & Oude Voshaar, Citation2016) and the Netherlands Study of Depression in Older Persons (NESDO) (Comijs et al., Citation2011). This offers the unique opportunity (1) to compare the prevalence of chronic pain in older patients that are diagnosed with either MUS, Medically Explained Symptoms (MES) and Major Depressive Disorder (MDD), (2) to examine the characteristics of chronic pain in the context of either MUS, MES and MDD, and finally (3) to examine the association between severity indices of MUS and chronic pain in MUS-patients.
Methods
Study designs
Older persons with medically unexplained symptoms (OPUS) study
The Older Persons with medically Unexplained Symptoms (OPUS) study aims to explore possible determinants of late-life MUS. Specifically, we performed a case-control study consisting of 118 older (>60 years) persons with MUS and 154 older persons with MES. In the OPUS study, MUS were defined as ‘physical symptoms that have existed for more than several weeks and for which adequate medical examination has not revealed any condition that sufficiently explains the symptoms’ (Olde Hartman et al., Citation2013). Initially, the older person's own general practitioner decided whether or not this person met the definition of MUS. Subsequently, a multidisciplinary team (consisting of a geriatrician (Benraad et al., Citation2013), an old-age psychiatrist and a psychologist) thoroughly assessed the older MUS-patient to confirm the unexplained origin of the physical complaints. MES were defined as physical symptoms that are present for more than several weeks for which adequate medical examination has revealed a physical disease (e.g. asthma, rheumatoid arthritis) that sufficiently explains the symptoms. Exclusion criteria for both cases and controls were the presence of a primary psychotic condition; presence of cognitive impairment, defined as a Mini Mental State Examination (MMSE; Folstein, Folstein, & McHugh, Citation1975) total score below 19 or an established diagnosis of dementia; living with terminal illness; not sufficiently mastering the Dutch language; and severe auditory and/or visual limitations. Participants were recruited in the community by advertisements in local newspapers, via general practices, and in secondary health care, particularly via an outpatient mental health clinic and the geriatrics department of the Radboud University Medical Center (Nijmegen, the Netherlands).
After the multidisciplinary screening, MUS-patients were visited at home for further assessment of social and cognitive determinants as well as care needs. In addition, all MUS-patients filled out self-report questionnaires. Older MUS-patients rejecting the multidisciplinary assessment (38.1%) (e.g. because they were not willing to visit a psychiatrist or psychologist), but agreed to participate in the study, received an additional home-visit by a researcher during which all standardized measurements of the multidisciplinary team were conducted (except for the full geriatric assessment). Older persons with MES were visited at home twice to complete all measures, similarly to the MUS-patients. All data were gathered between 2011 and 2014.
102/118 (86.4%) Older participants with MUS and 145/154 older participants with MES (94.2%) completed the Graded Chronic Pain Scale. The main reason for not completing this questionnaire was dropout between the first and the second research interview due to no interest in a home-visit (anymore).
Netherlands study of depression in older adults (NESDO)
The Netherlands Study of Depression in Older adults (NESDO Study; Comijs et al., Citation2011) is a prospective cohort study that aims to study late-life depression, its course and comorbidities.
In the current study, the baseline data of 275 older participants that fulfilled the criteria of a DSM-IV diagnosis of MDD in the past month were used as a second comparison group. Exclusion criteria for the NESDO study were a MMSE (Folstein et al., Citation1975) score below 19 or a (suspected) primary diagnosis of dementia, and insufficient command of the Dutch language. In the NESDO study, depressed participants were recruited via general practices, and via out- and inpatient clinics in different regions of the Netherlands. Baseline data were acquired with questionnaires, interviews and physical assessments between 2007 and 2010. All of the participants that fulfilled the criteria of the DSM-IV diagnosis of MDD in the past month (100%) completed the questionnaire on pain characteristics.
In both the OPUS and the NESDO study, all participants gave informed consent after receiving written and oral information about the study. Furthermore, local Medical Ethics Committees approved both the OPUS and the NESDO study.
Measures
General characteristics
| – | Basic demographic variables: We assessed age, gender and highest level of education achieved (categorized as low, middle and higher education level). | ||||
| – | Severity of depressive symptoms: We used the 30-item Inventory of Depressive Symptomatology (IDS-SR; Rush et al., Citation1985) to assess the severity of depressive symptoms over the past seven days (max. score: 84 points, with higher scores indicative of more severe depressive symptoms). | ||||
Physical characteristics
| – | Physical comorbidity: The presence of physical comorbidity was assessed by asking the presence of nine common diseases in later life: cardiac failure, rheumatoid arthritis, vascular disease, diabetes mellitus, stroke, cancer, peptic ulcer, liver disease and lung diseases (asthma, COPD). The total number of physical diseases was calculated, resulting in a maximum score of 9. | ||||
| – | Medication: The patient's medication was listed to determine the total number of different medicines, including psychiatric medications. To increase reliability, the researcher inspected drug containers and/or asked for a list of medication use from the person's pharmacist. | ||||
| – | Cognitive functioning: The MMSE (Folstein et al., Citation1975) was assessed in all participants to provide a global indication of cognitive functioning (max. score: 30, with higher scores indicative of better cognitive functioning). | ||||
Pain characteristics
The Graded Chronic Pain Scale (GCPS; von Korff & Miglioretti, Citation2005; Von Korff, Ormel, Keefe, & Dworkin, Citation1992) was used to assess pain characteristics in the three patient groups. This self-report questionnaire questions the presence of seven pain locations: ‘In the past six months, did you experience any -back pain, neck pain, headache/migraine, face-ache, abdominal pain, joint pain, chest pain -?’ (yes/no). In addition, the current use of pain medication use was charted.
If pain was present in one or more pain locations in the past six months, first, the most prominent pain location was asked: ‘If you had any pain in the past six months, which pain did affect you most?’. Next, additional questions were asked to examine characteristics of the most prominent pain location, specifically pain intensity, the degree to which one feels disabled, and the number of days in pain. In line with the suggestions of the International Association for the Study of Pain (Citation1986) and consistent with previous studies (e.g. Gerrits et al., Citation2012; Hanssen et al., Citation2014), we defined chronic pain as pain that was present for 90 days or more in the past six months.
Three pain characteristics were derived from the GCPS:
| (1) | Pain intensity score – The pain intensity score was calculated by taking the average of three individual item scores on the pain intensity of the most prominent pain location, namely ‘How would you describe your pain at this moment?’, ‘How intense was your worst pain over the past six months?’, and ‘How intense was your average pain over the past six months?’ Answers were given on a 0–10 scale. The average score on these three items was multiplied by ten, resulting in a pain intensity score ranging from 0 (no pain) to 100 (high pain intensity). | ||||
| (2) | Pain disability score – Three questions regarding pain disability were asked: ‘To what extent have you been limited by your chronic pain in carrying out your daily activities over the past six months?’, ‘To what extent have you been limited in your spare time, your social life, and during family activities because of your chronic pain over the past six months?’, and ‘To what extent have you been limited in carrying out your work (housework included) because of your chronic pain over the past six months?’. Answers were given on a 0–10 scale. The average score on these three items was multiplied by ten, resulting in a scale from 0 (no pain disability) to 100 (high pain disability). | ||||
| (3) | Number of pain locations – The number of pain locations was assessed by counting the number of pain locations that were present in the past six months, resulting in a score ranging from 0 to 7. | ||||
Severity indices of MUS
Among patients with MUS, several measures were used as a severity indicator of the MUS.
| – | Severity of the primary physical complaint: This was assessed using a Visual Analogue Scale (VAS) from 0 (not severe at all) to 100 (very severe): ‘How severe was your primary physical complaint over the past six months, on average?’ | ||||
| – | Presence of a Somatoform Disorder according to DSM-IV criteria: The Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., Citation1998) was used to assess the presence of a Somatoform Disorder (American Psychiatric Association [APA], Citation2000). With this semi-structured interview, the DSM-IV criteria of Somatization Disorder, Pain Disorder, and Hypochondria were assessed. | ||||
| – | Presence of a comorbid disorder according to DSM-IV criteria: This was assessed with the MINI as well, by asking the sections about depressive disorders, anxiety disorders, obsessive-compulsive disorder, and alcohol and drugs dependence/abuse. | ||||
| – | Hypochondriac cognitions: The Whitely Index (Pilowsky, Citation1967), a questionnaire consisting of 14 statements with answering categories yes/no (range 0–14), was used to assess the levels of hypochondriac cognitions. | ||||
| – | Somatization: Levels of somatization were assessed using the Brief Symptom Inventory's (BSI) somatization subscale that consists of seven items with answering categories from 1 (not present at all) to 5 (present all the time) in the past week. This scale reflects ‘psychological distress arising from perception of bodily dysfunction’ (Derogatis, Citation1975). | ||||
Statistical analyses
Descriptive statistics, such as mean scores (M) and standard deviations (SD), are reported for all demographic and pain characteristics. Univariate Analyses of Variance (ANOVAs) (for continuous variables) and Chi-Square tests (for categorical variables) were performed to assess between-group (MUS/MDD/MES) differences regarding patient- and pain characteristics. In addition, Analyses of Covariance (ANCOVAs) were performed to assess between-group differences of the pain intensity score, the pain disability score, and the number of pain locations, adjusted for demographic (age, gender, education) and physical characteristics (physical comorbidity, medication use, cognitive functioning). In case of statistically significant differences (p < .05), Bonferroni post hoc analyses were performed to assess contrasts, using the older MUS-group as a reference category. For all ANCOVAs, degrees of freedom (df), F-values and p-values are reported, as well as the eta2 value as an estimation of the effect size.
In order to assess whether the presence of chronic pain was associated with the three patient groups, a binary logistic regression analysis was performed with chronic pain (yes/no) as dependent variable and dummies for group status as the independent variable (with MUS as the reference group), adjusted for age, gender, education, physical comorbidity, number of medication use and cognitive functioning. This analysis was repeated with additional adjustments for the severity of depressive symptoms (IDS-score), since depressive symptoms in itself may also affect the experience of pain. Odds ratios (ORs), 95% confidence interval (CI) values, and significance values (p-values) of these binary logistic regression models are reported.
To examine the association between severity measures of MUS and pain characteristics in older MUS-patients with chronic pain, multiple linear regression analyses were performed with outcomes pain intensity, pain disability and the number of pain locations. These models were all adjusted for demographic (age, gender, education) and physical variables (physical comorbidity, number of medication use, cognitive functioning). For all regression models B-values, Standard Errors (SE), Beta-values, p-values and R2's are presented.
P-values under .05 were considered statistically meaningful. All statistical analyses were performed using IBM SPSS version 20.
Results
Sample characteristics
presents patient characteristics per patient group. In sum, MUS-patients were significantly younger than MES-patients and MDD-patients. Furthermore, whereas MUS-patients and MDD-patients were more often female, MES-patients were more often male. MDD-patients reported a significantly higher severity of depressive symptoms than MUS-patients, which in turn reported a significantly higher severity of depressive symptoms than MES-patients. The three groups did not differ regarding physical comorbidity and the number of medication use. Compared to older MES- and MDD-patients, older MUS-patients most often had chronic pain.
Table 1. Patient characteristics of the study sample.
Adjusted for demographic (age, gender, education) and physical variables (physical comorbidity, number of medication use and level of cognitive functioning), the odds ratio of having chronic pain was about two times greater for older MUS-patients as opposed to older MES-patients (OR = 2.01; 95% CI: 1.16–3.49; p = .013), but similar for older MUS and MDD -patients (OR = .83; 95% CI: .52–1.33; p = .438). When additionally adjusted for depressive symptom severity, again, the odds of having chronic pain were increased for older MUS-patients as opposed to older MES-patients (OR = 3.24; 95% CI: 1.66–6.32; p = .001), but not as opposed to depressed older adults (OR = 1.48; 95% CI: .84–2.59; p = .175).
Characteristics of chronic pain in the context of either MUS, MES or MDD
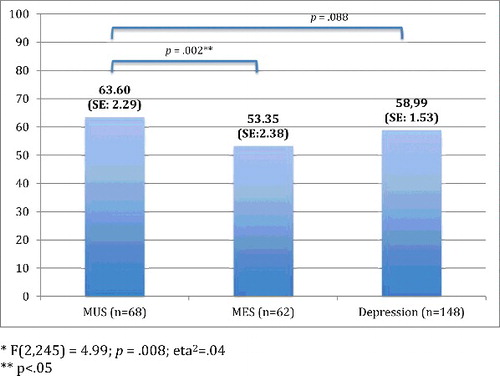
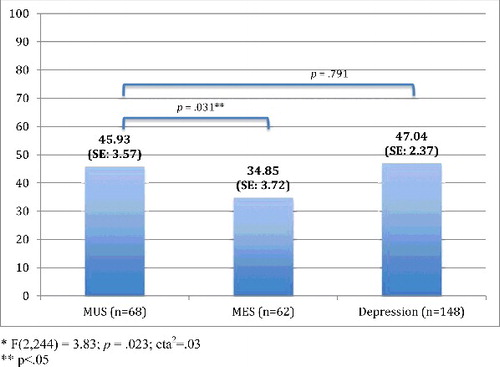
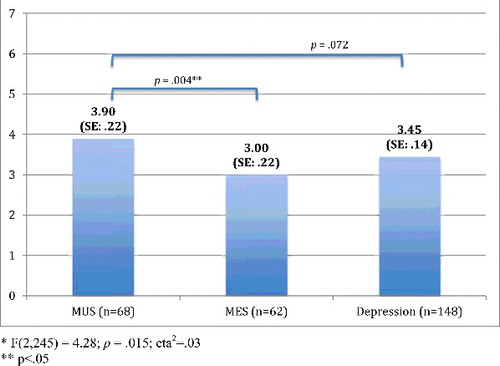
presents the chronic pain characteristics per patient group. In sum, the frequency of the most prominent pain locations did not differ significantly between the three groups, whereas the other characteristics did. Post hoc tests showed that chronic pain had fewer locations and was less intense in MES-patients compared with either MUS-patients as well as MDD-patients, whereas disability due to pain only differed significantly between MES- and MDD-patients, with lower disability scores in MES patients. MUS- and MDD-patients did not differ on any of the characteristics.
Table 2. Chronic pain characteristics in the context of MUS, MES and MDD.
Figures – present these group differences, based on MANOVAs adjusted for demographic (age, gender, education) and physical variables (physical comorbidity, number of medication use and level of cognitive functioning). In comparison to the results shown in , the differences between MUS and MDD weakened after adjustments for demographic and physical variables. However, differences between MUS- and MES-patients remained statistically different.
Associations between severity indices MUS and chronic pain characteristics
Multiple linear regression analyses were performed with outcomes pain intensity, pain disability and the number of pain locations and severity measures of MUS, adjusted for demographic and physical variables (). In sum, in older MUS-patients with chronic pain, the self-perceived severity of the primary physical complaint was associated with higher pain intensity scores, whereas the presence of a primary somatoform disorder and the presence of a comorbid diagnosis according to DSM-IV criteria were associated with pain disability scores. Furthermore, a higher number of pain locations was associated with both a higher level of hypochondriac cognitions, as well as higher levels of somatization in older MUS-patients with chronic pain.
Table 3. Associations between severity measures of MUS and pain intensity, pain disability and the number of pain locations in older MUS-patients with chronic pain (n = 68).
Discussion
Main findings
In our study, two-thirds of older patients with MUS had chronic pain and half of the depressed older patients. Older persons with MUS have approximately two times more chance of chronic pain when compared to older persons with MES, and have equal chances of having chronic pain as opposed to older persons with MDD. Older persons with MUS and older persons with MDD report equal chronic pain intensity levels and an equal number of pain locations, but more intense chronic pain and pain in more sites when compared to older MES-patients.
Among MUS-patients with chronic pain, severity measures of MUS were differentially related to the specific pain characteristics under study: (1) higher pain intensity scores were associated with higher-self-perceived severity of the primary physical complaint; (2) higher pain intensity scores were associated with the presence of a psychiatric condition, namely both a primary somatoform disorder as well as comorbid (other) psychiatric conditions; and last, (3) more pain locations were associated with higher levels of somatization as well as hypochondria.
Integration with previous findings
The present study clearly shows that pain characteristics in older MUS-patients and MDD-patients are comparable with respect to intensity, number of pain locations and disability. These similarities in the phenotypic expression of pain may point to shared underlying pathways for chronic pain in patients with either MUS or MDD, as was previously mentioned by Katona et al. (Citation2005).
By studying the associations between pain characteristics and MUS-severity indicators in older MUS-patients, we found that pain intensity was associated with the severity of the primary unexplained symptom. This makes sense, acknowledging that pain was the primary physical complaint in most of our MUS-patients. The absence of an association between pain intensity and all other indicators of MUS-severity, however, may be more interesting: we found that with increasing perceived severity of MUS, pain intensity is not the pain characteristic that amplifies. Rather, older MUS-patients express the increased severity of MUS by a higher level of pain disability and by more pain locations.
More specific, pain disability in older MUS-patients is associated with the presence of a (co-morbid) psychiatric condition, irrespective whether this is a somatoform, anxiety or depressive disorder. At first sight, this might not be surprising as (severe) functional disability is a prerequisite for having a psychiatric condition (APA, Citation2000). Nonetheless, we measured pain disability using a self-report questionnaire (GCPS); clinicians, on the other hand, set the psychiatric diagnoses. Although several studies have shown that patients and clinicians often disagree about the level of the patient's disability (e.g. Rothwell, McDowell, Wong, & Dorman, Citation1997), this cannot simply be generalized to older MUS patients according to our findings. Moreover, when older MUS-patients experience high pain disability, this might be an indication for psychiatrists to consider the presence and treatment of psychiatric comorbidity.
Last, a higher number of pain locations was associated with more hypochondriac cognitions and a higher level of somatization. The direction of this association, however, cannot be established within the present study. It seems most likely that a high level of somatization or health anxiety results in attentional bias for physical signals and subsequently in the emergence of more pain locations. If true, psychological treatment might reduce the burden of chronic pain, in this case by reducing the number of pain sites. On the other hand, we cannot exclude the possibility that more pain locations simply result in increased levels of health anxiety and somatization.
Strengths and limitations of the current study
As far as we know, this is the first study to explore differences in chronic pain characteristics between older persons with MUS, MES and MDD. To interpret our findings correctly, several limitations should be taken into account.
First, in the current study we combined data of the OPUS and the NESDO study. Participants were thus recruited using slightly different study protocols, increasing the chance of selection bias. Over- or underrepresentation of specific subgroups in the studies, such as patients with very mild or very severe complaints, could distort our research findings, herewith possibly reducing external validity. As older persons living with terminal illness were excluded from both the OPUS and the NESDO studies, our current findings cannot be generalized to this specific patient group.
In line with this, the presence of psychiatric diagnoses was based on the MINI (Sheehan et al., Citation1998) in the OPUS study, and on the CIDI (Wittchen et al., Citation1991) in the NESDO study. Furthermore, in the NESDO study only the parts of the CIDI about depressive and anxiety disorders were assessed. Therefore, we do not know how many of the depressed older adults actually had a comorbid somatoform disorder. In the OPUS study, participants were allowed to have comorbid depression and anxiety next to the primary MUS. As we did not compare psychiatric diagnoses in the current study and a previous study has demonstrated a high level of agreement between psychiatric diagnoses based on both instruments (Lecrubier et al., Citation1997), consequences of the use of different measurements are probably limited.
Implications for clinical practice
Compared to chronic pain in MES-patients, chronic pain in older patients with MUS or MDD has a higher intensity, presents in a higher number of locations and is more disabling. These findings might indicate the importance of an intensive dual-track pain-management program for patients with MUS or depression. Good somatic health care is crucial for relieving pain, while it is known that psychiatric patients often receive suboptimal health care (Corrigan, Citation2004; Kohn, Saxena, Levav, & Saraceno, Citation2004). Pain management could be focused on learning adequate coping skills as offered in mental health care programs for chronic pain or somatoform conditions (Ashburn & Staats, Citation1999; Campbell, Clauw, & Keefe, Citation2003), while taking into account the treatment preferences of the older age group (Lansbury, Citation2000). Our results point to several potential treatment targets in older adults, namely the level of hypochondria, somatization and comorbid psychiatric conditions, as for all of these aspects evidence based interventions are available (e.g. Allen, Woolfolk, & Escobar, Citation2006; McGuire, Nicholas, Asghari, Wood, & Main, Citation2014; Speckens et al., Citation1995). Moreover, integrated programs combining optimal medical and mental management are in line with the DSM-5 classification of Somatic Symptom Disorder that emphasized the interaction between medically (un)explained symptoms and psychological symptoms, such as depressive symptoms (American Psychiatric Association, Citation2013).
The effects of multidisciplinary chronic pain management programs are promising, e.g. in achieving more effective coping strategies (Jensen, Turner, & Romano, Citation2001). The positive effects of these chronic pain management programs are also endorsed for older adults (McGuire et al., Citation2014). Nonetheless, the high prevalence of chronic pain identified in our patient samples – two-thirds of all older MUS-patients and half of all persons with MDD -- suggests underutilization of these programs for older adults. Our findings may not only stress the importance of such treatment programs for older patients, but may also help to refine treatment programs for this age group.
Disclosure statement
The authors have nothing to disclose.
Additional information
Funding
References
- Allen, L. A., Woolfolk, R. L., & Escobar, J. L. (2006). Cognitive-behavioral therapy for somatization disorder: A randomized controlled trial. Archives of Internal Medicine, 166, 1512–1518.
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: Author.
- Arola, H., Nicholls, E., Mallen, C., & Tomas, E. (2010). Self-reported pain interference and symptoms of anxiety and depression in community-dwelling older adults: Can a temporal relationship be determined ? European Journal of Pain, 14, 966–971.
- Ashburn, M. A., & Staats, P. S. (1999). Management of chronic pain. The Lancet, 353, 1865–1869.
- Benraad, C. E. M., Hilderink, P. H., Van Driel, D. T. J. W., Disselhorst, L. G., Lubberink, B., van Wolferen, L., … Oude Voshaar, R. C. (2013). Physical functioning in older persons with somatoform disorders: A pilot study. Journal of the American Medical Directors Association, 14, 75.e9–75.e13.
- Blazer, D. G. (2003). Depression in late life: Review and commentary. The Journals of Gerontology: Medical Sciences, 58A, 249–265.
- Burke, A. L., Mathias, J. L., & Denson, L. A. (2015). Psychological functioning of people living with chronic pain. British Journal of Clinical Psychology, 54, 345–360.
- Campbell, L. C., Clauw, D. J., & Keefe, F. J. (2003). Persistent pain and depression: A biopsychosocial perspective. Biological Psychiatry, 54, 399–409.
- Casten, R. J., Parmelee, P. A., Kleban, M. H., Lawton, M. P., & Katz, I. R. (1995). The relationships among anxiety, depression, and pain in a geriatric institutionalized sample. Pain, 61, 271–276.
- Chou, K. (2007). Reciprocal relationship between pain and depression in older adults: Evidence from the English Longitudinal Study of Ageing. Journal of Affective Disorders, 102, 115–123.
- Comijs, H. C., van Marwijk, H. W., van der Mast, R. C., Naarding, P., Oude Voshaar, R. C., Beekman, A. T. F., … Smit, J. H. (2011). The Netherlands study of depression in older persons (NESDO); a prospective cohort study. BMC Research Notes, 4, 524.
- Corrigan, P. (2004). How stigma interferes with mental health care. American Psychologist, 59, 614–625.
- Derogatis, L. R. (1975). The brief symptom inventory. Baltimore, MD: Clinical Psychometric Research.
- Fishbain, D. A., Cutler, R., Rosomoff, H. L., & Rosomoff, R. S. (1997). Chronic pain-associated depression: Antecedent or consequence of chronic pain? A review. The Clinical Journal of Pain, 13, 116–137.
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). Mini-mental state – A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198.
- Geerlings, S. W., Twisk, J. W. R., Beekman, A. T. F., Deeg, D. J. H., & van Tilburg, W. (2002). Longitudinal relationship between pain and depression in older adults: Sex, age and physical disability. Social Psychiatry and Psychiatric Epidemiology, 37, 23–30.
- Gerrits, M. M. J. G., Vogelzangs, N., van Oppen, P., van Marwijk, H. W. J., van der Horst, H., & Penninx, B. W. J. H. (2012). Impact of pain on the course of depressive and anxiety disorders. Pain, 153, 429–436.
- Hanssen, D. J., Lucassen, P. L., Hilderink, P. H., Naarding, P., & Oude Voshaar, R. C. (2016). Health-related quality of life in older persons with medically unexplained symptoms. American Journal of Geriatric Psychiatry, 24(11), 1117–1127
- Hanssen, D. J. C., Naarding, P., Collard, R. M., Comijs, H. C., & Oude Voshaar, R. C. (2014). Physical, lifestyle, psychological and social determinants of pain intensity, pain disability, and the number of pain locations in depressed older adults. Pain, 155, 2088–2096.
- Hilderink, P. H., Burger, H., Deeg, D. J., Beekman, A. T., & Oude Voshaar, R. C. (2012). The temporal relationship between pain and depression: Results from the Longitudinal Aging Study Amsterdam. Psychosomatic Medicine, 74, 945–951.
- Institute of Medicine, Committee on Advancing Pain Research, Care and Education. (2011). Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press.
- International Association for the Study of Pain. (1986). Classification of chronic pain – descriptions of chronic pain syndromes and definitions of pain terms. Pain, 3, 1–222.
- Jensen, M. P., Turner, J. A., & Romano, J. M. (2001). Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. Journal of Consulting and Clinical Psychology, 69, 655–662.
- Katona, C., Peveler, R., Dowrick, C., Wessely, S., Feinmann, C., Gask, L., … Wager, E. (2005). Pain symptoms in depression: Definition and clinical significance. Clinical Medicine, 5, 390–395.
- Kohn, R., Saxena, S., Levav, I., & Saraceno, B. (2004). The treatment gap in mental health care. Bulletin of the World Health Organization, 82, 858–866.
- Lansbury, G. (2000). Chronic pain management: A qualitative study of elderly people's preferred coping strategies and barriers to management. Disability and Rehabilitation, 22, 2–14.
- Lecrubier, Y., Sheehan, D. V., Weiller, E., Amorim, P., Bonora, I., Harnett Sheehan, K., … Dunbar, G. C. (1997). The mini international neuropsychiatric interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry, 12, 224–231.
- McGuire, B. E., Nicholas, M. K., Asghari, A., Wood, B. M., & Main, C. J. (2014). The effectiveness of psychological treatments for chronic pain in older adults: Cautious optimism and an agenda for research. Current Opinion in Psychiatry, 27, 380–384.
- Merskey, H., Lau, C. L., Russell, E. S., Brooke, R. I., James, M., Lappano, S., … Tilsworth, R. H. (1987). Screening for psychiatric morbidity. The pattern of psychological illness and premorbid characteristics in four chronic pain populations. Pain, 30, 141–157.
- Olde Hartman, T. C., Blankenstein, A. H., Molenaar, A. O., Bentz van den Berg, D., Van der Horst, H., Arnold, I., … Woutersen-Koch, H. (2013). NHG guideline on medically unexplained symptoms (MUS). Huisarts en Wetenschap, 56, 222–230.
- Pilowsky, I. (1967). Dimensions of hypochondriasis. The British Journal of Psychiatry, 113, 39–43.
- Rothwell, P. M., McDowell, Z., Wong, C. K., & Dorman, P. J. (1997). Doctor's and patients don't agree: Cross sectional study of patient's and doctor's perceptions and assessments of disability in multiple sclerosis. British Medical Journal, 314, 1580.
- Rush, A. J., Giles, D. E., Schlesser, M. A., Fulton, C. L., Weissenburger, J., & Burns, C. (1985). The inventory for depressive symptomatology (IDS): Preliminary findings. Psychiatry Research, 18, 65–87.
- Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Harnett, K., Amorim, P., Janavs, J., … Dunbar, G. C. (1998). The mini-international neuropsychiatric interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-V and ICD-10. Journal of Clinical Psychiatry, 59, 22–33.
- Speckens, A. E., van Hemert, A. M., Spinhoven, P., Hawton, K. E., Bolk, J. H., & Rooijmans, H. G. (1995). Cognitive behavioural therapy for medically unexplained physical symptoms: A randomised controlled trial. British Medical Journal, 311, 1328.
- Turner, J. A., Ersek, M., & Kemp, C. (2005). Self-efficacy for managing pain is associated with disability, depression, and pain coping among retirement community residents with chronic pain. The Journal of Pain, 6, 471–479.
- Tyrer, S. P., Capon, M., Peterson, D. M., Charlton, J. E., & Thompson, J. W. (1989). The detection of psychiatric illness and psychological handicaps in a British pain clinic population. Pain, 30, 63–74.
- Von Korff, M., & Miglioretti, D. L. (2005). A prognostic approach to defining chronic pain. Pain, 117, 304–313.
- Von Korff, M., Ormel, J., Keefe, F. J., & Dworkin, S. F. (1992). Grading the severity of chronic pain. Pain, 50, 133–149.
- Wijeratne, C., Brodaty, H., & Hickie, I. (2003). The neglect of somatoform disorders in old-age psychiatry: Some explanations and suggestions for future research. International Journal of Geriatric Psychiatry, 18, 812–819.
- Wittchen, H. U., Robins, L. N., Cottler, L. B., Sartorius, N., Burke, J. D., & Regier, D. (1991). Cross-cultural feasibility, reliability and sources of variance of the composite international diagnostic interview (CIDI). The multicentre WHO/ADAMHA field trials. The British Journal of Psychiatry, 159, 645–653.