 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives: Poor social connections may be associated with poor cognition in older people who are not experiencing mental health problems, and the trajectory of this association may be moderated by cognitive reserve. However, it is unclear whether this relationship is the same for older people with symptoms of depression and anxiety. This paper aims to explore social relationships and cognitive function in older people with depression and anxiety.
Method: Baseline and two-year follow-up data were analysed from the Cognitive Function and Ageing Study–Wales (CFAS-Wales). We compared levels of social isolation, loneliness, social contact, cognitive function, and cognitive reserve at baseline amongst older people with and without depression or anxiety. Linear regression was used to assess the relationship between isolation and cognition at baseline and two-year follow-up in a subgroup of older people meeting pre-defined criteria for depression or anxiety. A moderation analysis tested for the moderating effect of cognitive reserve.
Results: Older people with depression or anxiety perceived themselves as more isolated and lonely than those without depression or anxiety, despite having an equivalent level of social contact with friends and family. In people with depression or anxiety, social isolation was associated with poor cognitive function at baseline, but not with cognitive change at two-year follow-up. Cognitive reserve did not moderate this association.
Conclusion: Social isolation was associated with poor cognitive function at baseline, but not two-year follow-up. This may be attributed to a reduction in mood-related symptoms at follow-up, linked to improved cognitive function.
Background
Cognitive ageing refers to a normal process in healthy ageing in which some subtle changes in cognitive function are observed (Liverman, Yaffe, & Blazer, Citation2015). Trajectories of cognitive change can vary from healthy cognitive ageing to more advanced unhealthy cognitive decline or progression to dementia (Gow, Pattie, Whiteman, Whalley, & Deary, Citation2007; Wilson et al., Citation2002).
Cognitive reserve theory can account for differences in late-life cognitive trajectories and suggests that individuals differ in their resilience against damage caused by age-related brain pathology (Stern, Citation2002, Citation2012). Hence, individuals with an equivalent level of pathology may present with differing levels of cognitive ability (Stern, Citation2009). These differences may be accounted for by factors across the lifespan that build cognitive reserve, including experiences and opportunities such as education or complex occupations, lifestyle factors such as physical exercise, and participation in social and cognitively stimulating activities (Stern, Citation2002, Citation2012). This reserve can act as a protective mechanism that compensates for damage and recruits alternative neural networks if required (Siedlecki et al., Citation2009). Individuals differ in the level of reserve built across the lifespan, which results in differences in the level of resilience against pathology and hence differences in trajectories of late-life cognition (Siedlecki et al., Citation2009).
Compared to other factors that are thought to build cognitive reserve, the relationship between social isolation and cognitive function is less well studied. Social isolation is defined as having few social contacts and little engagement with others and the wider community (Nicholson, Citation2009). Social isolation may not be a choice, particularly for people with physical and mental health problems who may desire social contact but are unable to engage due to the impact of illness and stigma (Corrigan & Rao, Citation2012). Additional barriers such as poor transport, living in rural areas, having no children or family nearby, and a lack of opportunity for social engagement in the community are also highly influential in determining social isolation (Bowling & Stafford, Citation2007; Rosso, Taylor, Tabb & Michael, Citation2013; Wen, Hawkley, & Cacioppo, Citation2006).
From a cognitive reserve perspective, having a wide range of social contacts and frequent engagement in social activities may provide mental stimulation through challenging and complex interactions with others and hence build reserve (Bennett, Schneider, Tang, Arnold, & Wilson, Citation2006; Fratiglioni, Wang, Ericsson, Maytan, & Winblad, Citation2000). Evidence suggests that being isolated may be associated with poor cognitive function in healthy older people (DiNapoli, Wu, & Scogin, Citation2014; Shankar, Hamer, McMunn, & Steptoe, Citation2013; Wilson et al., Citation2007), however, some studies do not find this association (Holwerda et al., Citation2012; Simning, Conwell, & van Wijngaarden, Citation2014; Wilson et al., Citation2007).
The association between social isolation and cognitive function may be different in people with depression or anxiety, who may have fewer social interactions due to symptoms of, or underlying reasons for, illness (García-Peña, Citation2013; Litwin, Citation2012; Segrin, Citation2000). In turn, having less frequent social contact may increase feelings of loneliness and isolation (Domènech-Abella et al., Citation2017; Luanaigh & Lawlor, Citation2008; Yaacob, Juhari, Talib, & Uba, Citation2017), hypervigilance to social threats, or expectations of negative social interactions, which may intensify and reinforce feelings of loneliness and isolation (Cacioppo & Hawkley, Citation2009; Granerud & Severinsson, Citation2006). In addition to possibly benefitting cognitive function, social connections are fundamental for good mental health and may reduce symptoms of depression or anxiety (Diener & Seligman, Citation2004; Kuchibhatla, Fillenbaum, Hybels, & Blazer, Citation2012; Santini, Koyanagi, Tyrovolas, Mason, & Haro, Citation2015; Sonnenberg et al., Citation2013).
The association between depression and anxiety, social relationships, and cognitive health is complex, as although good social relationships may enhance wellbeing, individuals who have greater wellbeing may experience better social relationships (Diener & Seligman, Citation2004). In addition, depression and anxiety are associated with poor cognitive function (Aggarwal, Kunik, & Asghar-Ali, Citation2017; Pietrzak et al., Citation2012; Potvin, Forget, Grenier, Préville, & Hudon, Citation2011; Yochim, Mueller, & Segal, Citation2013) and an increased risk of dementia (Burton, Campbell, Jordan, Strauss, & Mallen, Citation2012; Diniz, Butters, Albert, Dew, & Reynolds, Citation2013; Gulpers et al., Citation2016). However, findings are inconsistent and some studies report that although depression or anxiety may accompany poor cognitive function, symptoms of depression and anxiety do not necessarily precede poor cognitive function (Andreescu et al., Citation2014; Okereke & Grodstein, Citation2013; Potvin et al., Citation2013; Richard et al., Citation2013).
The evidence reviewed suggests that older people with depression or anxiety may have more negative experiences of social relationships than those without depression or anxiety, which may intensify feelings of loneliness and isolation. In addition, previous studies report that people with mood disorders may have poorer cognitive function. Previous work with older people without depression or anxiety suggests that good social relationships may be associated with better cognitive function and higher levels of cognitive reserve. It is possible that the negative experiences of social relationships in those with depression or anxiety may influence how social relationships contribute to cognitive reserve, and hence cognition. Therefore, the present study aimed to investigate the following:
Do older people with depression or anxiety experience greater feelings of loneliness, more social isolation, and less social contact than those without depression or anxiety?
Do people with depression or anxiety have poorer cognitive function and lower cognitive reserve than those without depression or anxiety?
Is social isolation associated with poor cognitive function in older people with depression or anxiety?
Does cognitive reserve moderate the association between social isolation and cognition in older people with depression or anxiety?
Method
Design
Data from the Cognitive Function and Ageing Study–Wales (CFAS-Wales), a longitudinal population-based study, were used to address the study aims. The North Wales - West NHS research ethics committee granted ethical approval for data collection (Ref No: 10/WNo01/37; IRAS Project No: 40092).
Study population
People ≥65 years were randomly selected from general practice databases across two study sites (Gwynedd/Ynys Môn and Neath Port Talbot). To ensure that participants were age representative of the general population, participants were stratified into two age groups (65–74 years and ≥75 years). People who agreed to participate completed an extensive questionnaire administered by a trained research assistant at the participant’s home. Baseline data were collected between 2011 and 2013 and follow-up interviews were conducted two-years later, between 2013 and 2015.
Baseline data was collected for 3,593 participants and follow-up data was collected for 2,236 participants. To reduce the risk of reverse causation we excluded participants with an Automated Geriatric Examination Assisted Taxonomy (AGECAT) classification of dementia (N = 185) or cognitive impairment (Mini-Mental State Examination score ≤25; N = 908) at baseline. The AGECAT is a diagnostic algorithm embedded in the CFAS-Wales interview that assesses symptoms to determine whether a person has a diagnosis of dementia, depression, anxiety, or no diagnosis (Copeland et al., Citation1986). We excluded participants living in an institution at baseline (N = 95) as social relationships are experienced differently in institutional care. Participants with missing data at baseline (N = 796) and follow-up (N = 686) were also excluded. This gave a final baseline sample of 2,135, of which 154 (7%) participants had an AGECAT classification of depression, 32 (1.5%) participants had an AGECAT classification of anxiety, and 1,949 (91%) participants had no AGECAT diagnosis. The final sample size at two-year follow-up was 1,449 participants, of whom 101 (7%) participants had a classification of depression, 22 (2%) had a classification of anxiety, and 1,326 (92%) had no AGECAT diagnosis. A comparison of the participants included in baseline analyses but excluded from longitudinal analyses because of missing data is presented in . Those excluded at follow-up had poorer cognitive scores, but there were no differences in any other baseline variables.
Table 1. Comparison of included and excluded participants at two-year follow-up
Measures
Depression and anxiety
Depression and anxiety were assessed at baseline using the AGECAT (Copeland et al., Citation1986), a semi-structured interview designed to assess whether older people have symptoms of depression, anxiety, dementia, or no illness. An algorithm is used to assign scores ranging from 0–5 and diagnoses are given based on the severity of symptoms, reflecting either no or few symptoms (0–1), some symptoms and a probable sub-threshold illness (2), or clinically relevant symptoms and a probable clinically significant case (3–5). The algorithm uses a hierarchical system to determine one main diagnosis for which the individual exhibits the most symptoms. Given that symptoms of depression and anxiety are assessed in the same cluster and may overlap, this algorithm may give precedence to a classification of depression rather than anxiety. The AGECAT has high concordance with diagnoses given by trained psychiatrists (Cohen’s k = 0.84: Copeland et al., Citation1986; Copeland et al., Citation2002).
Cognitive function
Cognitive function was assessed at baseline and follow-up using the Cambridge Cognitive Assessment (CAMCOG: Roth et al., Citation1986). The CAMCOG assesses cognition under the following dimensions: orientation, language (comprehension and expression), memory (remote, recent, and learning), praxis, attention, abstract thinking, perception, and calculation. Scores range from 0–107 and a lower score indicates poorer cognitive function. The CAMCOG has good inter-rater reliability (r = 0.97) and high sensitivity (92%) and specificity (96%) in detecting cognitive impairment (Roth et al., Citation1986).
Cognitive reserve
A composite measure of cognitive reserve was calculated using three proxy measures: education, occupational complexity, and cognitive activity, based on previous cognitive reserve scores created in MRC-CFAS (Valenzuela, Brayne, Sachdev, Wilcock, & Matthews, Citation2011) and CFAS-II (Opdebeeck et al., Citation2018). Education was measured as the number of years in full time education. Occupational complexity was determined by the complexity and socioeconomic group of the participant’s main occupation and social class (Valenzuela et al., Citation2011). Complexity scores ranged from 1 (low occupational complexity, e.g. unskilled and low socioeconomic group, such as a cleaner) to 14 (high occupational complexity, e.g. skilled and high socioeconomic group, such as a lawyer or doctor). Cognitive activity was assessed by seven questions asking about engagement in cognitive activities (e.g. reading, playing games). Responses were recorded on a five-point scale ranging from once a year or less to everyday and higher scores indicated greater cognitive activity.
Scores for each of the proxy measures were weighted based on the interquartile range to ensure that each item contributed equally to determine the cognitive reserve score. This gave the following formula:
Higher scores indicate higher levels of reserve.
Social isolation
Social isolation was assessed at baseline using the Lubben Social Network Scale–6 (LSNS-6: Lubben et al., Citation2006), a standardised measure consisting of three questions assessing isolation from family and three comparable questions assessing isolation from friends. The questions ask participants to report the number of relatives/friends they have seen or heard from at least once in the past month, can call on for help, and can speak with about private matters. Participants respond on a six-item category response scale ranging from 0 (no relatives/friends) to 5 (nine or more relatives/friends). Total scores range from 0 to 30 and lower scores indicate more social isolation. The LSNS-6 can be scored for family and friends separately to indicate level of isolation from kin and non-kinship relationships. Questions specific to family and friends are summed separately, scores for each sub-scale range from 0-15, and social isolation is indicated by a lower score.
Social contact
Social contact was assessed at baseline based on the frequency of contact with friends and family. For each question, participants could respond as daily (5), 2–3 times a week (4), at least weekly (3), at least monthly (2), less often (1), or never/no relatives (0). Scores for each question were combined, giving a possible range from 0-10, with zero indicating less social contact and ten indicating greater social contact.
Loneliness
Loneliness was assessed at baseline using the six-item De Jong Gierveld scale (De Jong Gierveld & Van Tilburg, Citation2006). This scale assesses social and emotional loneliness using three questions for each type of loneliness. Participants respond either yes, more or less, or no. Total scores range from 0 to 6 and higher scores indicate greater feelings of loneliness. For the social and emotional loneliness subscales, scores range from 0 to 3 and higher scores indicate greater feelings of social or emotional loneliness.
Covariates
Covariates included were age (years), gender, and education (years) at baseline as these are established covariates of late-life cognition (Barnes et al., Citation2003; Tervo et al., Citation2004; Tilvis et al., Citation2004) and cardiovascular risk factors including stroke, heart attack, and hypertension (Grodstein, Citation2007).
Statistical analysis
All analyses were conducted in Stata version 15.0. T-tests were conducted to examine the differences in social isolation, social contact, loneliness, cognitive function, and cognitive reserve across older people with and without depression or anxiety. Means and standard deviations of scores were reported, along with the t value, degrees of freedom, and the p value.
The relationship between social isolation and cognitive reserve was examined in a subgroup of people with depression and anxiety (N = 186 for baseline analyses, N = 123 for longitudinal analyses). A linear regression was conducted to determine whether there was a relationship between social isolation and cognitive function at baseline. To assess this relationship using longitudinal data, a cognitive change score was calculated by subtracting the CAMCOG score at baseline from the CAMCOG score at follow-up. Each participant’s cognitive change score was then standardised by the standard deviation value of the baseline CAMCOG score. A linear regression was conducted to determine whether social isolation at baseline was associated with cognitive change over two-year follow-up. These analyses were adjusted for all covariates. Adjusted R2 values were reported for regression models to indicate the proportion of variance explained by variables in the model. Regression coefficients were also reported, along with 95% confidence intervals. All measures were standardised to provide comparable coefficients.
Moderation analyses were conducted to determine whether cognitive reserve moderates the association between social isolation and cognition or cognitive change in the subgroup of people with depression and anxiety. These analyses tested for an interaction between social isolation and the cognitive reserve score and were adjusted for all covariates except for education as this was a component of the cognitive reserve score.
Results
At baseline, the mean age of participants was 73.26 years and 51% were women. The mean cognitive reserve score was 60.76 and ranged from 33.53–109.30. Scores on the CAMCOG ranged from 63–105 with a mean of 93.49 at baseline and 76–103 with a mean of 92.99 at follow-up. People with depression or anxiety were significantly more likely to be women, and to have poorer cognitive scores and lower cognitive reserve scores than people without depression or anxiety, but there were no differences in age, education, cognitive activity, or occupational complexity ().
Table 2. Summary of baseline characteristics of participants in CFAS-Wales
Social relationships in older people with and without depression or anxiety
Older people with depression or anxiety were classified as significantly more isolated on the total LSNS-6 scale, and on the family and friends subscales, than people without depression or anxiety. Older people with depression or anxiety indicated significantly higher feelings of overall loneliness, social, and emotional loneliness than people without depression or anxiety. There was no significant difference in social contact scores ().
Cognitive function and cognitive reserve in older people with and without depression or anxiety
Older people without depression or anxiety scored significantly higher on the CAMCOG than people with depression or anxiety. Likewise, people without depression or anxiety scored significantly higher on the cognitive reserve measure than people with anxiety or depression. There was no significant difference in the scores for each individual component of the cognitive reserve score (education, occupational complexity, and cognitive activity: ).
Social isolation and cognitive function in older people with depression or anxiety
Baseline
The association between social isolation and cognitive function at baseline was assessed in the subgroup of people with depression or anxiety. Social isolation was significantly associated with CAMCOG scores, adjusted R2 = 0.17, F(7, 178) = 6.41, p < 0.001. The model suggested that people with depression or anxiety who were less socially isolated had better CAMCOG scores (0.11; 95% CI: 0.03, 0.19) and the model explained 17% of the variance in CAMCOG scores (, ).
Figure 1. (a) The significant association between social isolation and cognitive function at baseline in people with depression or anxiety (N = 186), adjusted for all covariates. (b) The non-significant association between social isolation and cognitive function at two-year follow-up in people with depression or anxiety (N = 123), adjusted for all covariates.

Table 3. Cross-sectional association between social isolation and cognition in older people with depression or anxiety (N = 186).
Longitudinal
The association between social isolation and cognitive change over two-year follow-up was assessed in the subgroup of people with depression or anxiety at baseline. Social isolation was not significantly associated with cognitive change, adjusted R2 = 0, F(7, 115) = 1.06, p = 0.396 (, ).
Table 4. Longitudinal association between social isolation and cognitive change score over two-years in older people with depression or anxiety (N= 186).
Of the 123 participants with an AGECAT classification of either depression or anxiety at baseline, 53 (43%) of these participants had a healthy AGECAT classification at follow-up.
Social isolation, cognitive reserve, and cognitive function in older people with depression or anxiety
Baseline
The moderating effect of cognitive reserve on the association between social isolation and cognitive function was assessed in the subgroup of people with depression or anxiety. The interaction term between social isolation and the cognitive reserve score did not explain a significant increase in cognitive function (−0.01; 95% CI: −0.10, 0.07; , ).
Figure 2. (a) The non-significant moderating effect of cognitive reserve on the association between social isolation and cognitive function at baseline in people with depression or anxiety (N = 186), adjusted for covariates. (b) The non-significant moderating effect of cognitive reserve on the association between social isolation and cognitive function at two-year follow-up in people with depression or anxiety (N = 123), adjusted for covariates.
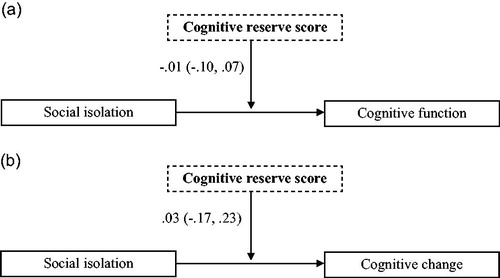
Table 5. Cross-sectional association between social isolation, cognitive reserve, and cognition in older people with depression or anxiety (N = 123).
Longitudinal
The moderating effect of cognitive reserve on the association between social isolation and cognitive change was assessed in the subgroup of people with depression or anxiety at baseline. The interaction term between social isolation and cognitive reserve score did not significantly moderate the cognitive change score (0.02; 95% CI: −0.18, .23; , ).
Table 6. Longitudinal association between social isolation, cognitive reserve, and cognitive change score over two-years in older people with depression or anxiety (N = 123).
Discussion
This study aimed to assess differences in experiences of social relationships across those with and without symptoms of depression or anxiety, as determined by the AGECAT classification. We also aimed to examine differences in cognitive function and cognitive reserve. Finally, in a subgroup of people with symptoms of depression or anxiety at baseline, we aimed to assess the relationship between social isolation and cognition, and to consider the moderating role of cognitive reserve in this relationship. Findings suggest that people with depression or anxiety experience poorer social relationships and poorer cognitive function, and have lower levels of cognitive reserve. Social isolation was associated with poor cognitive function in older people with depression or anxiety at baseline, but not with cognitive change at two-year follow-up. Cognitive reserve did not moderate this association.
Our results suggest that people with depression or anxiety experience higher levels of social isolation and loneliness compared to those without depression or anxiety. This is in line with previous research that suggests loneliness and social isolation often co-occur with depression (Cacioppo & Hawkley, Citation2009; Luanaigh & Lawlor, Citation2008). Interestingly, however, there was no significant difference in the frequency of social contact with friends and family across participants. This suggests that although people with and without depression or anxiety have the same frequency of contact, people with depression or anxiety report significantly higher feelings of loneliness and are more isolated. This may be explained by the tendency for people with depression to recall more negative than positive information about social relationships and interactions than people without depression (Beck, Citation2008; Lewis et al., Citation2017). People with depression may hold more negative social expectations, which may increase feelings of loneliness and isolation, despite having opportunities to engage in social contact (Cacioppo & Hawkley, Citation2009; Granerud & Severinsson, Citation2006).
We also found that people with depression or anxiety scored lower on a test of cognitive function than people without depression or anxiety. This finding is in line with previous research that suggests depression and anxiety are associated with poor cognitive function (Aggarwal et al. Citation2017; Pietrzak et al., Citation2012; Yates, Clare, & Woods, Citation2017; Yochim et al., Citation2013) and a greater risk of Alzheimer’s disease (Diniz et al., Citation2013; Gulpers et al., Citation2016). We also found that scores for cognitive reserve were lower for people with depression or anxiety.
We found that social isolation was associated with poor cognitive function in people with depression or anxiety at baseline, but not with cognitive change at two-year follow-up. One explanation for the difference in finding at follow-up may be the variance in mood-related symptoms experienced by participants. 57% of people who were experiencing clinically relevant symptoms of depression or anxiety at baseline were experiencing no, or few, symptoms two-years later. Previous work suggests that people with depression may experience significant cognitive impairment during episodes of illness, which may persist after symptom reduction (Airaksinen, Wahlin, Larsson, & Forsell, Citation2006) or remission (Gruber, Rathgeber, Bräunig, & Gauggel, Citation2007; Nakano et al., Citation2008). Further work suggests that, over time, cognitive ability may improve following the reduction of mood related symptoms (Biringer et al., Citation2005; Rock, Roiser, Riedel, & Blackwell, Citation2014). The reduction of such symptoms at follow-up in the present sample may therefore account for the non-significant relationship between social isolation and cognitive change at follow-up. In addition, there was very little cognitive change observed across the sample as a whole between baseline and two-year follow-up. This may also account for why there was an association at baseline but not with cognitive change at follow-up. Alternatively, as the number of people with depression or anxiety at follow-up was small, this may have limited the power to detect interaction effects in the longitudinal analysis.
We found that cognitive reserve does not moderate the association between social isolation and cognitive function or cognitive change at two-year follow-up. It may be that there are other mechanisms that are more important in explaining the relationship between social isolation and cognition. For example, it has been proposed that social networks may be used in the transmission of health information (Berkman & Glass, Citation2000; Kim, Kreps, & Shin, Citation2015; Masic, Sivic, Toromanovic, Borojevic, & Pandza, Citation2012). It is also suggested that good social relationships may directly influence positive psychological states, such as a sense of belonging, purpose, or security, and acknowledgement of self-worth (Cohen, Underwood, & Gottlieb, Citation2000; Thoits, Citation2011). In turn, these positive psychological states may benefit mental health, due to an increased motivation for self-care (e.g. regular exercise, not smoking, moderate alcohol consumption) which may indirectly benefit cognition (Thoits, Citation2011).
These findings have several implications. First, people within the social networks of those with depression or anxiety may be encouraged to engage more actively with such individuals in attempt to alter their perceptions of isolation and loneliness. It is likely that people within the networks of older people are also aged and may face several barriers to engaging in social contact themselves, such as limited mobility and transport, poor health, geographical distance, living in rural areas, poverty, and limited opportunities within the community to engage (Bowling & Stafford, Citation2007; Rosso et al., Citation2013; Wen et al., Citation2006). Therefore, this approach may be too simplistic; instead, it may be necessary to engage the wider community and address the physical and psychosocial barriers (Weden, Carpiano, & Robert, Citation2008).
Second, the study confirms that people with depression or anxiety may have poorer cognitive function compared to healthy older people. In addition, the observation that over half of the individuals with clinically relevant depression or anxiety at baseline were experiencing no mood-related symptoms two-years later is positive. The small number of participants with such problems in the present sample at baseline suggests that mood problems are relatively uncommon and the reduction in symptoms at follow-up indicates that mood problems are not necessarily stable over time. This is consistent with prevalence rates in similar study samples, which have reported prevalence rates of 8.7% for depression (McDougall et al., Citation2007). However, the prevalence of anxiety was particularly low in the present sample and previous studies of similar cohorts usually find prevalence rates of 3.1% (Kvaal, McDougall, Brayne, Matthews, & Dewey, Citation2008). This may be accounted for by the use of the AGECAT for diagnoses in the present study. The AGECAT uses a hierarchical system to determine a main diagnosis of either depression, anxiety, dementia, or no diagnosis, and may give precedence to a diagnosis of depression over anxiety when overlapping symptoms are reported (Opdebeeck et al., Citation2018). The observed change in symptoms can account for the finding that social isolation was associated with poor cognitive function at baseline, but not with change over time. It suggests that during episodes of depression or anxiety, being isolated may be detrimental to cognitive function. It may be that isolated individuals who also have depression or anxiety do not receive the benefits of cognitive stimulation through frequent and complex social contact with others (Bennett et al., Citation2006; Fratiglioni et al., Citation2000). However, when individuals are not experiencing symptoms of depression or anxiety, social isolation does not play such an important role.
This study has many strengths. First, CFAS-Wales is a large scale, representative, population-based cohort of older people. Secondly, participants were recruited through general practice registers. This ensures that individuals who are extremely isolated and who have depression or anxiety are more adequately represented than is the case with studies that recruit participants on a voluntary basis. This study also has limitations. We used the AGECAT algorithm to provide a diagnosis of depression or anxiety. This algorithm provides a diagnosis of either dementia, anxiety, depression, or no illness based on the presence of symptoms associated with these illnesses. Only one diagnosis is made, which is problematic as participants may have symptoms of more than one illness, but the algorithm can only identify the illness for which the participant presents the most symptoms. Given this, many diagnoses may have been missed. This is particularly problematic as many symptoms of depression and anxiety overlap and the disorders are often co-morbid (Beaudreau & O’hara, Citation2008). An additional limitation is the small number of people with anxiety compared those with depression, and the significantly larger group of people without depression or anxiety in CFAS-Wales. This may limit the power of the study and reduce comparability across the three groups. Future work may consider more comprehensive assessments of depression or anxiety that may capture co-morbidity of mood related symptoms and ensure that each group of participants is adequately represented. Finally, it was not possible to determine the quality of social relationships or whether social interactions were negative in CFAS-Wales. Understanding this component of social relationships and how this relates to the quality of social interactions may provide a more comprehensive understanding of how social relationships may contribute to health outcomes, such as cognitive function and mood disorders. This is a relatively under-researched area and would serve well for further exploration (Santini et al., Citation2015).
We have demonstrated that older people with depression or anxiety are more isolated and feel lonelier than people without depression or anxiety, despite reporting a comparable level of social contact. In addition, we have reported that social isolation is associated with poor cognitive function in people with depression or anxiety at baseline. This relationship may not be observed longitudinally due to the transient nature of mood disorders and a reduction in related symptoms, leading to improvements in cognitive function. Cognitive reserve does not seem to play a role in the association between isolation and cognitive function in people experiencing depression or anxiety. It may be that symptoms of depression and anxiety are more associated with cognitive outcomes than social relationships.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Aggarwal, R., Kunik, M., & Asghar-Ali, A. (2017). Anxiety in later life. Focus, 15, 157–161.
- Airaksinen, E., Wahlin, Å., Larsson, M., & Forsell, Y. (2006). Cognitive and social functioning in recovery from depression: results from a population-based three-year follow-up. Journal of Affective Disorders, 96, 107–110.
- Andreescu, C., Teverovsky, E., Fu, B., Hughes, T. F., Chang, C. C. H., & Ganguli, M. (2014). Old worries and new anxieties: Behavioral symptoms and mild cognitive impairment in a population study. The American Journal of Geriatric Psychiatry, 22, 274–284.
- Barnes, L. L., Wilson, R. S., Schneider, J. A., Bienias, J. L., Evans, D. A., & Bennett, D. A. (2003). Gender, cognitive decline, and risk of AD in older persons. Neurology, 60, 1777–1781.
- Beaudreau, S. A., & O’hara, R. (2008). Late-life anxiety and cognitive impairment: A review. The American Journal of Geriatric Psychiatry, 16, 790–803.
- Beck, A. T. (2008). The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry, 165, 969–977.
- Bennett, D. A., Schneider, J. A., Tang, Y., Arnold, S. E., & Wilson, R. S. (2006). The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: A longitudinal cohort study. The Lancet Neurology, 5, 406–412.
- Berkman, L. F., & Glass, T. (2000). Social integration, social networks, social support, and health. Social Epidemiology, 1, 137–173.
- Biringer, E., Lundervold, A., Stordal, K., Mykletun, A., Egeland, J., Bottlender, R., & Lund, A. (2005). Executive function improvement upon remission of recurrent unipolar depression. European Archives of Psychiatry and Clinical Neuroscience, 255, 373–380.
- Bowling, A., & Stafford, M. (2007). How do objective and subjective assessments of neighbourhood influence social and physical functioning in older age? Findings from a British survey of ageing. Social Science & Medicine, 64, 2533–2549.
- Burton, C., Campbell, P., Jordan, K., Strauss, V., & Mallen, C. (2012). The association of anxiety and depression with future dementia diagnosis: a case-control study in primary care. Family Practice, 30, 25–30.
- Cacioppo, J. T., & Hawkley, L. C. (2009). Perceived social isolation and cognition. Trends in Cognitive Sciences, 13, 447–454.
- Cohen, S., Underwood, L. G., & Gottlieb, B. H. (Eds.). (2000). Social support measurement and intervention: A guide for health and social scientists. Oxford: Oxford University Press.
- Copeland, J. R. M., Dewey, M. E., & Griffiths-Jones, H. M. (1986). A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychological Medicine, 16, 89–99.
- Copeland, J. R. M., Prince, M., Wilson, K. C. M., Dewey, M. E., Payne, J., & Gurland, B. (2002). The geriatric mental state examination in the 21st century. International Journal of Geriatric Psychiatry, 17, 729–732.
- Corrigan, P. W., & Rao, D. (2012). On the self-stigma of mental illness: Stages, disclosure, and strategies for change. The Canadian Journal of Psychiatry, 57, 464–469.
- De Jong Gierveld, J., & Tilburg, T. V. (2006). A 6-item scale for overall, emotional, and social loneliness: Confirmatory tests on survey data. Research on Aging, 28, 582–598.
- Diener, E., & Seligman, M. E. (2004). Beyond money: Toward an economy of well-being. Psychological Science in the Public Interest, 5, 1–31.
- DiNapoli, E. A., Wu, B., & Scogin, F. (2014). Social isolation and cognitive function in Appalachian older adults. Research on Aging, 36, 161–179.
- Diniz, B. S., Butters, M. A., Albert, S. M., Dew, M. A., & Reynolds, C. F. (2013). Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. The British Journal of Psychiatry, 202, 329–335.
- Domènech-Abella, J., Lara, E., Rubio-Valera, M., Olaya, B., Moneta, M. V., Rico-Uribe, L. A., Ayuso-Mateos, J. L., Mundo, J., & Haro, J. M. (2017). Loneliness and depression in the elderly: the role of social network. Social Psychiatry and Psychiatric Epidemiology, 52, 381–390.
- Fratiglioni, L., Wang, H. X., Ericsson, K., Maytan, M., & Winblad, B. (2000). Influence of social network on occurrence of dementia: a community-based longitudinal study. The Lancet, 355, 1315–1319.
- García-Peña, C., Wagner, F. A., Sánchez-García, S., Espinel-Bermúdez, C., Juárez-Cedillo, T., Pérez-Zepeda, M., Arango-Lopera, V., Franco-Marina, F., Ramirez-Aldana, R., & Gallo, J. J. (2013). Late-life depressive symptoms: Prediction models of change. Journal of Affective Disorders, 150, 886–894.
- Gow, A. J., Pattie, A., Whiteman, M. C., Whalley, L. J., & Deary, I. J. (2007). Social support and successful aging: Investigating the relationships between lifetime cognitive change and life satisfaction. Journal of Individual Differences, 28, 103–115.
- Granerud, A., & Severinsson, E. (2006). The struggle for social integration in the community–the experiences of people with mental health problems. Journal of Psychiatric and Mental Health Nursing, 13, 288–293.
- Grodstein, F. (2007). Cardiovascular risk factors and cognitive function. Alzheimer's & dementia: the Journal of the Alzheimer's Association, 3, S16–S22.
- Gruber, S., Rathgeber, K., Bräunig, P., & Gauggel, S. (2007). Stability and course of neuropsychological deficits in manic and depressed bipolar patients compared to patients with major depression. Journal of Affective Disorders, 104, 61–71.
- Gulpers, B., Ramakers, I., Hamel, R., Köhler, S., Voshaar, R. O., & Verhey, F. (2016). Anxiety as a predictor for cognitive decline and dementia: A systematic review and meta-analysis. The American Journal of Geriatric Psychiatry, 24, 823–842.
- Hendrie, H. C., Albert, M. S., Butters, M. A., Gao, S., Knopman, D. S., Launer, L. J., Yaffe, K., Cuthbert, B. N., Edwards, E., & Wagster, M. V. (2006). The NIH cognitive and emotional health project: Report of the critical evaluation study committee. Alzheimer's & Dementia, 2, 12–32.
- Holwerda, T. J., Deeg, D. J., Beekman, A. T., van Tilburg, T. G., Stek, M. L., Jonker, C., & Schoevers, R. A. (2012). Feelings of loneliness, but not social isolation, predict dementia onset: Results from the Amsterdam Study of the Elderly (AMSTEL). Journal of Neurology, Neurosurgery & Psychiatry, 85, 135–142.
- Kawachi, I., & Berkman, L. F. (2001). Social ties and mental health. Journal of Urban health, 78, 458–467.
- Kim, W., Kreps, G. L., & Shin, C. N. (2015). The role of social support and social networks in health information–seeking behavior among Korean Americans: A qualitative study. International Journal for Equity in Health, 14, 40.
- Kuchibhatla, M. N., Fillenbaum, G. G., Hybels, C. F., & Blazer, D. G. (2012). Trajectory classes of depressive symptoms in a community sample of older adults. Acta Psychiatrica Scandinavica, 125, 492–501.
- Kvaal, K., McDougall, F. A., Brayne, C., Matthews, F. E., & Dewey, M. E. (2008). Co‐occurrence of anxiety and depressive disorders in a community sample of older people: Results from the MRC CFAS (Medical Research Council Cognitive Function and Ageing Study). International Journal of Geriatric Psychiatry, 23, 229–237.
- Lewis, G., Kounali, D. Z., Button, K. S., Duffy, L., Wiles, N. J., Munafo, M. R., Harmer, C. J., & Lewis, G. (2017). Variation in the recall of socially rewarding information and depressive symptom severity: A prospective cohort study. Acta Psychiatrica Scandinavica, 135, 489–498.
- Litwin, H. (2012). Physical activity, social network type, and depressive symptoms in late life: An analysis of data from the National Social Life, Health and Aging Project. Aging & Mental Health, 16, 608–616.
- Liverman, C. T., Yaffe, K., & Blazer, D. G. (Eds.). (2015). Cognitive aging: Progress in understanding and opportunities for action. National Academies Press.
- Lubben, J., Blozik, E., Gillmann, G., Iliffe, S., von Renteln Kruse, W., Beck, J. C., & Stuck, A. E. (2006). Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. The Gerontologist, 46, 503–513.
- Luanaigh, C. Ó., & Lawlor, B. A. (2008). Loneliness and the health of older people. International Journal of Geriatric Psychiatry, 23, 1213–1221.
- Masic, I., Sivic, S., Toromanovic, S., Borojevic, T., & Pandza, H. (2012). Social networks in improvement of health care. Materia Socio-Medica, 24, 48–53.
- McDougall, F. A., Kvaal, K., Matthews, F. E., Paykel, E., Jones, P. B., Dewey, M. E., & Brayne, C. (2007). Prevalence of depression in older people in England and Wales: the MRC CFAS Study. Psychological Medicine, 37, 1787–1795.
- Nakano, Y., Baba, H., Maeshima, H., Kitajima, A., Sakai, Y., Baba, K., Suzuki, T., & Arai, H. (2008). Executive dysfunction in medicated, remitted state of major depression. Journal of Affective Disorders, 111, 46–51.
- Nicholson Jr, N. R. (2009). Social isolation in older adults: An evolutionary concept analysis. Journal of Advanced Nursing, 65, 1342–1352.
- Nishiguchi, S., Yamada, M., Sonoda, T., Kayama, H., Tanigawa, T., Yukutake, T., & Aoyama, T. (2013). Cognitive decline predicts long-term care insurance requirement certification in community-dwelling older Japanese adults: A prospective cohort study. Dementia and Geriatric Cognitive Disorders Extra, 3, 312–319.
- Okereke, O. I., & Grodstein, F. (2013). Phobic anxiety and cognitive performance over 4 years among community-dwelling older women in the Nurses' Health Study. The American Journal of Geriatric Psychiatry, 21, 1125–1134.
- Opdebeeck, C., Matthews, F. E., Wu, Y. T., Woods, R. T., Brayne, C., & Clare, L. (2018). Cognitive reserve as a moderator of the negative association between mood and cognition: Evidence from a population-representative cohort. Psychological Medicine, 48, 61–71.
- Pietrzak, R. H., Maruff, P., Woodward, M., Fredrickson, J., Fredrickson, A., Krystal, J. H., Southwick, S. M., & Darby, D. (2012). Mild worry symptoms predict decline in learning and memory in healthy older adults: A 2-year prospective cohort study. The American Journal of Geriatric Psychiatry, 20, 266–275.
- Potvin, O., Bergua, V., Meillon, C., Le Goff, M., Bouisson, J., Dartigues, J. F., & Amieva, H. (2013). State anxiety and cognitive functioning in older adults. The American Journal of Geriatric Psychiatry, 21, 915–924.
- Potvin, O., Forget, H., Grenier, S., Préville, M., & Hudon, C. (2011). Anxiety, depression, and 1‐year incident cognitive impairment in community‐dwelling older adults. Journal of the American Geriatrics Society, 59, 1421–1428.
- Richard, E., Reitz, C., Honig, L. H., Schupf, N., Tang, M. X., Manly, J. J., Mayeux, R., Devanand, D., & Luchsinger, J. A. (2013). Late-life depression, mild cognitive impairment, and dementia. JAMA Neurology, 70, 383–389.
- Rock, P. L., Roiser, J. P., Riedel, W. J., & Blackwell, A. D. (2014). Cognitive impairment in depression: a systematic review and meta-analysis. Psychological Medicine, 44, 2029–2040.
- Rosso, A. L., Taylor, J. A., Tabb, L. P., & Michael, Y. L. (2013). Mobility, disability, and social engagement in older adults. Journal of Aging and Health, 25, 617–637.
- Roth, M., Tym, E., Mountjoy, C. Q., Huppert, F. A., Hendrie, H., Verma, S., & Goddard, R. (1986). CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. The British Journal of Psychiatry, 149, 698–709.
- Santini, Z. I., Koyanagi, A., Tyrovolas, S., Mason, C., & Haro, J. M. (2015). The association between social relationships and depression: a systematic review. Journal of Affective Disorders, 175, 53–65.
- Segrin, C. (2000). Social skills deficits associated with depression. Clinical Psychology Review, 20, 379–403.
- Shankar, A., Hamer, M., McMunn, A., & Steptoe, A. (2013). Social isolation and loneliness: relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosomatic Medicine, 75, 161–170.
- Siedlecki, K. L., Stern, Y., Reuben, A., Sacco, R. L., Elkind, M. S., & Wright, C. B. (2009). Construct validity of cognitive reserve in a multiethnic cohort: The Northern Manhattan Study. Journal of the International Neuropsychological Society, 15, 558–569.
- Simning, A., Conwell, Y., & van Wijngaarden, E. (2014). Cognitive impairment in public housing residents living in Western New York. Social Psychiatry and Psychiatric Epidemiology, 49, 477–485.
- Singh‐Manoux, A., Marmot, M. G., Glymour, M., Sabia, S., Kivimäki, M., & Dugravot, A. (2011). Does cognitive reserve shape cognitive decline? Annals of Neurology, 70, 296–304.
- Sonnenberg, C. M., Deeg, D. J., van Tilburg, T. G., Vink, D., Stek, M. L., & Beekman, A. T. (2013). Gender differences in the relation between depression and social support in later life. International Psychogeriatrics, 25, 61–70.
- St John, P. D., Tyas, S. L., & Montgomery, P. R. (2015). Cognition, even in the normal range, predicts disability: cross‐sectional and prospective analyses of a population‐based sample. International Journal of Geriatric Psychiatry, 30, 1008–1016.
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8, 448–460.
- Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47, 2015–2028.
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. The Lancet Neurology, 11, 1006–1012.
- Tervo, S., Kivipelto, M., Hänninen, T., Vanhanen, M., Hallikainen, M., Mannermaa, A., & Soininen, H. (2004). Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dementia and Geriatric Cognitive Disorders, 17, 196–203.
- Thoits, P. A. (2011). Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior, 52, 145–161.
- Tilvis, R. S., Kähönen-Väre, M. H., Jolkkonen, J., Valvanne, J., Pitkala, K. H., & Strandberg, T. E. (2004). Predictors of cognitive decline and mortality of aged people over a 10-year period. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 59, M268–M274.
- Tinetti, M. E., McAvay, G. J., Chang, S. S., Newman, A. B., Fitzpatrick, A. L., Fried, T. R., & Peduzzi, P. N. (2011). Contribution of multiple chronic conditions to universal health outcomes. Journal of the American Geriatrics Society, 59, 1686–1691.
- Valenzuela, M., Brayne, C., Sachdev, P., Wilcock, G., & Matthews. F. (2011). Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. American Journal of Epidemiology, 173, 1004–1012.
- Weden, M. M., Carpiano, R. M., & Robert, S. A. (2008). Subjective and objective neighborhood characteristics and adult health. Social Science & Medicine, 66, 1256–1270.
- Wen, M., Hawkley, L. C., & Cacioppo, J. T. (2006). Objective and perceived neighborhood environment, individual SES and psychosocial factors, and self-rated health: An analysis of older adults in Cook County, Illinois. Social Science & Medicine, 63, 2575–2590.
- Wilson, R. S., De Leon, C. F. M., Barnes, L. L., Schneider, J. A., Bienias, J. L., Evans, D. A., & Bennett, D. A. (2002). Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA, 287, 742–748.
- Wilson, R. S., Krueger, K. R., Arnold, S. E., Schneider, J. A., Kelly, J. F., Barnes, L. L., Tang, Y., & Bennett, D. A. (2007). Loneliness and risk of Alzheimer disease. Archives of General Psychiatry, 64, 234–240.
- Yaacob, S. N., Juhari, R., Talib, M. A., & Uba, I. (2017). Loneliness, stress, self-esteem and depression among Malaysian adolescents. Jurnal Kemanusiaan, 7, 85–95.
- Yates, J. A., Clare, L., & Woods, R. T. (2017). “You’ve got a friend in me”: can social networks mediate the relationship between mood and MCI? BMC Geriatrics, 17, 144.
- Yochim, B. P., Mueller, A. E., & Segal, D. L. (2013). Late life anxiety is associated with decreased memory and executive functioning in community dwelling older adults. Journal of Anxiety Disorders, 27, 567–575.
