Abstract
Objective: This study aimed to estimate the association between tobacco smoking and risk for dementia in seven low- and middle-income countries.
Methods: Secondary analysis of the 10/66 population-based cohort study was conducted with 11,143 dementia-free individuals aged 65 years and older who were followed-up for an average of 3.8 years totalling 42,715 person-years. Cox regression with competing-risk analyses was used, controlling for age, gender, number of assets, past hazardous drinking, exercise and self-report of heart disease. Exposure was measured in packyears and smoking status. The number of packyears was calculated by multiplying the average number of packs per day by years of consumption up to 50 years old and up to age at baseline.
Results: Meta-analysis of the results from each country yielded non-significant pooled relative risk ratios for all comparisons. There was no difference in risk for any dementia between ‘ever smokers’ compared to ‘never smokers’ (HR 0.96; 95% CI 0.82–1.13); ‘current smokers’ compared to ‘never smokers’ (HR 0.83; 95% CI 0.66–1.06); ‘former smokers’ compared to ‘never smokers’ (HR 1.06; 95% CI 0.88–1.27); ‘current smokers’ compared to ‘former smokers’ (HR 0.86; 95% CI 0.66–1.13). Results were similar for Alzheimer’s disease (AD) and Vascular Dementia (VaD) as outcomes. Lifetime tobacco consumption (packyears) was not associated with any dementia (HR 1.00; 95% CI 0.99–1.00), nor with AD or VaD.
Conclusion: Pooled results from all the countries showed no significant association between smoking and the onset of any dementia. Selective quitting in later-life might have biased the results towards no effect.
Introduction
The world population is aging rapidly, particularly in low- and middle-income countries (LMIC). With increased longevity, there is an increase in the prevalence of non-communicable chronic diseases, such as dementia, which is important cause of disability and dependence among older people (Sousa et al., Citation2009; Sousa et al., Citation2010). Whilst there is no cure for dementia, several modifiable risk factors have been identified (Prince, Albanese, Guerchet, & Prina, Citation2014), and it has been argued that targeting such factors could reduce up to a third of dementia cases worldwide (Livingston et al., Citation2017). Tobacco smoking is a risk factor for several other non-communicable conditions, such high blood pressure, heart failure, stroke and cancer. Even though tobacco is currently considered to be a risk factor for dementia, studies using different methodologies have yielded different results: case-control studies have shown that smoking is associated with a lower prevalence of cases of Alzheimer’s disease (AD) and cohort studies have shown that tobacco smoking may increase dementia risk (Almeida, Hulse, Lawrence, & Flicker, Citation2002). It is important to clarify the relationship between tobacco smoking and dementia risk so that public health services and governments can generate and implement appropriate responses to it.
Even though more than half of all the people living with dementia are from LMIC (Alzheimer’s Disease International, Citation2018), most of the evidence on the relationship between tobacco smoking and dementia risk comes from high-income countries and from studies using different methodologies (Deckers et al., Citation2015; Hazar, Seddigh, Rampisheh, & Nojomi, Citation2016; Hersi et al., Citation2017; Zhong, Wang, Zhang, Guo, & Zhao, Citation2015). In light of these findings, and to better clarify the protective/risk factor role of tobacco with regards to dementia incidence, this study aimed to estimate the association between tobacco smoking and dementia risk in a cohort of 11,143 dementia-free older persons from seven LMIC using the same methodology. We hypothesised that increased tobacco smoking is associated with higher risk for dementia and that this association is stronger for VaD than to AD.
Materials and methods
Study design
This is a population-based cohort study conducted in seven LMIC as part of the 10/66 Dementia Research Group (Prince et al., Citation2007). Detailed study protocol can be found elsewhere (Prince et al., Citation2007). The study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (von Elm et al., Citation2007).
Sample and procedure
This study was undertaken in 10 geographically defined catchment sites in seven LMIC [China (urban and rural sites), Cuba, Dominican Republic, Mexico (urban and rural sites), Peru (urban and rural sites), Puerto Rico and Venezuela]. Although the catchment area samples were not statistically representative of the respective countries, all persons aged 65 and over were invited to take part in the study.
Comprehensive one-phase population-based surveys of all residents aged 65 years and older were undertaken between 2003 and 2007. These included a clinical interview, an informant interview and a physical examination. In the four-year average follow up period we sought to re-interview all baseline survey participants using a similar protocol to the baseline. The study questionnaires are available at the 10/66 Dementia Research Group website (https://www.alz.co.uk/1066/). The data used in this current study included all baseline dementia-free participants (n = 11,143).
Outcome variables
Dementia diagnosis
For diagnosis of dementia, DSM-IV criteria and the 10/66 diagnosis procedures, validated by the 10/66 Dementia Research Group for LMIC populations, were adopted (Prince et al., 2003). An algorithm based on the 10/66 protocol was used, and included: clinical interview and informant reports; the Community Screening Instrument for Dementia (CSI-D); the CERAD 10 word list learning and animal naming tests; the Geriatric Mental State (Copeland, Dewey, & Griffiths-Jones, Citation1986; Prince et al., Citation2004); and the History and Aetiology Schedule – Dementia Diagnosis and Subtype (Dewey & Saz, Citation2001). AD and VaD were identified with an algorithm using NINCDS-ADRDA criteria (McKhann et al., Citation1984) and NINDS-AIREN criteria (Roman et al., Citation1993), respectively.
Tobacco smoking
Participants were asked about their current and past tobacco-smoking habit. Exposure was measured using smoking status and packyears. Responses to smoking status were classified as ‘never’, ‘former’, ‘current’ or ‘ever smoker’. The number of packyears was calculated by multiplying the average number of packs per day by years of consumption. The total number of packyears was calculated for each participant up to the follow-up date. Number of packyears up to the age of 50 was also calculated to ensure that those who stopped smoking did not do so due to dementia onset. This may have also helped minimise confounding due to smokers quitting because of experiencing chronic diseases, which can be independently associated with dementia risk.
Covariables
Sociodemographic status
Included age, marital status and education (none; minimal: did not complete primary; completed primary; secondary; tertiary). Socio-economic data included a household assets index (calculated based on; the number of cars; number of refrigerators; television; among others). The number of assets in the household was categorized as follows: 0–2; 3–5 and 6+ assets).
Physical impairments
Self-reported physical impairments were rated according to whether they interfered with day-to-day activities ‘a little’ or ‘a lot’. These were then categorized in 11 limiting physical impairments (arthritis or rheumatism; eyesight problems; hearing difficulty or deafness; persistent cough; breathlessness, difficulty breathing or asthma; high blood pressure; heart trouble or angina; stomach or intestine problems; faints or blackouts; paralysis, weakness or loss of one leg or arm; and skin disorders such as pressure sores, leg ulcers or severe burns) (Fillenbaum & Smyer, Citation1981).
Alcohol consumption
Participants were asked if they had ever drunk alcohol before the age of 65. If answered yes, participants were then asked about the number of units consumed in a typical week. The consumption of 14 or more units per week for women and 21 or more units for men, before the age of 65, was considered as hazardous drinking. A unit of alcohol was measured according to the World Health Organization recommended AUDIT (The Alcohol Use Disorders Identification Test) questionnaire (Babor, Higgins-Biddle, Saunders, & Monteiro, Citation2001), which considers one alcohol unit to be as much as one can of beer, one glass of wine or one shot of spirits.
Physical activity
Participants reported level of physical activity in four categories: ‘very’, ‘fairly’, ‘not very’ and ‘not at all’ physically active.
Cardiovascular risk factors
Included self-report of ischemic heart disease (conflating angina and myocardial infarction).
Data analysis
Participants’ characteristics (age, sex, marital status, educational level, physical impairments, self-report of ischemic heart diseases, tobacco and alcohol use and incidence of any dementia and its subtypes) were described for the total study population and for each site. To estimate the association (Hazard Ratio – HR) between tobacco smoking and dementia, Cox regression with competing-risk analyses were modelled for each site, controlling for age, gender, educational level, number of assets, physical exercise, past hazardous drinking, number of physical impairments and self-report of angina and myocardial infarction. Fixed-effects meta-analyses were then conducted to combine the estimates from each site, resulting in a pooled estimate for each smoking comparison (lifetime packyears; packyears up to age of 50 years; current versus never; ever versus never; former versus never; current versus former) and for each dementia outcome (any dementia; AD; VaD). The degree of heterogeneity in each analysis was estimated using Higgins’ I2 (Higgins & Thompson, Citation2002) with an approximate 95% confidence interval.
Ethical approval
All participants signed an informed consent form. When participants were illiterate, their oral consent was independently witnessed. Each country’s local ethics committees, as well as the ethical committee of the Institute of Psychiatry at King’s College London, approved the study.
Results
Sample characteristics
We analysed a mean (±SD) of 3.8 (±1.3) years of follow-up, totalling 42,715 person-years. shows the demographic characteristics, the socioeconomic, health and behaviour status of participants at baseline, as well as the incidence of dementia and mortality rate for each site. Mean age was 73.8 (from 65 to 106), with similar values across each site, and there was a higher proportion of women than men in all countries. Most participants were married/cohabiting. Overall, most participants had no to minimal education, except for participants in Peru (urban site) and Puerto Rico, where more than half completed secondary or tertiary school. More than 50% of participants from the rural sites of Peru and China reported no physical illnesses, while in the other sites most participants reported one or more physical illnesses.
Table 1. Baseline characteristics and incidence of dementia and mortality for the total sample, and by each site.
A high proportion of participants had never smoked tobacco, ranging from 52.2% in the Dominican Republic to 87.3% in the urban site of Peru. Less than a quarter of participants were current smokers at baseline, except for China (rural site), where 30.4% reported being smokers at the time. Average lifetime pack-years was 8.9 (16.7 for men and 4.3 for women), while average pack-years up to 50 years of age was 6.2 (11.3 for men and 2.8 for women). There was also a difference in the proportion of smoking status between men and women: 60.9% of men were current smokers, and only 24.9% have never smoked tobacco. With regards to pack-years among ever smokers, we found an average of 26.2 lifetime pack-years and 18.3 pack-years up to 50 years of age. The prevalence of alcohol consumption varied from 4.2% in China (urban site) to 40.9% in the Dominican Republic, and hazardous drinking varied from 1.3% in Peru (urban site) to 32.1% in the Dominican Republic.
At the follow-up visit, 1,069 (9.6%) participants were diagnosed with dementia of any subtype, 3.0% of whom had AD and 0.7% had VaD. The highest number of cases was identified in China (12.5% in the urban site and 10.2% in the rural site). Mortality rate varied from 6.7% in Peru (urban site) to 24.5% in China (rural site).
Packyears
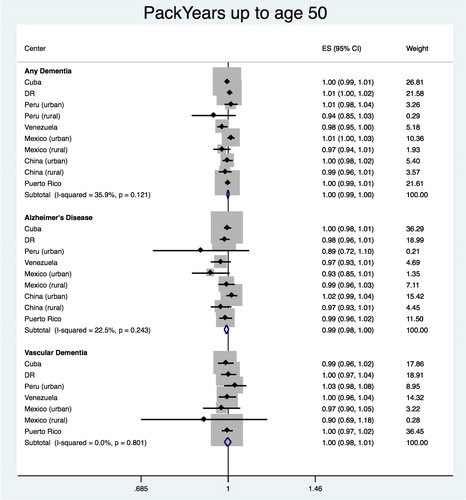
The pooled estimate of HR for the association between packyears up to the age of 50 and any dementia is shown in . We found a score of 0.999 (95% CI 0.994–1.005), with heterogeneity chi-squared = 14.19 (df = 9); p = 0.115; I2 = 36.6% (95% CI 0–70). Significant association was found for Venezuela (HR = 0.969; 95% CI 0.942–0.997), but not for the other countries. No significant association was found for AD (HR = 0.994; 95% CI 0.984–1.003; heterogeneity = 9.04 (df = 8); p = 0.338; I2 = 11.5%; 95% CI 0–53) or VaD (HR = 0.997; 95% CI 0.981–1.012; heterogeneity = 4.46 (df = 6); p = 0.614; I2 = 0.0%; 95% CI 0–71).
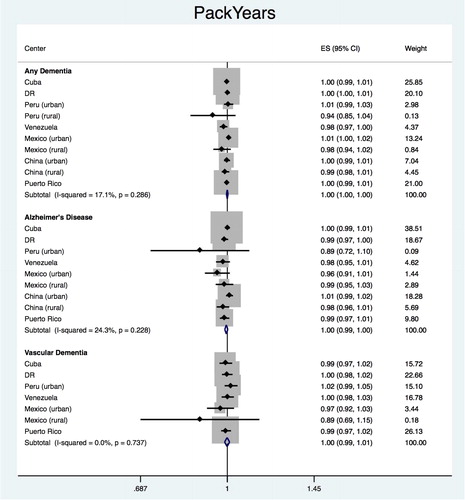
The same analyses were repeated using total packyears (up to age at baseline interview) (). The pooled estimate for the association of total packyears with any dementia was HR = 1.000 (95% CI 0.996–1.003), with heterogeneity chi-squared = 10.91 (df = 9); p = 0.282; I2 = 17.5% (95% CI 0–59). None of the countries showed a significant association between packyears and incidence of any dementia. The pooled estimates did not show a significant association between packyears and the dementia subtypes (AD: HR = 0.996, 95% CI 0.990–1.003, heterogeneity = 9.66, df = 8, p = 0.289, I2 = 17.1%, 95% CI 0–59; VaD: HR = 0.997, 95% CI 0.986–1.009, heterogeneity = 4.49, df = 6, p = 0.610, I2 = 0.0%, 95% CI 0–71).
Smoking status
Current vs. never
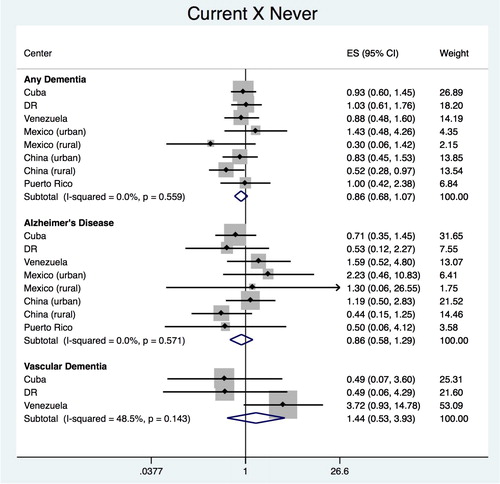
The pooled estimate for the association of ‘current’ vs. ‘never’ smokers with any dementia was HR = 0.838 (95% CI 0.663–1.061), with heterogeneity chi-squared = 6.03 (df = 7); p = 0.536; I2 = 0.0% (95% CI 0–68) (). The analysis of each country did not show any significant association with any dementia, except for China (rural site), with HR = 0.521 (95% CI 0.280–0.966). No significant association was found for dementia subtypes, with pooled estimate of HR = 0.805 (95% CI 0.531–1.221), with heterogeneity chi-squared = 4.56 (df = 7); p = 0.713; I2 = 0.0% (95% CI 0–68) for AD and HR = 0.646 (95% CI 0.196–2.123), with heterogeneity chi-squared = 0.4 (df = 2); p = 0.818; I2 = 0.0% (95% CI 0–90) for VaD.
Ever vs. never
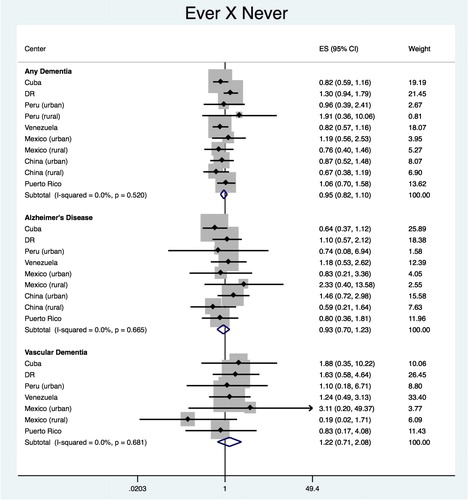
The pooled estimate for the association between ‘ever’ vs. ‘never’ smokers with any dementia was HR = 0.969 (95% CI 0.828–1.133), with heterogeneity chi-squared = 7.49 (df = 9); p = 0.586; I2 = 0.0% (95% CI 0–62) (). No significant association with any dementia was found for any of the countries. As for dementia subtypes, pooled estimates of HR was 0.911 (95% CI 0.680–1.221), with heterogeneity chi-squared = 5.66 (df = 8); p = 0.685; I2 = 0.0% (95% CI 0–65) for AD and 1.051 (95% CI 0.581–1.900), with heterogeneity chi-squared = 4.85 (df = 6); p = 0.563; I2 = 0.0% (95% CI 0–71) for VaD, showing no significant association.
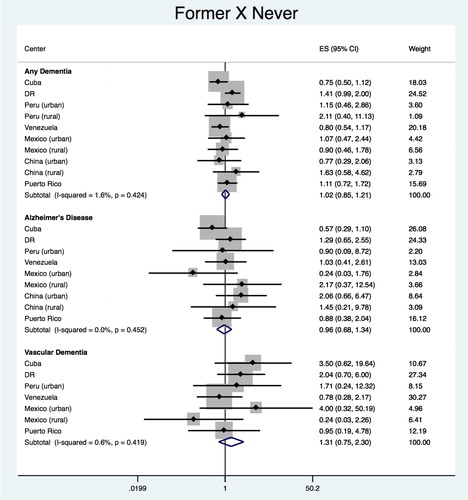
Former vs. never
The pooled estimate for the association between ‘former’ vs. ‘never’ smokers with any dementia was HR = 1.062 (95% CI 0.886–1.274), with heterogeneity chi-squared = 7.51 (df = 9); p = 0.584; I2 = 0.0% (95% CI 0–62) (). The analysis of each country did not show any significant association with any dementia or its subtypes. Meta-analysis of each country’s estimates produced a pooled estimate of HR = 0.964 (95% CI 0.679–1.369), with heterogeneity chi-squared = 7.96 (df = 8); p = 0.437; I2 = 0.0% (95% CI 0–65) for AD and HR = 1.343 (95% CI 0.724–2.492), with heterogeneity chi-squared = 6.72 (df = 6); p = 0.347; I2 = 0.0% (95% CI 0–74) for VaD.
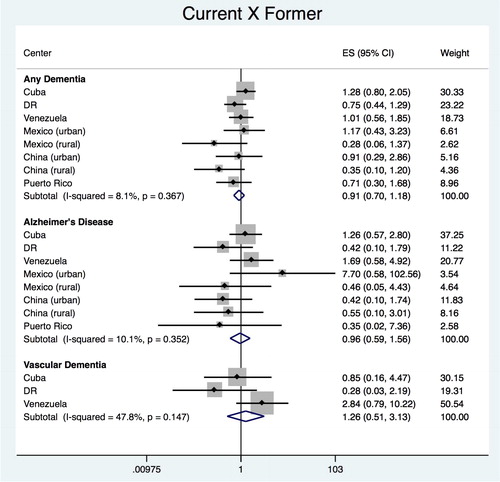
Current vs. former
The pooled estimate for the association between ‘current’ vs. ‘former’ smokers with any dementia was HR = 0.868 (95% CI 0.662–1.138), with heterogeneity chi-squared = 7.67 (df = 7); p = 0.362; I2 = 8.7% (95% CI 0–70) (). No significant association with any dementia was found for any of the countries. Similarly, for dementia subtypes, no significant association was found, demonstrated by the pooled estimates of HR = 0.847 (95% CI 0.503–1.427), with heterogeneity chi-squared = 6.52 (df = 7); p = 0.480; I2 = 0.0% (95% CI 0–68) for AD and of HR = 0.904 (95% CI 0.307–2.656), with heterogeneity chi-squared = 2.56 (df = 2); p = 0.278; I2 = 21.9% (95% CI 0–92) for VaD.
Discussion
Pooled results from all the countries showed no significant association between tobacco exposure and onset of any dementia, including AD and VaD. Individual site estimations showed no association between packyears up to age 50 and onset of any dementia in Venezuela. No association was also found between current smokers and any dementia, compared to never smokers, in rural China.
Tobacco smoking has been associated with both increased and reduced risk for AD in previous studies, which is reinforced by plausible biological mechanisms to explain both protective and risk effects. Nicotine crosses the blood-brain barrier and binds to nicotinic receptors in the cortex, which is associated with acute improvement in cognitive function (Campos, Serebrisky, & Castaldelli-Maia, Citation2017). However, smoking also leads to other processes that may injure neurons, such as oxidative stress caused by free radicals (e.g. reactive oxygen species) and other oxidizing substances, altered mitochondrial respiratory chain function and induction of proinflammatory cytokine release by the central nervous system glial cells (Durazzo, Mattsson, Weiner, & Alzheimer’s Disease Neuroimaging Initiative, 2014). Additionally, studies have shown that oxidative stress is also associated with increased production of amyloid-β and abnormal tau protein phosphorylation in the brain, which are currently hypothesized to be central to AD pathology (Mondragon-Rodriguez et al., Citation2010; Pratico et al., Citation2002).
The possibility of tobacco smoking having both protective or risk effects is supported by previous studies which tried to estimate the association between tobacco exposure and dementia risk in case-control and cohort studies. A systematic review published in 2002 (Almeida et al., Citation2002) comparing case-control studies showed that pooled estimates of case-control studies published in the 80s estimated a protective association between tobacco consumption and dementia (or cognitive decline). The review reported that more robust evidence from cohort studies were however showing the opposite, i.e. a risk association (Almeida et al., Citation2002). Since then, several longitudinal studies and other systematic reviews have been published with different focus, including a potential role of a tobacco industry affiliation (Cataldo, Prochaska, & Glantz, Citation2010), which suggested that studies with this potential conflict of interest tended to show no association or a protective association.
Anstey et al. examined 19 longitudinal studies and showed that, compared with people who had never smoked and with former smokers, current smokers had an increased risk of AD, but there was no difference between them regarding VaD risk (Anstey, von Sanden, Salim, & O'Kearney, Citation2007). In 2015, Zhong et al. published a meta-analysis, which included 37 cohort studies published in English or Chinese, suggesting that current and ever smokers had an increased dementia risk compared to never smokers (Zhong et al., Citation2015).
In 2015, another review (Xu et al., Citation2015) of several modifiable risk factors found a risk association of AD with heavy smoking (more than 55.5 packyears). This review also showed an increased risk for those who were current smokers compared to never smokers in the Asian population and a protective association in the western population. In our study, we found the opposite in rural China where, despite the fact that prevalence of current smokers among older people was higher (30.4%) compared to other sites (from 2.9% in Peru’s rural site to 19.7% in Cuba), current smokers were at a lower risk of dementia compared to never smokers. Although our findings in China could be a result of selection bias due to the death of more vulnerable smokers who would have developed dementia if they had survived, they could also be a result of the combination of other risk factors, such as residence in rural areas, which was shown to reduce dementia risk by roughly 30% in the same systematic review referred above(Xu et al., Citation2015).
Limitations
Our study has several limitations, which include the short duration of follow-up and the cut-off at 65 years of age. A lower cut-off for age (to include mid-life data) would be optimal when evaluating chronic lifestyle risk factors for dementia. To account for this, we also included the packyears up to 50 years of age in our analyses. Another limitation of our study was that the measurement of tobacco consumption history was obtained by questionnaires, making the findings susceptible to recall bias. To reduce the unreliability of this variable, we also asked a close informant about the individual’s tobacco use or asked them to confirm the information we had collected. The number of potential confounding variables we were able to include in the analysis was also limited and we were unable to account for other known risk factors for dementia, such as body mass index, type 2 diabetes and apolipoprotein E ε4. Lastly, although this was a retrospective analysis, the methods used to collect data from all 10 centres were standardized and followed the same protocol, providing multicentric comparable data.
Using only the baseline cross-sectional data of the current study, we have previously shown a risk association between tobacco consumption (packyears) and dementia (Ferri et al., Citation2011). The current analysis using longitudinal data is more robust, and showed no association; however, selective quitting in later life due to chronic conditions, which are also risk factors for dementia, might have mitigated the potential risk effects of tobacco on dementia incidence. Despite progress in tobacco control, its consumption has increased steadily worldwide (Ng et al., Citation2014), mainly due to population growth, and it is still a threat to health on a global scale. Public health efforts are still needed to control its use worldwide.
Conclusion
We conducted secondary data analysis of 11,143 dementia-free older persons from seven LMIC using the same methodology (10/66 Dementia Research Group data) to estimate the association between tobacco smoking and dementia risk. We hypothesised that increased tobacco smoking is associated with higher risk for dementia and that this association is stronger for VaD than to AD. We found no significant association between tobacco exposure and onset of any dementia, including AD and VaD. Individual site estimations showed no association between packyears up to age 50 and onset of any dementia in Venezuela. We also found no association between current smokers and any dementia, compared to never smokers, in rural China. Selective quitting in later-life might have biased the results towards no effect. Future studies investigating the effects of smoking on dementia risk should consider following up people for longer periods of time to reduce potential biases.
Acknowledgment
We thank the 10/66 Dementia Research Group for providing us with the database used in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Almeida, O. P., Hulse, G. K., Lawrence, D., & Flicker, L. (2002). Smoking as a risk factor for Alzheimer’s disease: Contrasting evidence from a systematic review of case-control and cohort studies. Addiction, 97(1), 15–28.
- Alzheimer’s Disease International. (2018). World Alzheimer Report 2018: The state of the art of dementia research: New frontiers. London, UK: ADI.
- Anstey, K. J., von Sanden, C., Salim, A., & O'Kearney, R. (2007). Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. American Journal of Epidemiology, 166(4), 367–378.
- Babor, T., Higgins-Biddle, J., Saunders, J., & Monteiro, M. (2001). AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for use in primary care (2nd ed.). Geneva, Switzerland: WHO.
- Campos, M. W., Serebrisky, D., & Castaldelli-Maia, J. M. (2017). Smoking and Cognition. Current Drug Abuse Reviews, 9(2), 76–79.
- Cataldo, J. K., Prochaska, J. J., & Glantz, S. A. (2010). Cigarette smoking is a risk factor for Alzheimer’s Disease: An analysis controlling for tobacco industry affiliation. Journal of Alzheimer’s Disease, 19(2), 465–480.
- Copeland, J. R., Dewey, M. E., & Griffiths-Jones, H. M. (1986). A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychological Medicine, 16(1), 89–99.
- Deckers, K., van Boxtel, M. P. J., Schiepers, O. J. G., de Vugt, M., Muñoz Sánchez, J. L., Anstey, K. J., … Köhler, S. (2015). Target risk factors for dementia prevention: A systematic review and Delphi consensus study on the evidence from observational studies. International Journal of Geriatric Psychiatry, 30(3), 234–246.
- Dewey, M. E., & Saz, P. (2001). Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. International Journal of Geriatric Psychiatry, 16(8), 751–761.
- Durazzo, T. C., Mattsson, N., & Weiner, M. W, & Alzheimer’s Disease Neuroimaging Initiative. (2014). Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimer’s & Dementia, 10(3), S122–S145.
- Ferri, C. P., West, R., Moriyama, T. S., Acosta, D., Guerra, M., Huang, Y., … Prince, M. J. (2011). Tobacco use and dementia: Evidence from the 1066 dementia population-based surveys in Latin America, China and India. International Journal of Geriatric Psychiatry, 26(11), 1177–1185.
- Fillenbaum, G. G., & Smyer, M. A. (1981). The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. Journal of Gerontology, 36(4), 428–434.
- Hazar, N., Seddigh, L., Rampisheh, Z., & Nojomi, M. (2016). Population attributable fraction of modifiable risk factors for Alzheimer disease: A systematic review of systematic reviews. Iran Journal of Neurology, 15(3), 164–172.
- Hersi, M., Irvine, B., Gupta, P., Gomes, J., Birkett, N., & Krewski, D. (2017). Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. NeuroToxicology, 61, 143–187.
- Higgins, J. P., & Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558.
- Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., … Mukadam, N. (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673–2734.
- McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34(7), 939–944.
- Mondragon-Rodriguez, S., Basurto-Islas, G., Lee, H. G., Perry, G., Zhu, X., Castellani, R. J., & Smith, M. A. (2010). Causes versus effects: The increasing complexities of Alzheimer’s disease pathogenesis. Expert Review of Neurotherapeutics, 10(5), 683–691.
- Ng, M., Freeman, M. K., Fleming, T. D., Robinson, M., Dwyer-Lindgren, L., Thomson, B., … Gakidou, E. (2014). Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA, 311(2), 183–192.
- Pratico, D., Clark, C. M., Liun, F., Rokach, J., Lee, V. Y., & Trojanowski, J. Q. (2002). Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer disease. Archives of Neurology, 59(6), 972–976.
- Prince, M., Acosta, D., Chiu, H., Copeland, J., Dewey, M., Scazufca, M., & Varghese, M. (2004). Effects of education and culture on the validity of the Geriatric Mental State and its AGECAT algorithm. British Journal of Psychiatry, 185(5), 429–436.
- Prince, M., Acosta, D., Chiu, H., Scazufca, M., & Varghese, M, & Dementia Research Group. (2003). Dementia diagnosis in developing countries: A cross-cultural validation study. The Lancet, 361(9361), 909–917.
- Prince, M., Albanese, E., Guerchet, M., & Prina, M. (2014). World Alzheimer Report 2014: Dementia and risk reduction: An analysis of protective and modifiable factors. London, UK: Alzheimer’s Disease International.
- Prince, M., Ferri, C. P., Acosta, D., Albanese, E., Arizaga, R., Dewey, M., … Uwakwe, R. (2007). The protocols for the 10/66 dementia research group population-based research programme. BMC Public Health, 7(1), 165.
- Roman, G. C., Tatemichi, T. K., Erkinjuntti, T., Cummings, J. L., Masdeu, J. C., Garcia, J. H., … Scheinberg, P. …. (1993). Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology, 43(2), 250–260.
- Sousa, R. M., Ferri, C. P., Acosta, D., Albanese, E., Guerra, M., Huang, Y., … Prince, M. (2009). Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: A 10/66 Dementia Research Group population-based survey. The Lancet, 374(9704), 1821–1830.
- Sousa, R. M., Ferri, C. P., Acosta, D., Guerra, M., Huang, Y., Jacob, K. S., … Prince, M. (2010). The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: A 10/66 Dementia Research Group population-based survey. BMC Geriatrics, 10(1), 53.
- von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., & Vandenbroucke, J. P. (2007). Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ: British Medical Journal, 335(7624), 806–808.
- Xu, W., Tan, L., Wang, H. F., Jiang, T., Tan, M. S., Tan, L., … Yu, J. T. (2015). Meta-analysis of modifiable risk factors for Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 86(12), 1299–1306.
- Zhong, G., Wang, Y., Zhang, Y., Guo, J. J., & Zhao, Y. (2015). Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One, 10(3), e0118333.