Abstract
Objectives
Previous studies indicated that stress diagnoses increase the risk of dementia. However, previous results may be biased by confounding, reverse causation and misclassification. Therefore, the main aim of this study was to investigate the association between clinically diagnosed stress in midlife and later dementia risk, while addressing limitations of previous studies.
Methods
The study population was selected from all individuals in Denmark born 1935–1956. Individuals diagnosed with stress in midlife (aged 37–58 years) were matched (1:5) with individuals without stress diagnoses based on sex and birthdate (N = 103,484). Data were retrieved from national registers. Cox regression models were adjusted for socio-demographic factors and different morbidities.
Results
We found a 2.20 (95% CI: 1.93–2.50) times higher rate of dementia among individuals with any stress diagnosis registered in midlife compared with no stress diagnosis. Hazard rate ratios of dementia were 1.73 (95% CI: 1.13–2.65) among individuals with acute stress reactions, 2.37 (95% CI: 2.05–2.74) among individuals with adjustment disorders, and 2.20 (95% CI: 1.73–2.80) among individuals with unspecified stress reactions. Individuals with PTSD and other stress reactions had non-significantly elevated rates of dementia. Adjustment for confounding only slightly attenuated the association, and reverse causation did not appear to bias the results substantially.
Conclusion
Our results support the hypothesis that severe stress in midlife is an important risk factor for dementia. This finding emphasizes the importance of identifying and treating severe stress in midlife to reduce potential detrimental consequences for brain health in later life.
Introduction
Despite indications of stagnation or even decline in dementia incidence in high-income countries (Prince et al., Citation2013; Taudorf et al., Citation2019), dementia continues to constitute a major threat to public health in the upcoming years (Prince et al., Citation2015). Dementia is caused by neurodegenerative and vascular changes often evolving over decades, and therefore the search for modifiable risk factors starts in midlife or even earlier (Livingston et al., Citation2017).
One branch of dementia epidemiology has focused on stress as a risk factor for dementia, as there are several plausible neuropathological pathways linking stress and dementia (Cameron & Schoenfeld, Citation2018; Greenberg, Tanev, Marin, & Pitman, Citation2014; Johansson et al., Citation2019; Linz et al., Citation2019; Mohlenhoff, O’Donovan, Weiner, & Neylan, Citation2017; Piirainen et al., Citation2017; Sussman, Pang, Jetly, Dunkley, & Taylor, Citation2016). A long-term activation of the physiological stress response can lead to long-term changes in the brain and thereby influence the risk of dementia (Ouanes & Popp, Citation2019). Although the precise mechanisms linking stress and dementia are not fully understood, different mechanisms have been suggested to link stress to neurodegeneration, e.g. through elevated cortisol levels and through direct effects on different brain areas. Also, metabolic dysfunction reflected as the metabolic syndrome, neuroinflammation, and dysregulation of the Hypothalamic-Pituitary-Adrenal axis have been suggested as mediators of neurodegeneration (Ouanes & Popp, Citation2019). Furthermore, some brain regions, especially the hippocampus, are suggested to be particularly vulnerable to the effects of stress hormones during midlife and late-life (+30 years) (Lupien, Juster, Raymond, & Marin, Citation2018).
Indeed, previous studies support an association between self-reported psychological stress and dementia (Islamoska et al., Citation2019; Johansson et al., Citation2010; Nabe-Nielsen et al., Citation2019; Skogen, Bergh, Stewart, Knudsen, & Bjerkeset, Citation2015), and between clinical diagnoses of stress and dementia (Flatt, Gilsanz, Quesenberry Jr., Albers, & Whitmer, 2018; Gradus et al., Citation2018; Greenberg et al., Citation2014; Mawanda, Wallace, McCoy, & Abrams, Citation2017; Meziab et al., Citation2014; Qureshi et al., Citation2010; Rafferty, Cawkill, Stevelink, Greenberg, & Greenberg, Citation2018; Yaffe et al., Citation2010). A stress diagnosis denotes severe stress reactions and adjustment disorders as maladaptive responses to severe or continued exposure to stressors (World Health Organization, Citation2016). Among these diagnoses, particularly Post-Traumatic Stress Disorder (PTSD) has shown to be associated with a decrease in cognitive function and increased risk of dementia in populations aged ≥ 55 years at the time of exposure assessment (Flatt et al., Citation2018; Mawanda et al., Citation2017; Meziab et al., Citation2014; Qureshi et al., Citation2010; Rafferty et al., Citation2018; Sumner et al., Citation2017; Yaffe et al., Citation2010).
Animal studies on stress suggest that stress in midlife may accelerate the progression of Alzheimer’s disease (AD) pathology and cognitive decline (Sandi, Citation2007; Wheelan et al., Citation2018). In humans, midlife is suggested to denote the age range of 40–60 years (Lachman, Teshale, & Agrigoroaei, Citation2015; Livingston et al., Citation2017; Ritchie, Ritchie, Yaffe, Skoog, & Scarmeas, Citation2015). A recent Danish register-based study found that stress diagnoses among individuals aged ≥ 40 years was associated with a higher risk of dementia (Gradus et al., Citation2018). Yet, the authors noted that the validity of their findings was limited by lack of adjustment for confounding by educational level (Gradus et al., Citation2018). Furthermore, the previous study did not obtain information about dementia from all available sources (Gradus et al., Citation2018).
Against this background, the main aim of this Danish nationwide register-based study was to investigate the association between a stress diagnosis and the risk of dementia, while adjusting for education and obtaining dementia diagnosis information from multiple sources. Furthermore, we focused specifically on stress in midlife (37–58 years) and the risk of dementia after 60 years in order to clearly separate exposure from outcome and reduce the risk of stress being a consequence of dementia-related cognitive decline. Additionally, in order to address limitations from the previously published Danish study, information about morbidities from a longer time span and individuals of all ethnic origins were included as well as expansion of the follow-up time with six calendar years.
Methods
Design and study population
We included national register-based data on inhabitants of all countries of origin in Denmark born from 1935 to 1956 (N = 1,657,890). We recorded information on stress diagnoses according to the 10th revision of the International Classification of Diseases (ICD-10) from 1994 onwards among individuals aged 37–58 years and obtained register information on dementia cases from the age of 60 years onwards (). We recorded dementia diagnoses for all individuals aged ≥ 60 years in order to clearly separate the timing of exposure from timing of the outcome. Furthermore, validation studies have demonstrated a lower validity of dementia diagnoses among younger individuals (Nielsen, Vogel, Phung, Gade, & Waldemar, Citation2011; Salem et al., Citation2012), thus, individuals with dementia before the age of 60 years were excluded. Individuals were followed until dementia diagnosis, death, emigration or end of follow-up (31 December 2017), whichever came first.
Figure 1. Study design. Individuals born between 1935 and 1956 were followed from when they turned 60 years until death, emigration, dementia diagnosis, or end of follow-up in 2017. Stress diagnoses were registered from 1994 onwards. This figure illustrates the life course of a single individual from 6 of the 21 included birth years exemplifying individuals turning 60 years and being followed in registers until endpoints.
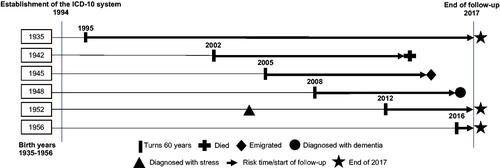
Individuals with dementia, who died or emigrated, or had missing information on covariates before the age of 60 years were excluded (). We included individuals of all ethnic origins to obtain a study population reflecting the Danish population and to be able to explore potential differences between sub-populations. Due to irregularities in national education data (Jensen & Rasmussen, Citation2011) and population data in general, some individuals had missing data for some or all calendar years and were therefore excluded.
Figure 2. Flow chart of the selection of study population (N = 103,484). After excluding individuals with missing information from the start, some individuals had missing information on education and marital status for specific index years and were therefore excluded after matching.
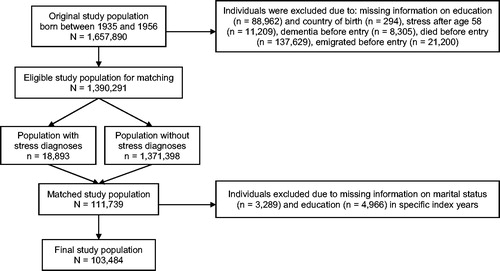
We applied an exposure-matching procedure in which we matched one individual with a stress diagnosis with five reference individuals without a stress diagnosis by sex and birthdate (± 30 days) (Grandits & Neuhaus, Citation2010). To be included, individuals had to be alive on their 60 years’ birthday. We defined the year of the first registered stress diagnosis as the index year for all exposed individuals and used the same index year for the matched unexposed individuals in the same generalized pair. Information on covariates were extracted one year before the index year in order to ensure the temporal relation between assessment of potential confounders and assessment of exposure status. Our final study population included 103,484 individuals ().
Stress diagnoses
We identified individuals with a stress diagnosis using patient data from the Danish National Patient Register (NPR) and the Danish Psychiatric Central Research Register (PCRR) (Lynge, Sandegaard, & Rebolj, Citation2011; Mors, Perto, & Mortensen, Citation2011). We extracted data from ICD-10 codes for the following diagnoses from 1994 onwards: acute stress reactions (F43.0), PTSD (F43.1), adjustment disorders (F43.2), other reactions to severe stress (F43.8), and unspecified reactions to severe stress (F43.9) (Gradus et al., Citation2014; World Health Organization, Citation2016). The NPR and PCRR include data on a combination of all in- and outpatient contacts at somatic and psychiatric departments or wards in the secondary healthcare system all over Denmark (Mors et al., Citation2011; Schmidt et al., Citation2015). In Denmark, there is free and public access to the healthcare system, which improves the coverage of the register-based information. However, mild to moderate psychiatric disorders are diagnosed and treated in clinics of general practitioners or specialists in psychiatry and are not included in registers (Mors et al., Citation2011). We assume that individuals who are treated for their stress diagnosis in the secondary healthcare system are more severe cases compared with individuals treated in the primary healthcare system. Information on stress diagnoses was derived from primary diagnoses (i.e., the reason for hospital contact) or secondary diagnoses (i.e., additional diagnoses registered at the same time as the primary diagnosis) registered in the secondary healthcare system.
Dementia
We defined dementia as either the first ever redeemed anti-dementia medication or registration of a dementia diagnosis, whichever came first. Dementia diagnoses were obtained by using patient data from the NPR (Lynge et al., Citation2011) and the PCRR (Mors et al., Citation2011), and mortality data from the Danish Register of Causes of Death (Helweg-Larsen, Citation2011) using ICD-8 and ICD-10 codes for AD, vascular dementia, frontotemporal dementia, Lewy body dementia, and unspecified and other dementia (). We did not differentiate between the sub-types of dementia due to poor validity of the individual diagnoses, with the exception of AD, and, additionally, a great part of the patients has unspecific diagnoses (Phung et al., Citation2007; Taudorf et al., Citation2019). Information on redeemed prescription medication data was based on Anatomical Therapeutic Chemical Classification System (ATC) codes () of cholinesterase-inhibitors or glutamate-receptor antagonists and was obtained from the Danish National Prescription Registry (Kildemoes, Sørensen, & Hallas, Citation2011). In Denmark, all medical doctors can prescribe anti-dementia medication, but it must be prescribed by a specialist in geriatrics, neurology or psychiatry for patients to receive reimbursement for these medications (Johannsen, Waldorff, Pedersen, & Wermuth, Citation2019).
Covariates
Information on dementia risk factors was obtained from national registers including socio-demographic factors, i.e., birthdate, sex, country of origin (Denmark/Western countries/Non-Western countries), marital status (unmarried/married), and highest attained educational level (low educational level defined by primary school/medium educational defined by upper secondary education, business high school, and vocational education and training/high educational level defined by short-term further education, middle-range education, bachelor’s degree, extended education, and research degree).
Based on previous literature (Gradus, Citation2017; Pedersen et al., Citation2014) and expert opinion, we generated a combined measure of the following psychiatric diagnoses from both ICD-8 and ICD-10: schizophrenia, schizotypal and delusional disorders, mood affective disorders, other or unspecified psychoses, neuroses, transient situational disturbances, and mental and behavioral disorders due to psychoactive substance use (). Data on psychiatric morbidities were included for all individuals until the index date and dated back to 1968.
Likewise, we defined three binary variables including diagnoses of cardiovascular and metabolic morbidities (myocardial infarction, heart failure, peripheral vascular disease, cerebral vascular accident, type 1 and type 2 diabetes), head injuries, and sleep disorders, respectively (). Data were dated back to 1970.
All data in this study were obtained with approval from Statistics Denmark and the Danish Health Data Authority.
Statistical analysis
Descriptive analyses were used to investigate the distribution of socio-demographic factors and different morbidities among individuals with and without stress diagnoses ().
Table 1. Baseline characteristics of the study population with and without a stress diagnosis. Prevalence and medians with 25-75% interquartile ranges (IQR) are shown (N = 103,484).
To investigate the association between stress diagnoses and dementia, we applied the Cox regression model and used time after the age of 60 years as underlying time scale for the analyses. Stress diagnoses were recorded from 1994 onwards, thus, individuals with stress before 1994 will be misclassified as non-exposed. We used data from individuals born across a wide range of years, and because the probability of exposure misclassification depended on birth cohort, we analyzed data using a Cox model stratified on birth cohorts to ensure that comparisons were made among individuals with the same risk of misclassification.
We conducted two sets of main analyses: First, we used one binary exposure variable combining all five stress diagnoses and defined as having any stress diagnosis. Second, as 79 individuals were registered with more than one stress diagnosis at the same occasion, we also defined five binary exposure variables defining the five different stress diagnoses. Matched unexposed individuals were used as reference group in all analyses. We adjusted for potential confounding in two steps: 1) Model 1 included sex; 2) Model 2 included sex, country of origin, marital status, educational level, psychiatric morbidities, cardiovascular and metabolic morbidities, head injuries, and sleep disorders (). We conducted supplementary analyses focusing specifically on AD. In these analyses, individuals were censored, if the first dementia diagnosis was any of the other previously defined dementia outcomes. In addition, we adjusted for all morbidities occurring after the index date to investigate whether potential mediating effects could explain the association between stress diagnoses and dementia.
Table 2. Hazard ratios (HR) for dementia with 95% confidence intervals (95% CI). Results for any stress diagnosis and the five specific stress diagnoses vs. no stress diagnosis are presented (N = 103,484).
In additional interaction analyses, we investigated whether the association between having any stress diagnosis (yes/no) and dementia was stronger for a specific sex (men and women), educational level (low, medium and high), birth cohort (1935–1939, 1940–1944, 1945–1949 and 1950–1956), and country of origin (Denmark, Western countries and Non-Western countries). The resulting p-values are shown in . Furthermore, we also made a refinement of the exposure to investigate whether the stress-dementia association depended on age at the time of stress diagnosis. We created a variable defining individuals registered with stress diagnoses at either age 37–48 years, 49–54 years or 55–58 years, respectively, and compared them with individuals without stress diagnoses.
Table 3. Hazard ratios (HR) for dementia with 95% confidence intervals (95% CI). Results for any stress diagnosis vs. no stress diagnosis grouped by sex, educational level, country of origin and the birth cohorts 1935-1956 (N = 103,484) and corresponding interaction tests expressed by p-values.
We were particularly concerned about reverse causation, i.e., that stress was an early indicator of dementia pathology. Therefore, we conducted sensitivity analyses and considered individuals at risk of dementia only when at least 5, 10, 15, and 20 years had passed after the index date ().
Since a key assumption of the Cox regression model is that the rate ratio of hazards for any two groups is constant over time (Altman, Citation1991), we tested the proportionality of hazards. In a Cox regression model including covariates of Model 2, we included a time dependent interaction between time and covariates and tested its significance (Hosmer, Lemeshow, & May, Citation2008).
Results
In our study population of 103,484 individuals, 17,928 (17%) individuals were registered with a clinical stress diagnosis (median age: 52 years). More specifically, 56% were registered with an adjustment disorder, 24% were registered with unspecified stress reactions, and less than 10% were diagnosed with acute stress reactions, PTSD, or other stress reactions (). Among individuals with any stress diagnosis compared with none, there were slightly more individuals who were from Non-Western countries, unmarried, and had other psychiatric morbidities. Among individuals with PTSD compared with other stress diagnoses, there was an equal distribution of men and women with PTSD and more individuals were from Non-Western countries.
The median follow-up time from the age of 60 years was 5.7 years (interquartile range (IQR): 3.0–9.4). The median number of years between stress diagnosis and dementia was 12.9 (IQR: 8.8–17.3). We observed that 369 individuals with any stress diagnosis were registered with dementia at a median age of 66 years (IQR: 62–70). In the unexposed cohort, 784 individuals were registered with dementia with a median age of 67 years (IQR: 64–71). Individuals were mainly registered with dementia for the first time in patient data (85%) followed by prescription data (14%) and mortality data (1%).
The main analyses showed that among individuals with any stress diagnosis, a 2.20 times higher rate of dementia was observed (95% CI: 1.93–2.50). We observed a 1.73 times higher rate of dementia among individuals with acute stress reactions (95% CI: 1.13–2.65), a 2.37 times higher rate of dementia among individuals with adjustment disorders (95% CI: 2.05–2.74), and a 2.20 times higher rate of dementia among individuals with unspecified stress reactions (95% CI: 1.73–2.80). The rate of dementia in individuals with PTSD and other stress reactions pointed to a marginally higher risk, but these estimates were statistically non-significant. Adjustment for socio-demographic factors and morbidities only slightly attenuated the association (). In additional analyses, we investigated all confounders from Model 2 as potential mediators by adjusting for these factors, when they occurred after the index date. The results showed that throughout the whole follow-up period, there was a lower but significantly higher rate of dementia among individuals with stress diagnoses compared with not having stress diagnoses (HR = 1.17; 95% CI: 1.02–1.36).
Our interaction analyses did not show any significant differences in the association between stress diagnoses and dementia among men and women and across educational levels, countries of origin, and birth cohorts ().
We found the highest rate of dementia among individuals aged 49–58 years when registered with a stress diagnosis compared with individuals without any stress diagnosis (). However, the difference in HRs across age groups were not significantly different (p = 0.48).
Table 4. Hazard ratios (HR) for dementia with 95% confidence intervals (95% CI). Results for any stress diagnosis at age 37-48, 49-50 and 50-58 years vs. no stress diagnosis (N = 103,484) and homogeneity test expressed by p-value.
In our sensitivity analyses, the association between stress diagnoses and dementia were slightly attenuated, but remained statistically significant when postponing start of follow-up to at least 5, 10, 15, and 20 years after index date (). The estimates for the association between stress diagnoses and AD had the same direction as in the main analyses of stress and the combined measure of all dementia diagnoses, but the HR for AD was somewhat lower (Model 2: HR = 1.75; 95% CI: 1.31–2.32).
The tests for proportional hazards showed that this assumption was violated for some of the covariates, however, the overall HR observed for the association between any stress diagnosis and the rate of dementia (: HR = 2.20; 95% CI: 1.93–2.50) remained robust when allowing for non-proportionality of dementia hazards for these covariates. More importantly, the HR for any stress diagnosis varied significantly with time and tended to decrease with time. Thus, the reported HRs of dementia should be interpreted as average estimates of the association between stress diagnoses and the rate of dementia after the age of 60 years.
Discussion
Main results
In this national longitudinal follow-up study of 103,484 individuals, the main results showed a higher rate of dementia over time when registered with any clinical stress diagnosis. In analyses of specific stress diagnoses, individuals with acute stress reactions, adjustment disorders and unspecified stress reactions had significantly higher rates of dementia than individuals without any stress diagnosis. The highest rate of dementia was found among individuals registered with a stress diagnosis at age 49–58 years. Overall, adjustment for confounding by socio-demographic factors and morbidities only slightly attenuated the associations, as did the application of a longer time-window between index date and start of follow-up.
Comparison with previous research
Overall, our main findings are in alignment with results of previous studies demonstrating a longitudinal association between dementia and measures of self-reported psychological stress (Islamoska et al., Citation2019; Johansson et al., Citation2010; Nabe-Nielsen et al., Citation2019; Skogen et al., Citation2015) and a clinical diagnosis of stress (Gradus et al., Citation2018).
The validity of the results from our and previous observational studies is supported by evidence regarding plausible physiological mechanisms including neurodegenerative processes in the brain linking chronic stress to dementia (Greenberg et al., Citation2014; Johansson et al., Citation2019; Ouanes & Popp, Citation2019). Furthermore, the discrepancies in the risk estimates between stress and AD, and stress and all dementia diagnoses may suggest a difference in the underlying mechanism, which have not been fully unveiled yet (Johansson et al., Citation2019).
Our results were comparable to results from the previous Danish study (Gradus et al., Citation2018) reporting an association between acute stress reactions, adjustment disorders and unspecified stress reactions and a higher risk of dementia. Yet, for the remaining diagnoses of stress, our results indicated a somewhat lower and non-significant association with dementia (PTSD: HR = 1.08 vs. 2.0; other stress reactions: HR = 1.41 vs. 2.2) (Gradus et al., Citation2018). These differences may stem from differences in the scope of our and the previous study yielding disparities in methodological approaches. Our aim was to investigate midlife risk factors for dementia, as midlife has been emphasized as the period in which dementia pathology is likely to have its onset (Livingston et al., Citation2017; Ritchie et al., Citation2015). In the previous Danish study, approximately 19% of the study population were ≥ 60 years by the time of stress diagnosis (Gradus et al., Citation2018). However, late-life neuropsychiatric symptoms, e.g. depressive, anxious, apathetic, irritable, or psychotic symptoms, leading to a stress diagnosis may be early signs of dementia (Livingston et al., Citation2017). We recorded information on stress diagnoses from individuals, who were aged 37–58 years to reduce the risk of reverse causation. We also adjusted for educational level, which did not, however, seem to cause severe confounding.
In general, previous studies have reported higher and significant dementia rates among individuals with PTSD compared with our results (Flatt et al., Citation2018; Mawanda et al., Citation2017; Meziab et al., Citation2014; Qureshi et al., Citation2010; Rafferty et al., Citation2018; Yaffe et al., Citation2010). In the majority of the previous studies, the study populations included US veterans (predominantly men) aged ≥ 55 years (Mawanda et al., Citation2017; Meziab et al., Citation2014; Qureshi et al., Citation2010; Yaffe et al., Citation2010) with estimates ranging from 1.35–2.31. Apart from our and the previous Danish study (Gradus et al., Citation2018), the only other population-based study was from the US and included individuals aged ≥ 60 years at baseline (Flatt et al., Citation2018). In this latter study, a 1.20 higher rate of dementia (95% CI: 1.02–1.41) was reported among individuals with PTSD (Flatt et al., Citation2018).
Strengths and limitations
This is the first study to investigate the association between clinical stress diagnoses specifically in midlife and the risk of dementia in later life. To ensure that information about covariates was obtained before the date of the stress diagnoses, we matched the exposed population with a non-exposed population on sex and birthdate and used register-based data prior to the index date. To reduce confounding, we adjusted for several dementia risk factors, such as education, country of origin, marital status, psychiatric, cardiovascular and metabolic morbidities, head injuries, and sleep disorders using hospitalization data dating back to 1968. We had available register data until 2017 making it possible to follow individuals with stress diagnoses in registers for more than 20 years with a low risk of loss to follow-up. The use of register-data yielded a large study population and high statistical power, although, some sub-analyses were based on smaller groups.
F43 stress diagnoses are valid measures applicable for research and diagnostics (Svensson, Lash, Resick, Hansen, & Gradus, Citation2015). The stress diagnoses registered in original medical records have been compared with register diagnoses, and 80% of the medical records noted a stressful or traumatic event (Svensson et al., Citation2015). Nevertheless, a main methodological limitation of our study is that exposure information only dated back to the inception year of ICD-10 in 1994. Thus, the risk of misclassifying individuals—particular in older birth cohorts—as non-exposed is pronounced. We addressed this limitation by taking birth cohort into account in our statistical model. Furthermore, we adjusted for psychiatric diagnoses that from an expert opinion might have been used for patients with severe stress reactions before ICD-10. In addition, we cannot rule out that individuals in the comparison cohort were treated for mild to moderate psychiatric diagnoses by general practitioners. Such treatment is not recorded in the available registers. Thus, our results only apply to severe cases of clinical stress reactions and adjustment disorders, and due to misclassification of exposed individuals, the stress-dementia association is likely to be underestimated, as the non-exposed group is diluted by exposed individuals.
The validity of the results of the present study is strengthened by the use of validated register data on dementia diagnoses (Phung et al., Citation2007). However, despite that the validity of dementia diagnoses and the diagnostic rate in secondary care have improved over time (Phung et al., Citation2007; Phung, Waltoft, Kessing, Mortensen, & Waldemar, Citation2010), only 40% of dementia patients are actually registered with a diagnosis (Jørgensen & Waldemar, Citation2014; Prince, Bryce, & Ferri, Citation2011), which limits our study’s ability to detect associations between the exposure and the outcome under study. We addressed this limitation by obtaining dementia information from all available register-based sources. It should be noted that some types of dementia medication may also be used for dementia in Parkinson’s disease and mild cognitive impairment, which were not defined as an outcome in our study. We cannot preclude that anti-dementia medications are prescribed to individuals with mild cognitive impairment, despite that national clinical guidelines did not recommend use of anti-dementia medications to patients with mild cognitive impairment, but without dementia (Sundhedsstyrelsen, Citation2013). Furthermore, it could be speculated that declining medication prices may lead to dementia patients being referred to specialists less often and instead being prescribed anti-dementia medications without an expert examination. Thus, the specificity of dementia based on information on anti-dementia medications may be decreasing over time. A primary concern is that we cannot rule out detection bias occurring, if dementia is more frequently diagnosed when in contact with the healthcare system, e.g. due to stress. Such a detection bias is likely to result in an overestimation of the association between stress and dementia. We partly took this bias into account by adjusting for a range of covariates which, apart from being potential confounders, were also likely to be associated with healthcare use.
Dementia is typically registered in individuals aged 80–89 years (Prince et al., Citation2015). Our study population was relatively young at the end of follow-up and the overall incidence of dementia was only registered until the age of 82 years among the oldest individuals in our population. Furthermore, in a recent Danish, national register-based study including all dementia cases above age 60, the median age was 82 years among dementia cases at the time of diagnosis (Taudorf et al., Citation2019). Thus, it is unknown whether results also apply to individuals above the age of 82 years, who comprise the majority of individuals with dementia (Taudorf et al., Citation2019). This is also supported by the proportional hazards tests showing a tendency of a decreasing dementia rate ratio after age 60. Thus, we cannot rule out that a longer follow-up time would yield other results.
When adjusting for confounding by different morbidities, the results only changed marginally. Yet, we cannot eliminate that morbidities developed after the stress diagnosis may have affected the risk of dementia. Taking other morbidities throughout the whole follow-up period into account resulted in a substantially lower, but still significant association between clinical stress diagnoses and dementia. However, due to the onset of such morbidities, these are considered potential mediators, which in principle should not be adjusted for.
Unfortunately, there was no available register-based information on other important confounders such as obesity, physical inactivity, smoking, hypertension, hearing loss, social isolation and personality (Livingston et al., Citation2017; Low, Harrison, & Lackersteen, Citation2013). Other studies adjusting for several of these confounders found slight attenuations of the association between self-reported stress and dementia (Islamoska et al., Citation2019; Nabe-Nielsen et al., Citation2019).
In most cases, dementia is a slowly developing syndrome with a long preclinical phase (Livingston et al., Citation2017). Hence, we were particularly concerned that our results were biased by reverse causation, i.e. that declining cognitive function increased the risk of being diagnosed with stress. Yet, excluding dementia cases and risk time during the first 5–20 years after the index date only slightly attenuated the associations. Thus, even though we cannot rule out that dementia pathology in its early stage could affect the risk of severe stress reactions, this effect did not seem to explain all of the observed association.
Conclusion
In this national register-based study, we found empirical support for the hypothesis that clinically diagnosed stress in midlife is a risk factor for dementia in later life. Although the majority of the sub-diagnoses of stress had rates ratios of dementia within the same range, we found the strongest association between acute stress reactions, adjustment disorders and unspecified stress reactions and dementia. In particular, individuals in late midlife had a higher rate ratio of dementia. Our study highlights the importance of identifying and treating severe stress in midlife in order to reduce potential detrimental consequences for brain health in later life.
Acknowledgements
We are grateful for the prudent discussions and assistance from Elsebet Steno Hansen, Senior consultant, MD, PhD from Geriatric Psychiatric Department, Vordingborg, Region Zealand, regarding the inclusion and adjustment of relevant psychiatric ICD-8 diagnoses in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Altman, D. (1991). Analysis of survival times. In D. Altman (Ed.), Practical statistics for medical research (1st ed., pp. 365–395): London, England: Chapman & Hall/CRC.
- Cameron, H., & Schoenfeld, T. (2018). Behavioral and structural adaptations to stress. Frontiers in Neuroendocrinology, 49, 106–113. doi:10.1016/j.yfrne.2018.02.002
- Flatt, J., Gilsanz, P., Quesenberry, C., Jr., Albers, K., & Whitmer, R. (2018). Post-traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimer's & Dementia, 14(1), 28–34. doi:10.1016/j.jalz.2017.04.014
- Gradus, J. (2017). Prevalence and prognosis of stress disorders: A review of the epidemiologic literature. Clinical Epidemiology, 9, 251–260. doi:10.2147/CLEP.S106250
- Gradus, J., Bozi, I., Antonsen, S., Svensson, E., Lash, T., Resick, P., & Hansen, J. (2014). Severe stress and adjustment disorder diagnoses in the population of Denmark. Journal of Traumatic Stress, 27(3), 370–374. doi:10.1002/jts.21926
- Gradus, J., Horváth-Puhó, E., Lash, T., Ehrenstein, V., Tamang, S., Adler, N., … Sørensen, H. (2018). Stress disorders and dementia in the Danish population. American Journal of Epidemiology, 188(3), 493–499. doi:10.1093/aje/kwy269
- Grandits, G., & Neuhaus, J. (2010). Using SAS® to perform individual matching in design of case-control studies. Seattle, WA: SAS Global Forum.
- Greenberg, M., Tanev, K., Marin, M., & Pitman, R. (2014). Stress, PTSD, and dementia. Alzheimer's & Dementia, 10(3 Suppl), S155–S165. doi:10.1016/j.jalz.2014.04.008
- Helweg-Larsen, K. (2011). The Danish register of causes of death. Scandinavian Journal of Public Health, 39(7_suppl), 26–29. doi:10.1177/1403494811399958
- Hosmer, D. W., Lemeshow, S., & May, S. (2008). Applied Survival Analysis: Regression Modeling of Time to Event Data. John Wiley & Sons, Inc., 2nd edition. doi:10.1002/9780470258019
- Islamoska, S., Ishtiak-Ahmed, K., Hansen, ÅM., Grynderup, M. B., Mortensen, E. L., Garde, A. H., … Nabe-Nielsen, K. (2019). Vital exhaustion and incidence of dementia: Results from the Copenhagen city heart study. Journal of Alzheimer's Disease, 67(1), 369–379. doi:10.3233/JAD-180478
- Jensen, V., & Rasmussen, A. (2011). Danish education registers. Scandinavian Journal of Public Health, 39(7_suppl), 91–94. doi:10.1177/1403494810394715
- Johannsen, P., Waldorff, F., Pedersen, H., & Wermuth, L. (2019). Midler mod demens. Retrieved from https://pro.medicin.dk/Laegemiddelgrupper/grupper/315685
- Johansson, L., Guo, X., Waern, M., Östling, S., Gustafson, D., Bengtsson, C., & Skoog, I. (2010). Midlife psychological stress and risk of dementia: A 35-year longitudinal population study. Brain, 133(8), 2217–2224. doi:10.1093/brain/awq116
- Johansson, L., Sacuiu, S., Kern, S., Guo, X., Zetterberg, H., Blennow, K., … Skoog, I. (2019). Longstanding psychological stress in relation to biomarkers of neuronal dysfunction in cerebrospinal fluid: A 25-year follow-up study in women. Neurobiology of Aging, 80, 111–115. doi:10.1016/j.neurobiolaging.2019.02.013
- Jørgensen, K., & Waldemar, G. (2014). Praevalens af demens i Danmark. Ugeskrift for Laeger, 177(11), 6140325.
- Kildemoes, H., Sørensen, H., & Hallas, J. (2011). The Danish National Prescription Registry. Scandinavian Journal of Public Health, 39(7), 38–41. doi:10.1177/1403494810394717
- Lachman, M., Teshale, S., & Agrigoroaei, S. (2015). Midlife as a pivotal period in the life course: Balancing growth and decline at the crossroads of youth and old age. International Journal of Behavioral Development, 39(1), 20–31. doi:10.1177/0165025414533223
- Linz, R., Puhlmann, L. M. C., Apostolakou, F., Mantzou, E., Papassotiriou, I., Chrousos, G. P., … Singer, T. (2019). Acute psychosocial stress increases serum BDNF levels: An antagonistic relation to cortisol but no group differences after mental training. Neuropsychopharmacology, 44(10), 1797–1804. doi:10.1038/s41386-019-0391-y
- Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., … Mukadam, N. (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673–2734. doi:10.1016/S0140-6736(17)31363-6
- Low, L., Harrison, F., & Lackersteen, S. (2013). Does personality affect risk for dementia? A systematic review and meta-analysis. The American Journal of Geriatric Psychiatry, 21(8), 713–728. doi:10.1016/j.jagp.2012.08.004
- Lupien, S., Juster, R., Raymond, C., & Marin, M. (2018). The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Frontiers in Neuroendocrinology, 49, 91–105. doi:10.1016/j.yfrne.2018.02.001
- Lynge, E., Sandegaard, J., & Rebolj, M. (2011). The Danish National Patient Register. Scandinavian Journal of Public Health, 39(7_suppl), 30–33. doi:10.1177/1403494811401482
- Mawanda, F., Wallace, R., McCoy, K., & Abrams, T. (2017). PTSD, psychotropic medication use, and the risk of dementia among US veterans: A retrospective cohort study. Journal of the American Geriatrics Society, 65(5), 1043–1050. doi:10.1111/jgs.14756
- Meziab, O., Kirby, K., Williams, B., Yaffe, K., Byers, A., & Barnes, D. (2014). Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimer's & Dementia, 10(3 Suppl), S236–S241. doi:10.1016/j.jalz.2014.04.004
- Mohlenhoff, B., O’Donovan, A., Weiner, M., & Neylan, T. (2017). Dementia risk in posttraumatic stress disorder: The relevance of sleep-related abnormalities in brain structure, amyloid, and inflammation. Current Psychiatry Reports, 19(11), 89. doi:10.1007/s11920-017-0835-1
- Mors, O., Perto, G., & Mortensen, P. (2011). The Danish psychiatric central research register. Scandinavian Journal of Public Health, 39(7_suppl), 54–57. doi:10.1177/1403494810395825
- Nabe-Nielsen, K., Rod, N. H., Hansen, Å. M., Prescott, E., Grynderup, M. B., Islamoska, S., … Westendorp, R. G. J. (2019). Perceived stress and dementia: Results from the Copenhagen city heart study. Aging & Mental Health, 1–9. [Epub ahead of print].doi:10.1080/13607863.2019.1625304
- Nielsen, T., Vogel, A., Phung, T., Gade, A., & Waldemar, G. (2011). Over- and under-diagnosis of dementia in ethnic minorities: A nationwide register-based study. International Journal of Geriatric Psychiatry, 26(11), 1128–1135. doi:10.1002/gps.2650
- Ouanes, S., & Popp, J. (2019). High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Frontiers in Aging Neuroscience, 11(43), 1-11. doi:10.3389/fnagi.2019.00043
- Pedersen, C. B., Mors, O., Bertelsen, A., Waltoft, B. L., Agerbo, E., McGrath, J. J., … Eaton, W. W. (2014). A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry, 71(5), 573–581. doi:10.1001/jamapsychiatry.2014.16
- Phung, T., Andersen, B., Hogh, P., Kessing, L., Mortensen, P., & Waldemar, G. (2007). Validity of dementia diagnoses in the Danish hospital registers. Dementia and Geriatric Cognitive Disorders, 24(3), 220–228. doi:10.1159/000107084
- Phung, T., Waltoft, B., Kessing, L., Mortensen, P., & Waldemar, G. (2010). Time trend in diagnosing dementia in secondary care. Dementia and Geriatric Cognitive Disorders, 29(2), 146–153. doi:10.1159/000269933
- Piirainen, S., Youssef, A., Song, C., Kalueff, A., Landreth, G., Malm, T., & Tian, L. (2017). Psychosocial stress on neuroinflammation and cognitive dysfunctions in Alzheimer’s disease: The emerging role for microglia? Neuroscience & Biobehavioral Reviews, 77, 148–164. doi:10.1016/j.neubiorev.2017.01.046
- Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., & Ferri, C. P. (2013). The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer's & Dementia, 9(1), 63–75. doi:10.1016/j.jalz.2012.11.007
- Prince, M., Bryce, R., & Ferri, C. (2011). World Alzheimer Report 2011: The benefits of early diagnosis and intervention. Alzheimer’s Disease International. Retrieved from https://www.alz.co.uk/research/WorldAlzheimerReport2011.pdf
- Prince, M., Wimo, A., Guerchet, M., Ali, G., Wu, Y., & Prina, M. (2015). World Alzheimer Report 2015: The Global Impact of Dementia - An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International. Retrieved from https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf
- Qureshi, S. U., Kimbrell, T., Pyne, J. M., Magruder, K. M., Hudson, T. J., Petersen, N. J., … Kunik, M. E. (2010). Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. Journal of the American Geriatrics Society, 58(9), 1627–1633. doi:10.1111/j.1532-5415.2010.02977.x
- Rafferty, L., Cawkill, P., Stevelink, S., Greenberg, K., & Greenberg, N. (2018). Dementia, post-traumatic stress disorder and major depressive disorder: A review of the mental health risk factors for dementia in the military veteran population. Psychological Medicine, 48(9), 1400–1409. doi:10.1017/S0033291717001386
- Ritchie, K., Ritchie, C., Yaffe, K., Skoog, I., & Scarmeas, N. (2015). Is late-onset Alzheimer’s disease really a disease of midlife? Alzheimer's & Dementia: Translational Research & Clinical Interventions, 1(2), 122–130. doi:10.1016/j.trci.2015.06.004
- Salem, L., Andersen, B., Nielsen, T., Stokholm, J., Jorgensen, M., Rasmussen, M., & Waldemar, G. (2012). Overdiagnosis of dementia in young patients - a nationwide register-based study. Dementia and Geriatric Cognitive Disorders, 34(5–6), 292–299. doi:10.1159/000345485
- Sandi, C. (2007). Memory impairments associated with stress and aging. In F. Bermúdez-Rattoni (Ed.), Neural plasticity and memory: From genes to brain imaging, 225-264. Boca Ration, FL: CRC PRess/Taylor & Francis.
- Schmidt, M., Schmidt, S., Sandegaard, J., Ehrenstein, V., Pedersen, L., & Sorensen, H. (2015). The Danish National Patient Registry: A review of content, data quality, and research potential. Clinical Epidemiology, 7, 449–490. doi:10.2147/clep.S91125
- Skogen, J., Bergh, S., Stewart, R., Knudsen, A., & Bjerkeset, O. (2015). Midlife mental distress and risk for dementia up to 27 years later: The Nord-Trøndelag Health Study (HUNT) in linkage with a dementia registry in Norway. BMC Geriatrics, 15(1), 1-10. doi:10.1186/s12877-015-0020-5
- Sumner, J., Hagan, K., Grodstein, F., Roberts, A., Harel, B., & Koenen, K. (2017). Posttraumatic stress disorder symptoms and cognitive function in a large cohort ofmiddle-aged women. Depression and Anxiety, 34(4), 356–366. doi:10.1002/da.22600
- Sundhedsstyrelsen. (2013). National klinisk retningslinje for udredning og behandling af demens. Copenhagen, Denmark.
- Sussman, D., Pang, E., Jetly, R., Dunkley, B., & Taylor, M. (2016). Neuroanatomical features in soldiers with post‐traumatic stress disorder. BMC Neuroscience, 17(1), 1-11. doi:10.1186/s12868-016-0247-x
- Svensson, E., Lash, T., Resick, P., Hansen, J., & Gradus, J. (2015). Validity of reaction to severe stress and adjustment disorder diagnoses in the Danish Psychiatric Central Research Registry. Clinical Epidemiology, 7, 235–242. doi:10.2147/clep.s80514
- Taudorf, L., Nørgaard, A., Islamoska, S., Jørgensen, K., Laursen, T., & Waldemar, G. (2019). Declining incidence of dementia: A national registry-based study over 20 years. Alzheimer’s & Dementia, 15(11), 1383-1391. doi:10.1016/j.jalz.2019.07.006
- Wheelan, N., Kenyon, C., Harris, A., Cairns, C., Al Dujaili, E., Seckl, J., & Yau, J. (2018). Midlife stress alters memory and mood-related behaviors in old age: Role of locally activated glucocorticoids. Psychoneuroendocrinology, 89, 13–22. doi:10.1016/j.psyneuen.2017.12.018
- World Health Organization. (2016). International statistical classification of diseases and related health problems 10th revision. Retrieved from https://icd.who.int/browse10/2016/en
- Yaffe, K., Vittinghoff, E., Lindquist, K., Barnes, D., Covinsky, K. E., Neylan, T., … Marmar, C. (2010). Posttraumatic stress disorder and risk of dementia among US veterans. Archives of General Psychiatry, 67(6), 608–613. doi:10.1001/archgenpsychiatry.2010.61
Appendix
Table A.1. Diagnostic codes for dementia and different morbidities including psychiatric, cardiovascular and metabolic morbidities, head injuries and sleep disorders based on diagnoses from the International Classification of Diseases (ICD) codes of the 8th and 10th revision.
Table A.2. Anatomical Therapeutic Chemical (ATC) codes for identification of anti-dementia medication.
