Abstract
Objective
Anticholinergic burden refers to the cumulative effect of medications which contain anticholinergic properties. We assessed how anticholinergic burden and different types of anticholinergic medications influence mortality rates among people with dementia in Northern Ireland. Our secondary aim was to determine what demographic characteristics predict the anticholinergic burden of people with dementia.
Methods
Data were extracted from the Enhanced Prescribing database for 25,418 people who were prescribed at least one dementia management medication between 2010 and 2016. Information was also extracted on the number of times each available anticholinergic drug was prescribed between 2010 and 2016, allowing the calculation of an overall anticholinergic burden. Cox proportional hazard models were used to determine how anticholinergic burden influenced mortality whilst multilevel model regression determined what demographic characteristics influence overall anticholinergic burden.
Results
Of the 25,418 people with dementia, only 15% (n = 3880) had no anticholinergic burden. Diazepam (42%) and risperidone (18%) were the two most commonly prescribed drugs. Unadjusted Cox proportional hazard models indicated that higher anticholinergic burden was associated with significantly higher mortality rates in comparison to people with dementia who had no anticholinergic burden (HR = 1.59: 95% CI = 1.07-2.36). In particular, urological (HR = 1.20: 95% CI = 1.05-1.38) and respiratory (HR = 1.17: 95% CI = 1.08-1.27) drugs significantly increased mortality rates. People with dementia living in areas with low levels of deprivation had significantly lower anticholinergic burden (HR=-.39: 95% CI=-.47:-30).
Conclusions
Reducing anticholinergic burden is essential for people with dementia. Further research should address the unfavourable prognosis of people living with dementia in highly deprived areas.
Background
Dementia is a major global issue with implications for health services, social provision and wider society (World Health Organization, Citation2012). Dementia is a neurocognitive disorder which causes a progressive or chronic decline in cognitive functioning often leading to disturbances in memory, thinking, language, judgement, and orientation as well as the ability to learn and calculate. In 2015, over 47 million people worldwide were living with dementia and this figure is expected to increase to 66 million in 2030 and 115 million in 2050 (Fox, Livingston, et al., Citation2011). In Northern Ireland, it is estimated that there are currently 19,000 people living with dementia, a number which could potentially increase to 60,000 by 2050 (Department of Health Social Services and Public Safety Northern Ireland, Citation2011). People with dementia are more likely to be taking more comorbidity medications than people without dementia, moreover, some of these comorbidity medications are likely to have anticholinergic properties (Fox, Richardson, et al., Citation2011). Anticholinergic drugs block the neurotransmitter acetylcholine (which is important for thinking and memory) in the peripheral and central nervous system. A well-established link between anticholinergic medication and cognitive decline has been described in the literature in people without (Fox et al., Citation2014; Fox, Richardson, et al., Citation2011) and with dementia.(Karimi, Dharia, Flora, & Slattum, Citation2012) Recently, research showed that in people with mild to moderate Alzheimer’s disease who were prescribed anticholinergic medication had significantly poorer cognition (Dyer et al., Citation2020).
Further observational (cohort and case control) studies have described an associated with anticholinergic medication and increased mortality in people without dementia (Cross et al., Citation2017; Gray et al., Citation2015) and people with dementia (Gray et al., Citation2015; Gray & Hanlon, Citation2016). For instance in a study of 25,825 people with dementia, researchers found that higher anticholinergic burden significantly increased mortality rates (Ah, Suh, Jun, Hwang, & Lee, Citation2019). Furthermore, anticholinergic burden has been shown to negatively affect the treatment response to cholinesterase inhibitors (Ah et al., Citation2019; Carnahan, Lund, Perry, & Chrischilles, Citation2004; Palmer et al., Citation2015). The risks of prescribing both anticholinergic medication and cholinesterase inhibitors have also been highlighted in the literature, as anticholinergic drugs have been found to counteract the effects of cholinesterase inhibitors. It is therefore vital to encourage clinicians to offer alternative treatments to anticholinergic drugs when prescribing medication for individuals with dementia. Anticholinergic drugs can be scored either one, two or three, with higher scores indicating higher anticholinergic properties. Therefore an important distinction is that of anticholinergic burden and anticholinergic drug use. A patient may be using one or several anticholinergic drugs (Salahudeen, Hilmer, & Nishtala, Citation2015). Whereas an individual’s anticholinergic burden refers to the cumulative score of all the prescribed anticholinergic drugs (Fox, Richardson, et al., Citation2011).
Previous research using medication data from Northern Ireland looked at medications prescribed to people with dementia over a one-year period (2013) found a high rate of inappropriate prescribing among people with dementia (Barry et al., Citation2016). Moreover, the same study also found that 25% of people with dementia had been prescribed at least one anticholinergic medication (Barry et al., Citation2016). However, it is not clear which demographic characteristics might influence anticholinergic burden. Our aims for this study were threefold, firstly to assess how anticholinergic burden influences mortality rates among people with dementia in NI. Secondly, to determine which categories of anticholinergic medication (such as ‘antidepressants’ and ‘antipsychotics’) influence mortality, and thirdly to determine what demographic characteristics (such as age, gender, area deprivation) influenced anticholinergic burden.
Methods
Study design and population
Similar to the rest of the United Kingdom the NHS is free at the point of use in Northern Ireland, including prescriptions prescribed by a general practitioner (GP) and dispensed by a pharmacist. In 2008, the Business Service Organisation in Northern Ireland implemented the Enhanced Prescribing database (EPD). The EPD adds a barcode to each medication prescribed by a GP; when the medication is dispensed by a pharmacist this information is then linked to the individual’s health care number and the GP who prescribed it. On a monthly basis, the information contained in each bar code is updated and added to the EPD. Prescriptions in the EPD are coded within the British National Formulary (BNF) sections allowing researchers to analyse the number of medications dispensed from each section of the BNF. Over-the-counter medication is not recorded in the EPD. The index date for this study was the first date that a dementia management medication (BNF section 4.11) was prescribed between January 1st, 2010 and December 31st, 2016, which was used a proxy for dementia diagnosis (Barry et al., Citation2016; McMichael, Zafeiridi, & McGuinness, Citation2020) Demographic characteristics such as gender, marital status, age, and whether the person lives in a rural or urban area were retrieved through other datasets and linked to the EPD by using the patient’s unique health care number. The Northern Ireland Multiple Deprivation Measure (NIMDM) was also available for most individuals in the dataset, the NIMDM ranges from 1 to 10 with 1 indicating the most deprived area and 10 indicating the least deprived areas, meaning that the NIMDM bracket of 7-10 encompasses the 4 least deprived areas (Northern Ireland Statistics and Research Agency, Citation2010). These particular demographic variables have been shown to influence mortality rates and were therefore included in the present study to assess whether they predict anticholinergic burden (McMichael, Zafeiridi, & McGuinness, Citation2020). As we were using secondary data for our analysis, ethical approval for the project was not needed. Moreover, due to the anonymity of the data, informed consent was not required.
Anticholinergic drug exposure
Determining the effect of anticholinergic drugs on the human brain is difficult. Research has shown that bioassays of serum anticholinergic activity does not necessarily correlate strongly with effects on cognition (Boustani, Campbell, Munger, Maidment, & Fox, Citation2008; Salahudeen, Chyou, & Nishtala, Citation2016). Due to this, the anticholinergic effect of drugs is classified using scales developed by literature reviews and expert consensus (Salahudeen et al., Citation2015). For the purpose of this study, we used the 2008 anticholinergic burden (AB) scale (Fox, Richardson, et al., Citation2011). Drugs with potentially anticholinergic effects are assigned a score of 1. Drugs are assigned a score of 2 if they have well established anticholinergic effects and scored a 3 if they have been associated with cognitive decline (Fox, Richardson, et al., Citation2011). Of the 83 drugs on the AB scale, 61 had been prescribed between 1st January 2010 – 31st December 2016 and were recorded in the EPD, 30 had an anticholinergic score of 1, six had a score of 2 and 23 had a score of 3. For each of these drugs we obtained the total number (if any) of times it was prescribed to each person between January 1st, 2010 – December 31st, 2016. We therefore calculated an overall anticholinergic burden (if any) for each person by totalling the scores of the anticholinergic medication that they had been prescribed and categorised the overall burden as 0, 1–4, 5–9, 10–14, ≥15. Using the BNF version 77 and methodology from past research (Fox, Richardson, et al., Citation2011) we further categorised each of the drugs into one of the following; antidepressants, antipsychotics, gastrointestinal, antiparkinsonian, respiratory, urological, antihistamines, drugs which did not fit into these categories were designated as other.
Statistical analysis
The frequencies and means for each of the demographic characteristics were assessed (). A series of independent t-tests were conducted to assess what demographic parameters influenced anticholinergic burden. In order to assess the influence of anticholinergic burden on mortality we conducted a series of unadjusted and adjusted cox proportional hazard model, firstly on overall anticholinergic burden. Secondly on each anticholinergic drug score (1, 2 or 3) and thirdly on each anticholinergic drug class (antipsychotics, antidepressants etc.). In each cox proportional hazard model analysis, the reference group was people with dementia who had not been prescribed any anticholinergic medications between 2010 and 2016. For each model, data from the 1st January 2010 until the date of death (through ICD10 codes) or follow-up (31st December 2016) was used. Before conducting the cox proportional hazard models, we assessed the assumption of proportionality.
Table 1. Demographic characteristics of study population.
Due to the hierarchal structure of the data (Hox et al., Citation2018), multilevel regression models were used. Anticholinergic burden was used as a continuous outcome to assess the influence of demographic characteristics on anticholinergic burden. All statistical analysis was conducted in Stata version 14 (Statacorp, CollegeStation, TX) at the safe setting within the BSO and all results received intermediate and final clearance prior to publication.
Results
The final dataset included 25418 people who were prescribed at least one dementia management medication between 2010 and 2016. highlights that at the time of analysis, 13289 people were alive. 65% of the study population was female (n = 16537) and the mean age was 77.2 (SD = 8.3) years. 32% of people were married, however, for 8973 people (35%), information on marital status was unavailable. Most people lived in an urban area (69%) and almost half (42%) lived in the least deprived areas.
3880 people were prescribed no anticholinergic medications between 2010 and 2016. In 56% of the cohort the anticholinergic burden ranged from 1 to 4; only 89 people (.35%) had an anticholinergic burden of 15 or above (). The most commonly prescribed anticholinergic medications were diazepam (42.4%), risperidone (18.05%), quetiapine (16.6%), isosorbride preparations (10.6%) and warfarin (10%). Independent t-tests showed that there was no significant difference in the average anticholinergic burden between males and females (t(25416)=-1.55; p = 0.11); people who lived in rural areas had significantly higher anticholinergic burden than those who lived in urban areas (t(24689)=8.42; p<.001).
, model 1 highlights that higher anticholinergic burden is associated with significantly higher mortality rates when compared to people with dementia who have no anticholinergic burden, this trend is also evidence in Increased age and being of male gender significantly increased mortality rates. In comparison to people who were married, people who were divorced/separated, single or widowed had significantly higher mortality. Urban/rural divide and deprivation measure did not significantly influence mortality rates. When comparing each anticholinergic medication score () we found that only anticholinergic drugs with a score of 1 significantly increased mortality (HR = 1.25; 95%CI = 1.20–1.31). Moreover, when we investigated anticholinergic drug class (), we found that ‘respiratory’ (HR = 1.17; 95%C = 1.08–1.27), ‘urological’ (HR = 1.20; 95%C = 1.05–1.38) and ‘other’ (HR = 1.28; 95%C = 1.22–1.34) categories significantly increased mortality. Multilevel regression modelling () indicated that increasing age was associated with significantly lower anticholinergic burden. Furthermore, when compared to people who were married, all other categories had a lower anticholinergic burden, with those who were single at the time of analysis having a significantly lower anticholinergic burden (despite being married significantly decreasing mortality rates). Gender or urban/rural divide did not influence anticholinergic burden. However, people in the least deprived areas had significantly lower anticholinergic burden, when compared to people in the most deprived areas (β=-.39; 95%CI=-.47:-.30) ().
Figure 1. Survival function derived from cox proportional hazard regression adjusted for covariates in . Y axis denotes estimated survival percentages. X axis is time in years. Increase in aggregate anticholinergic burden was associated with significantly higher mortality rates among people with dementia.
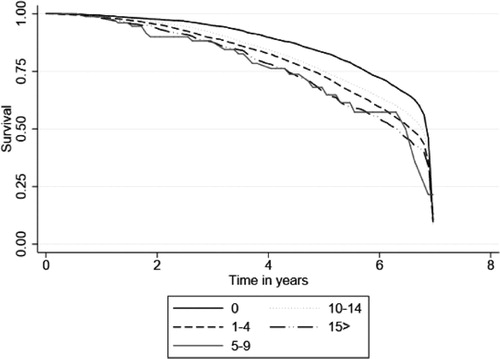
Table 2. Cox proportional hazard model assessing the influence of overall anticholinergic burden and demographic characteristics on mortality.
Table 3. Cox proportional hazard model assessing the influence of each anticholinergic drug score and demographic characteristics on mortality.
Table 4. Cox proportional hazard model assessing the influence of anticholinergic drug class and demographic characteristics on mortality.
Table 5. Multi-level regressions with total anticholinergic burden as the outcome variable and demographic characteristics as predictors.
Discussion
In this study, we aimed to determine the effect of anticholinergic medication on people with dementia in Northern Ireland and to determine what demographic parameters might influence anticholinergic burden. To the best of our knowledge this is the first study to use data from the Enhanced Prescribing Database (EPD) in Northern Ireland to investigate this research question over more than a one-year period. Previous population-based studies have indicated that sustained use of anticholinergic drugs has been associated with an elevated risk of receiving a dementia diagnosis and increased mortality (Chatterjee et al., Citation2016; Fox, Richardson, et al., Citation2011; Joung, Kim, Cho, & Cho, Citation2019). In this study, we also provide evidence that higher anticholinergic burden significantly increases mortality rates for people with dementia. Our results also showed that the majority of people with dementia had been prescribed at least one anticholinergic drug between 2010 and 2016. Indeed, only 15% (n = 3880) had no anticholinergic burden. However, it is also important to note that the majority of people with dementia had an anticholinergic burden of between one and four (56.6%) and only .35% (n = 89) had a total anticholinergic burden of 15 or above. Moreover, of the five most prescribed anticholinergic drugs in our dataset, four had an anticholinergic score of one (diazepam, risperidone, isosobride preparations and warfarin) and one had an anticholinergic score of three (quetiapine). Although antipsychotic medications such as quetiapine were routinely prescribed to treat agitation and aggression in people with dementia (Corbett, Burns, & Ballard, Citation2014), the rates at which these drugs are prescribed has been in sharp decline due to their high anticholinergic properties (Corbett et al., Citation2014). Indeed, a growing body of evidence has indicated that antipsychotics have questionable benefits for people with dementia, but can have serious side effects (Guthrie, Clark, & Mccowan, Citation2010; Schneider et al., Citation2006; Schulze et al., Citation2013). However, given the high anticholinergic score of quetiapine, future research could investigate alternative pharmacological or non-pharmacological treatments in dementia to help manage high levels of agitation in dementia. Nevertheless, these descriptive statistics may suggest that, were possible, clinicians in Northern Ireland are attempting to keep overall anticholinergic burden low and prescribing mild anticholinergic drugs when possible.
Our cox proportional hazard models showed that higher anticholinergic burden was associated with higher mortality rates among people with dementia in our cohort. Similar to past studies assessing mortality rates in dementia, we also noted that being of male gender and increased age significantly increases mortality rates (Tan et al., Citation2018; Garcia-Ptacek et al., Citation2014; McMichael, Zafeiridi, & McGuinness, Citation2020). On the other hand, being married and living in some of the least deprived areas, significantly decreased mortality (). However, in a somewhat paradoxical finding, our multi-level regression modelling suggested that although being married decreased mortality, married people were more likely to have significantly higher anticholinergic burden than people with dementia who were not married. This could be because people who are married may be more likely to have someone to notice the early signs of dementia and a carer throughout different stages of the disease. However, married people may also be more likely to have someone who ensures that they take their prescribed medication, some of which may be high in anticholinergic properties. We also found that people who were older than eighty-five had a lower anticholinergic than the younger age categories (), possibly suggesting that some anticholinergic medications may be withdrawn in the later stages of dementia, due to no longer being beneficial.
highlights that only anticholinergic drugs with a score of 1 significantly increased mortality rates (), anticholinergic drugs with a score of 3 also increased mortality rates, but the effect was not significant. Although counterintuitive, this finding could be explained by noting the number of drugs in each class, thirty had a score of one, six drugs had a score of two and twenty-five had a score of 3, therefore the non-significance significance of drugs with a score of three may be due to a lack of statistical power. In 2014 Richardson et al (Fox, Richardson, et al., Citation2011), found that different classes of anticholinergic drugs (predominantly urological and respiratory) significantly influenced the risk of receiving a dementia diagnosis. When the available drugs in our study were categorised we found that respiratory and urological drugs significantly increased mortality rates. With this combination of results in mind, it is essential for future research to determine why these categories of anticholinergic drugs in particular, increase both the incidence rates of dementia and mortality rates of people with dementia. One potential explanation could be the stage of dementia at which urological and respiratory drugs are prescribed. If such drugs are prescribed in the later stages of dementia to control for incontinence and respiratory difficulties, it is likely that this will cause an increase in mortality rates. Urinary incontinence is often a side-effect of cholinesterase inhibitors so clinicians should be alert to this before prescribing a urological drug to a patient with dementia on a cholinesterase inhibitor. A change to Memantine may be more appropriate.
Urban/rural classification did not significantly influence mortality rates in people with dementia in our cohort. However, t-test and multi-level regression analysis showed that in comparison to people living in an urban area, living in a rural area significantly increased overall anticholinergic burden (). Access to health resources (such as GP appointments) may be poorer in rural areas, which may lead to larger prescriptions of medications, due to the perceived time before another GP appointment is available. In Northern Ireland, we have a statistical measure known as the Northern Ireland Multiple Deprivation Measure (NIMDM) (Northern Ireland Statistics and Research Agency, Citation2010). The NIMDM ranges from one to ten with ten indicating the lowest level of deprivation and one indicating the highest. As with past research (McMichael, Zafeiridi, & McGuinness, Citation2020; van de Vorst, Koek, Stein, Bots, & Vaartjes, Citation2016), we found that people with dementia who live in more deprived areas had significantly higher mortality rates in comparison to people with dementia who lived in areas with the least deprivation (). However, we also showed that people in the most deprived areas are likely to have a significantly higher anticholinergic burden in comparison to people with dementia in the least deprived areas. Using data from Northern Ireland, past research has shown treatment inequalities in the prescription of anti-dementia drugs, with people living in the least deprived areas being 25% more likely to be initiated on anti-dementia quicker than people in the most deprived areas (Cooper et al., Citation2016). Here we provide initial evidence that there may be treatment inequalities in the prescription of anticholinergic drugs, as we found that people who lived in the most deprived areas have significantly higher anticholinergic burden in comparison to people in the least deprived areas ().
In the present study we found that among our dementia cohort, 82% of people had an anticholinergic burden and preliminary research has now begun to assess the anticholinergic burden among people without dementia. For instance, the Northern Ireland Cohort of Longitudinal Ageing (NICOLA) is a longitudinal, representative study, which is currently following 8,500 people from Northern Ireland across different waves of data collection to understand the dynamics of ageing in Northern Ireland (O’reilly, Rosato, Catney, Johnston, & Brolly, Citation2012). Preliminary analysis from wave 1 of the NICOLA study has shown that 82% of people have no anticholinergic burden (McMichael, Zafeiridi, & McGuinness, Citation2020), in comparison to 82% of people with dementia who do have an anticholinergic burden in the present study. This difference in anticholinergic burden between people in the general population and people with dementia raises concerns and questions for future research. In particular, future research needs to address the underlying reasons as to why anticholinergic burden increases after a dementia diagnosis, despite the existing body of evidence attesting to the dangers of prescribing anticholinergic drugs to people with dementia (Durán, Azermai, & Vander Stichele, Citation2013; Gray & Hanlon, Citation2016). Moreover, longitudinally following the current wave 1 NICOLA participants over future waves may allow us to establish whether those exposed to anticholinergic drugs in wave 1 are more likely to receive a dementia diagnosis.
We propose that de-prescribing may be an eligible method of reducing the number of medications people with dementia are being prescribed (Barry et al., Citation2016; Scott et al., Citation2015). As a case in point, ‘drug holidays’, whereby a medication is withdrawn for a given period of time to assess its treatment utility or withdrawal symptoms, could act as the first stepping stone in reducing anticholinergic burden until other potential non-pharmacological treatments are developed (Barry et al., Citation2016; Howland, Citation2009). However, we also appreciate the ethical and complicated considerations in de-prescribing for people with dementia, as people with dementia are likely to be have an irreversible capacity to be involved in their treatment decisions. Further research is needed to assess the efficacy of de-prescribing medications in an attempt to decrease anticholinergic burden.
Limitations
To the best of our knowledge, this is the first study in Northern Ireland to assess the effects of anticholinergic drugs on people with dementia in Northern Ireland. Although, the EPD holds a wealth of data which has great utility for researchers and whilst we have confidence in our results, it is important to note some potential limitations of our study. Due to the nature of the data, we had to use the first date a dementia management medication was prescribed as a date of diagnosis, although it is likely that the official date of diagnosis was before any medication was prescribed. Using our proxy measure, we were unable to assess the effects of anticholinergic drugs on different types of dementia. Additionally, using our proxy measure for date of diagnosis, it is likely that people with vascular and frontotemporal dementia were not included in our analysis, as cholinesterase inhibitors are not prescribed for these dementia subtypes. Although the EPD is a useful resource for researchers, it currently gathers between 80% and 90% of all medications dispensed by a pharmacist, meaning that some people with dementia may not be in the dataset. It is possible to buy over the counter medications (OTC) which have varying levels of anticholinergic properties, however, these medications are not recorded in the EPD, meaning that there could potentially be an under estimation of the total anticholinergic medications each person was taking between 2010 and 2016. Furthermore, the data for this study did not account for other comorbidity medications or health behaviours such as smoking or alcohol consumption. Moreover, given the different benefit-risk trade-off of each drug and the interaction between different drugs it is possible that the people in our study population may not have fully adhered to their prescribed treatment. It also must be taken into consideration that there are numerous different anticholinergic scales available, some of which give the same drugs different scores (Durán et al., Citation2013), therefore our results may have been influenced by our scale selection. Finally, we must emphasise for the marital status variable, 35% of the data was missing, furthermore, the marital status was recorded at baseline (2010) but at different times between 2010 and 2016, thus our findings with regard to the influence of marital status on mortality and anticholinergic burden, should be interpreted with caution.
Conclusions
Higher anticholinergic burden was significantly associated with higher mortality among people with dementia in Northern Ireland. Respiratory and urological drugs in particular significantly increased mortality. Clinicians and researchers should continue to assess and decrease the use of anticholinergic drugs among people with dementia.
Acknowledgements
The authors would like to acknowledge the help provided by the Health and Social Care Board in Northern Ireland, and by the staff of the Honest Broker Service.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ah, Y. M., Suh, Y., Jun, K., Hwang, S., & Lee, J. Y. (2019). Effect of anticholinergic burden on treatment modification, delirium and mortality in newly diagnosed dementia patients starting a cholinesterase inhibitor: A population-based study. Basic & Clinical Pharmacology & Toxicology, 124(6), 741–748. doi:10.1111/bcpt.13184
- Barry, H. E., Cooper, J. A., Ryan, C., Passmore, A. P., Robinson, A. L., Molloy, G. J., … Hughes, C. M. (2016). Potentially inappropriate prescribing among people with dementia in primary care: A retrospective cross-sectional study using the enhanced prescribing database. Journal of Alzheimer's Disease, 52(4), 1503–1513. doi:10.3233/JAD-151177
- Boustani, M., Campbell, N., Munger, S., Maidment, I., & Fox, C. (2008). Impact of anticholinergics on the aging brain: A review and practical application. Aging Health, 4(3), 311–320. (Vol. Issue doi:10.2217/1745509X.4.3.311
- Carnahan, R. M., Lund, B. C., Perry, P. J., & Chrischilles, E. A. (2004). The concurrent use of anticholinergics and cholinesterase inhibitors: Rare event or common practice? Journal of the American Geriatrics Society, 52(12), 2082–2087. doi:10.1111/j.1532-5415.2004.52563.x
- Chatterjee, S., Bali, V., Carnahan, R. M., Johnson, M. L., Chen, H., & Aparasu, R. R. (2016). Anticholinergic medication use and risk of dementia among elderly nursing home residents with depression. The American Journal of Geriatric Psychiatry : official Journal of the American Association for Geriatric Psychiatry, 24(6), 485–495. doi:10.1016/j.jagp.2015.12.011
- Cooper, C., Lodwick, R., Walters, K., Raine, R., Manthorpe, J., Iliffe, S., & Petersen, I. (2016). Observational cohort study: Deprivation and access to anti-dementia drugs in the UK. Age and Ageing, 45(1), 148–154. doi:10.1093/ageing/afv154
- Corbett, A., Burns, A., & Ballard, C. (2014). Don’t use antipsychotics routinely to treat agitation and aggression in people with dementia. Bmj, 349, g6420.
- Cross, A. J., George, J., Woodward, M. C., Ames, D., Brodaty, H., Wolfe, R., … Elliott, R. A. (2017). Potentially inappropriate medication, anticholinergic burden, and mortality in people attending memory clinics. Journal of Alzheimer's Disease, 60(2), 349–358. doi:10.3233/JAD-170265
- Department of Health Social Services and Public Safety Northern Ireland (2011). Improving Dementia Services in Northern Ireland. November 2011. http://www.dhsspsni.gov.uk/improving-dementia-services-in-northern-ireland-a-regional-strategy-november-2011.pdf.
- Durán, C. E., Azermai, M., & Vander Stichele, R. H. (2013). Systematic review of anticholinergic risk scales in older adults. European Journal of Clinical Pharmacology, 69(7), 1485–1496. doi:10.1007/s00228-013-1499-3
- Dyer, A. H., Murphy, C., Segurado, R., Lawlor, B., & Kennelly, S. P, for the NILVAD Study Group (2020). Is ongoing anticholinergic burden associated with greater cognitive decline and dementia severity in mild to moderate Alzheimer’s disease? The Journals of Gerontology: Series A, 75(5), 987–994. doi:10.1093/gerona/glz244
- Fox, C., Livingston, G., Maidment, I. D., Coulton, S., Smithard, D. G., Boustani, M., & Katona, C. (2011). The impact of anticholinergic burden in Alzheimer’s dementia-the laser-AD study. Age and Ageing, 40(6), 730–735. doi:10.1093/ageing/afr102
- Fox, C., Richardson, K., Maidment, I. D., Savva, G. M., Matthews, F. E., Smithard, D., … Brayne, C. (2011). Anticholinergic medication use and cognitive impairment in the older population: The medical research council cognitive function and ageing study. Journal of the American Geriatrics Society, 59(8), 1477–1483. doi:10.1111/j.1532-5415.2011.03491.x
- Fox, C., Smith, T., Maidment, I., Hebding, J., Madzima, T., Cheater, F., … Young, J. (2014). The importance of detecting and managing comorbidities in people with dementia? Age and Ageing, 43(6), 741–743. doi:10.1093/ageing/afu101
- Garcia-Ptacek, S., Farahmand, B., Kåreholt, I., Religa, D., Cuadrado, M. L., & Eriksdotter, M. (2014). Mortality risk after dementia diagnosis by dementia type and underlying factors: a cohort of 15,209 patients based on the Swedish Dementia Registry. Journal of Alzheimer's Disease, 41(2), 467–477. doi:10.3233/JAD-131856
- Gray, S. L., & Hanlon, J. T. (2016). Anticholinergic medication use and dementia: Latest evidence and clinical implications. Therapeutic Advances in Drug Safety, 7(5), 217–224. doi:10.1177/2042098616658399
- Gray, S. L., Anderson, M. L., Dublin, S., Hanlon, J. T., Hubbard, R., Walker, R., … Larson, E. B. (2015). Cumulative use of strong anticholinergics and incident dementia: A prospective cohort study. JAMA Internal Medicine, 175(3), 401–407. doi:10.1001/jamainternmed.2014.7663
- Guthrie, B., Clark, S. A., & Mccowan, C. (2010). The burden of psychotropic drug prescribingin people with dementia: a populationdatabase study. Age and Ageing, 39(5), 637–642. doi:10.1093/ageing/afq090
- Howland, R. H. (2009). Medication holidays. Journal of Psychosocial Nursing and Mental Health Services, 47(9), 15–18. doi:10.3928/02793695-20090804-01
- Hox, J. J., Moerbeek, M., & Schoot, R. V. (2018). Multilevel analysis: Techniques and applications. New York: Routledge Taylor & Francis Group.
- Joung, K., Kim, S., Cho, Y. H., & Cho, S. (2019). Association of anticholinergic use with incidence of Alzheimer’s disease: Population-based cohort study. Scientific Reports, 9(1), 6802. doi:10.1038/s41598-019-43066-0
- Karimi, S., Dharia, S., Flora, D., & Slattum, P. (2012). Anticholinergic burden: Clinical implications for seniors and strategies for clinicians. The Consultant Pharmacist®, 27(8), 564–582. doi:10.4140/TCP.n.2012.564
- McMichael, A. J., Zafeiridi, E., & McGuinness, B. (2020). Do anticholinergic drugs influence cognition: Data from wave 1 of the NICOLA study. Manchester, UK: British and Irish Longitudinal Studies.
- McMichael, A. J., Zafeiridi, E., Passmore, P., Cunningham, E. L., & McGuinness, B. (2020). Factors associated with mortality including nursing home transitions: A retrospective analysis of 25,418 people prescribed anti-dementia drugs in Northern Ireland. Journal of Alzheimer's Disease, 73(3), 1233–1242. doi:10.3233/JAD-190751
- Northern Ireland Statistics and Research Agency (2010). Northern Ireland Multiple Deprivation Measure 2017 (NIMDM2017) | Northern Ireland Statistics and Research Agency. Retrieved August 27, 2018, from https://www.nisra.gov.uk/statistics/deprivation/northern-ireland-multiple-deprivation-measure-2017-nimdm2017.
- O’reilly, D., Rosato, M., Catney, G., Johnston, F., & Brolly, M. (2012). Cohort description: The Northern Ireland longitudinal study (NILS). International Journal of Epidemiology, 41(3), 634–641.
- Palmer, J. B., Albrecht, J. S., Park, Y., Dutcher, S., Rattinger, G. B., Simoni-Wastila, L., … Zuckerman, I. H. (2015). Use of drugs with anticholinergic properties among nursing home residents with dementia: A national analysis of medicare beneficiaries from 2007 to 2008. Drugs & Aging, 32(1), 79–86. doi:10.1007/s40266-014-0227-8
- Salahudeen, M. S., Chyou, T. Y., & Nishtala, P. S. (2016). Serum anticholinergic activity and cognitive and functional adverse outcomes in older people: A systematic review and meta-analysis of the literature. PLoS One, 11(3), e0151084. doi:10.1371/journal.pone.0151084
- Salahudeen, M. S., Hilmer, S. N., & Nishtala, P. S. (2015). Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. Journal of the American Geriatrics Society, 63(1), 85–90. doi:10.1111/jgs.13206
- Schneider, L. S., Dagerman, K., & Insel, P. S. (2006). Efficacy and adverse effects of atypical antipsychotics for dementia: Meta-analysis of randomized, placebo-controlled trials. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 14(3), 191–210. doi:10.1097/01.JGP.0000200589.01396.6d
- Schulze, J., van den Bussche, H., Glaeske, G., Kaduszkiewicz, H., Wiese, B., & Hoffmann, F. (2013). Impact of safety warnings on antipsychotic prescriptions in dementia: Nothing has changed but the years and the substances. European Neuropsychopharmacology, 23(9), 1034–1042. doi:10.1016/j.euroneuro.2013.02.001
- Scott, I. A., Hilmer, S. N., Reeve, E., Potter, K., Le Couteur, D., Rigby, D., … Martin, J. H. (2015). Reducing inappropriate polypharmacy: The process of deprescribing. JAMA Internal Medicine, 175(5), 827–834. doi:10.1001/jamainternmed.2015.0324
- Tan, E. C., Eriksdotter, M., Garcia-Ptacek, S., Fastbom, J., & Johnell, K. (2018). Anticholinergic burden and risk of stroke and death in people with different types of dementia. Journal of Alzheimer's Disease, 65(2), 589–596.
- van de Vorst, I. E., Koek, H. L., Stein, C. E., Bots, M. L., & Vaartjes, I. (2016). Socioeconomic disparities and mortality after a diagnosis of dementia: Results from a nationwide registry linkage study. American Journal of Epidemiology, 184(3), 219–226. doi:10.1093/aje/kwv319
- World Health Organization. (2012). Dementia: A public health priority. Dementia, 112https://doi.org/9789241564458
