?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives:
Early detection of mild cognitive impairment (MCI) is necessary to prevent irreversible brain damage caused by incipient Alzheimer’s disease. It has been showing that amnestic MCI (a-MCI) subjects exhibit subtle deficits in executive function that can be tested using saccade eye movements. Eye-tracking technology is a sensitive method to measure cognitive impairments in dementia and MCI.
Methods
In this study, we used eye-tracking technology to explore saccade impairments to distinguish between a-MCI and the variants of reference controls. 21 patients with AD, 40 patients with a-MCI, and 59 normal participants were recruited in current study. We measured saccade reaction time, saccade errors, saccade omission, and uncorrected saccades using anti-saccade and pro-saccade tasks with ‘gap’ and ‘overlap’ procedures. These parameters were used as markers of executive function and visual attention deficits.Results: The findings revealed that more errors, more omissions, and fewer corrections characterized the saccade behavior of the a-MCI group compared to the reference group. These eye-tracking characteristics can be considered as inhibitory control and working memory deficits in a-MCI subjects. Our results thus demonstrate the applicability of the anti-saccade task as a cognitive marker in a-MCI.
Conclusion
The work provides further support for eye-tracking as a useful diagnostic biomarker in the assessment of executive function in aging with cognitive impairments.
Introduction
Increased life expectancy has led to some unwanted consequences such as an increase in the number of individuals affected by common chronic age-related diseases like Alzheimer disease (AD). It is estimated that the number of individuals with AD will double in the next 20 years. Mild cognitive impairment (MCI) is a state of cognitive changes that in most cases represents the initial phase of AD or other dementias (Donaghy et al., Citation2018; Pereira, Camargo, Aprahamian, & Forlenza, Citation2014), therefore, early diagnosis of MCI is recommended as a vital step in the management and timely interventions of dementia (Limongi et al., Citation2018).
Attention and executive impairment are frequent and disabling symptoms in MCI when measured with neuropsychological tests ranging from simple processing speed tasks to tasks of complex problem-solving (Peltsch, Hemraj, Garcia, & Munoz, Citation2014). These deficits have emotional and functional implications (Kiosses & Alexopoulos, Citation2005) and effects in activities of daily living these patients (Lee, Jang, & Chang, Citation2019). Moreover, it was suggested that patients with AD in a pre-clinical phase may have deficits in executive function, visuospatial skills and attentional control, before memory (Alichniewicz, Brunner, Klunemann, & Greenlee, Citation2013; Amieva, Phillips, Della Sala, & Henry, Citation2004; Greenwood, Parasuraman, & Alexander, Citation1997; Pereira et al., Citation2014). However, the applicability of neuropsychological measures to assess executive deficits is limited in this group because they are time consuming and participants may experience psychological distress during performing the test (Oyama et al., Citation2019). Also, they mostly require movement dexterity and language. Therefore, several researchers focused on attentional deficits in early identification of a-MCI through measuring changes in eye movements of the suspected cases (Nakashima, Morita, Ishii, Shouji, & Uchimura, Citation2010).
Eye movements are controlled by multiple cognitive functions, including working memory and inhibitory control (Crawford, Parker, Solis-Trapala, & Mayes, Citation2011; Crawford & Higham, Citation2016) that have been extensively used for the assessment of attention and cognitive control (10–12). Eye movement deficits can reveal cognitive impairment before routine neuropsychological measures (Crawford & Higham, Citation2016) and they are strongly correlated with severity of AD and degree of cortical atrophy in MCI (Crawford et al., Citation2005; Heuer et al., Citation2013). Recently it was investigated that eye-tracking technology focusing on gaze and region of interests (ROI) are highly correlated with neuropsychological measures and can be useful in distinguishing and timely intervention of people with MCI (Oyama et al., Citation2019). To investigate saccade characteristics in a-MCI, AD, and normal aging, we employed tasks ideal for testing executive function. The two levels of saccadic control which investigated in the present study were pro-saccadic and anti-saccadic control. The pro-saccade task requires fast automatic responses with a rapid reflex of the eye to a novel target. The anti-saccade task requires avoidance of the new target with an eye movement toward the opposite side of the target. This task requires a high level of executive processing (Peltsch et al., Citation2014) and is acknowledged as a sensitive tool to evaluate executive function (Hellmuth et al., Citation2012), cognitive control, inhibition and cognitive changes (Hallett, Citation1978; Luna, Velanova, & Geier, Citation2008; Munoz & Everling, Citation2004). Both saccade functions are related to frontal oculomotor circuits and can be evaluated by the gap paradigm and the overlap paradigm. During the gap condition, the central fixation offset for 200 ms before the onset of stimulus. In the overlap task, the central fixation and peripheralW target remained simultaneously for the period of 200 ms. (Yang, Wang, Su, Xiao, & Kapoula, Citation2013). This can evaluate automatic and controlled initiated saccades involving different cortical–subcortical ocular motor networks. Gap and overlap tasks were used to assess attentional disengagement (Crawford et al., Citation2013).
The current study aims to evaluate the extent of saccade deficits in a-MCI. In this study, we propose to test the capacity of saccade eye movement tasks to discriminate a-MCI patients from healthy older adults. More particularly, we measured several saccade parameters (e.g. saccade amplitude and reaction time, errors rates, omission, uncorrected saccades) to clarify whether these markers are sensitive enough to clearly distinguish between healthy aging and pathological conditions (i.e. MCI and AD vs controls). Ideally, we would distinguish the neurocognitive impairments in AD from any nonspecific effects due to poor comprehension, motivation or abnormalities in sensorimotor functions. Our main objective was to determine whether these saccade tasks would predict executive function in MCI patients and supporting the potential use of the saccade task to detect executive dysfunction and potentially predict cognitive decline in normal aging and MCI. This is important for clinical practice as we currently do not have the sensitive tools to detect these subtle changes while early diagnosis and timely intervention can delay cognitive decline.
Material and methods
Participants
There were 120 participants included in this experimental study.
Inclusion criteria for a-MCI
The a-MCI group consisted of 40 multiple-domain a-MCI patients who were recruited from Memory Clinic of the University Hospital (Rofaydeh) as well as University Brain and Cognition Center, Tehran, Iran. The MCI diagnosis was based on Petersen criteria that were performed in mentioned above centers. These criteria include memory problems, objective memory disorder, absence of other cognitive disorders or repercussions on daily life, normal general cognitive function, and absence of dementia (Mild cognitive impairment (MCI) in medical practice (Petersen et al., Citation1999). Moreover, a-MCI subjects had to have a Mini-Mental Examination (MMSE) ≥ 22 which was the best cut-off for use in assessing and differentiating control versus cognitively impaired individuals in the Persian-speaking population (Foroughan, Zahra, Bayan, Faraahani, & Mahdi, Citation2008). We also applied Addenbrooke’s Cognitive Examination (ACE) ≥ 85 for a-MCI group and (ACE) ≥ 78 for AD group (Pouretemad, Khatibi, Ganjavi, Shams, & Zarei, Citation2009). The AD group (N = 21) comprised mild to moderate AD patients recruited from above mentioned centers in Tehran (Iran) in 2017 ().
Table 1. Demographic information and psychometric test scores.
Inclusion criteria for AD
All the AD patients fulfilled the probable Alzheimer disease criteria based on the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-V) that were evaluated by psychiatrist or neurologist in both mentioned clinics. Furthermore, a review of clinical history and physical examination of patients added from the clinics as well.
Inclusion criteria for control
The control group was recruited from age-matched community-dwelling elder volunteers in Tehran (N = 59) with no known cognitive difficulties.
In next step all recruited patients along with control group were evaluated in the psychology laboratory of Shahid Beheshti University by neuropsychological testing. All neuropsychological tests were conducted by two independent gerontologists.
Exclusion criteria for all groups
Another neurological or neuropsychiatric disorder, depression, deficits in activities of daily living, head trauma, substance abuse or using a medication that is known to affect cognition, ophthalmological diseases, such as glaucoma or macular degeneration, abnormal visual acuity according to Snellen chart. All demographic and neuropsychological assessments of participants are summarized in .
Measures
General cognitive status was evaluated by MMSE and ACE. Episodic memory was assessed by Rey auditory verbal learning test (RVALT) (Jafari, Steffen Moritz, Zandi, Kamrani, & Malyeri, Citation2010). Patients with AD and a-MCI were interviewed to assess functional disabilities using the Persian version of the Clinical dementia rating scale (CDR) (Sadeghi, Noroozian, Khalaji, & Mokhtari, Citation2012) and the 15-Item Persian Geriatric Depression Scale (GDS) for screening depression (Malakouti, Fatollahi, Mirabzadeh, Salavati, & Zandi, Citation2006). The extent of deficits in activities of daily living was assessed by the Barthel Index (Hormozi et al., Citation2019).
Procedure
This study was approved by the ethics committee of the University of social welfare and rehabilitation sciences, Tehran (Iran) with ethics code: IR.USWR.REC.1395.250. After a providing a detailed description of the study, written informed consent was obtained from all participants. For the AD patients we also asked the caregivers to read and sign the informed consent
Eye-tracking procedure
Eye movements were recorded using a remote desktop eye tracker SMI RED system (SensoMotoric Instruments). The sampling rate of the SMI system was 220HZ and the optimal resolution was approximately 0.01°. The stimuli were presented on a 22-inch computer screen using iViewX (SensoMotoric Instruments) software and data were collected by BeGaze (SensoMotoric Instruments software). The subjects were seated approximately 60 cm in front of a flat monitor in an adjusted-lit room during the eye-tracking procedure.
A nine-point calibration sequence was performed at the start of both gap and overlap experiments and the participants were instructed both by text on the computer monitor and verbally. All recording and calibration were binocular. During the experiment, the central fixation point was a cross sign presented at the center of the computer screen with a white background. The target stimulus was a red dot at an eccentricity of 10°, randomly displayed at the left or right of the fixation cross. The participant was comfortably seated on a chair while they rested their chin on a chin rest with head support.
Assessment of saccadic eye movements
Each experiment was preceded by written and oral instructions. Then 5 practice trials were performed to ensure that the participants understood the task. In the pro-saccade trials (PST), the subjects were requested to look toward the target until it disappeared. While in the anti-saccade trials (AST) they were asked to look in the opposite direction from the visual target and to correct themselves if they made a mistake. All participants confirmed verbally that they understood the practice trials and asked to look as rapidly and accurately as possible. The experiment comprised two blocks, each block consisted only of a gap or an overlap paradigm (see below). The targets were presented randomly in each horizontal direction and in both PST and AST equally. In total, each block consisted of 96 trials and lasted about 5 min, making a total of 192 trials across the two blocks, with 10 min break between the tasks to avoid fatigue. Error rate (%) reflects the percentage of error trials over the total number of valid trials and is considered as a measure of inhibitory control, working memory and the ability to appropriately activate a volitional response. It must be mentioned that the ability to sustain attention is an important correlate of the ability to evoke correct erroneous response, the principal index of inhibitory control and error monitoring. In this study, the spatial precision of saccadic eye movements (i.e. saccade amplitudes) was measured, towards and away from the target, to identify any general deficits of sensorimotor function (Crawford et al., Citation2013). A saccade omission occurs when a participant fails to generate a saccade on a given trial; they provide an index of sustained attention and task compliance (Kaiser, Kuhlmann, & Bosnjak, Citation2018).
Gap saccade task
A central fixation cross appeared at the start of each trial, that was presented for 1000 or 1500 milliseconds randomly. In the last 500 ms, it converted to green in PST and to red in AST. The fixation cross disappeared for a period of 200 ms (i.e. Gap) before the onset of target stimuli and subsequently, the eccentric saccade target was visible for 2000 ms. Between trials, a blank page was shown for 1200 ms.
Overlap saccade task
The stimuli were identical to the gap paradigm except for the timing of central fixation offset. Here the fixation cross was displayed for 200 ms during the target presentation. The target stayed on for another 2000 ms.
In both tasks, identical stimuli were presented randomly and bottom-up and top-down cognitive control is used by a change of instruction of the tasks.
Data analysis
In this 2 × 2 experimental design using the following factors: saccadic paradigm (PTS and AST) + Task Format (GAP and Overlap), all raw eye-tracking data were analyzed offline by custom-made MATLAB software (Version R2013a; The MathWorks, Natick, MA, USA). Saccades with an amplitude of less than 1 degree also were filtered from the data. All frames that contained artifacts like noise and spike, loss of pupils and eye blinks were excluded. Saccade onset and offset were defined as the point in time at which the velocity crossed 30(Crawford et al., Citation2005, Citation2013). All saccades directed toward the target < 80 s after its appearance were defined as anticipation. Saccade measures included saccade reaction time (time to initiate saccades), saccade omission (fails to generate a saccade on a given trial), and number of anti-saccade errors (saccade in the direction of stimuli, the direction of saccades was defined by the eye position difference between the start and the end of the saccade) and uncorrected saccade (uncorrected triggered saccades toward the target).
Statistical analysis
Statistical analysis was completed with SPSS version.22. For each of the variables of interest (saccade reaction time, error proportion, omission and uncorrected saccades) a mixed repeated measures ANOVA was performed with two within-group factors with two level each, task (pro, anti) and material (gap, overlap), and one between-group factor at three levels (AD, MCI, Control). Post-hoc comparison applied the Bonferroni alpha adjustment. The Receiver Operating Characteristics (ROC) analysis, sensitivity, and specificity were calculated to assess the diagnostic capacity of error rates, omission and uncorrected responses that showed significant main effect between a-MCI and normal subjects. The effect size of saccade measures was calculated by Cohen’s d that was categorized as a large effect size when it was ≥ 0.8 (Cohen, Citation2013). Pearson correlation applies to assess the correlation between general cognitive measures and eye tracking measures.
Results
Neuropsychological data
There was no significant difference in age and gender among groups as shown in . However, the control group had significantly higher years of education compared to others. As expected, the AD group showed greater impairment on the cognitive scores compared to the controls and a-MCI subjects on the MMSE and ACE total score, Rey total score and CDR. Compared to the normal group, a-MCI and AD groups showed poorer performance on verbal fluency (both letter and animal) on the ACE sub-scales and performed worse in visuospatial function measured by the ACE task. All ACE sub-scales were significantly different between a-MCI and control groups. Furthermore, the overall degree of cognitive impairments was significantly different in the 3 groups.
Eye-tracking data
Saccade reaction time
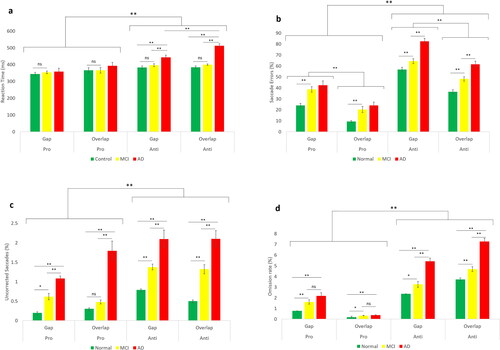
The results of mixed repeated measures ANOVA showed that there was not a significant interaction between group and task paradigms (p = 0.07), while there was a significant effect of task paradigm (gap versus overlap) (F (1,116) = 58.61, p < 0.001, η2 = 0.33) showing faster saccades in the gap task compared to the overlap task (gap effect) (). In terms of saccade direction, the reaction time was significantly higher in AST than PST (F (1,116) = 9.26, p = 0.003, η2 = 0.07). The result of three-way ANOVA revealed no interaction between task paradigms, direction of saccades and group of participants (F (2,117) = 1.0.8, p = 0.34). Moreover, group had significant main effect on saccade reaction time (F (2,117) = 18.23, p < 0.001, η2 = 0.12). Further post-hoc analysis showed no significant difference between a-MCI and healthy controls (p = 0.77) while saccades reaction time was significantly different between AD patients, control group and a-MCI subjects (p < 0.001).
Saccadic errors
Interestingly, we could indicate that the group showed significant interaction with task paradigm (gap versus overlap) in percentage of erroneous saccade, (F (2,111) = 4.23, p = 0.01, η2 = 0.07). In addition, the percentage of erroneous saccade were significantly different between task paradigms (gap versus overlap), (F (1,111) = 44.17, p < 0.001, η2 = 0.79) In contrast, there was no significant interaction between the group of participants and stimulus direction (AST vs PST) (F (2,111) = 0.35, p = 0.69, η2 = 0.006). Nevertheless, the error proportions were significantly different according to task direction (AST vs PST) (F (1,111) = 18.39, p < 0.001, η2 = 0.61) with higher percentage in AST compared to PST Furthermore, three-way ANOVA showed no significant interaction between group of participants, task paradigm and saccade directions (F (2,111) = 18.39, p = 0.34, η2 = 0.61). While the group had significant main effect on error proportion (F (2,111) = 63.69, p < 0.001, η2 = 0.53). Pairwise comparison indicated elevated errors in both a-MCI and AD (p < 0.001) compared to control ().
Uncorrected errors
Considering uncorrected errors, we found significant interaction between group and task paradigm (gap vs overlap) (F (2,117) = 6.14, p = 0.003, η2 = 0.09) and stimulus type (AST vs (PST) (F (2,117) = 5.58, p = 0.005, η2 = 0.08). In addition, number of uncorrected saccades showed a statistically significant interaction between task paradigm, stimulus direction and group of participants (F (2,117) = 4.42, p = 0.01, η2 = 0.07). Furthermore, a significant main effect of the group of participants was found (F (2,117) = 14.89, p < 0.001, η2 = 0.71). Pairwise comparison showed that the main effect of group on uncorrected saccades was significantly different between three groups of patients (p < 0.001) ()
Saccade omission
The results significant interaction between group and paradigm type (F (2,117) = 40.26, p < 0.001, η2 = 0.90), but there was not any significant interaction between stimulus direction and group of participants (F (2,117) = 0.37, p = 0.68, η2 = 0.006). Furthermore, 3-way ANOVA indicated significant interaction between group, direction of saccades and task paradigms direction (F (2,117) = 6.76, p = 0.002, η2 = 0.10). Group of participants had significant effect on saccade omission (F (2,117) = 11.45, p < 0.001, η2 = 0.60). Pairwise comparison showed that there was significantly different between 3 groups of participants (p < 0.001) ()
Diagnostic accuracy of saccade features
In ROC curve analysis, we found that the Area Under Curve (AUC) for overlap AST error rates was 0.70 (negative cases: control group, positive cases: a-MCI and cut-off score = 38.29) and for gap AST error rates, AUC was 0.75 (cut-off score = 26.36). ROC analysis for target omission also revealed that for the overlap AST, the AUC was 0.69 (cut-off score = 22.74), and for the gap AST, the AUC increased to 0.77(cut-off score = 8.71). Finally, for the uncorrected errors, ROC analysis was as follow: for AST overlap, the AUC was 0.82 (cut-off score = 0.62), the AUC decreased to 0.68 in gap AST (cut-off score = 0.44).
Likelihood analysis (LR) for saccade features accompanied by intermediate LR+. The results showed that error proportion in AST overlap and uncorrected saccades in AST gap and overlap revealed highest sensitivity in detection of a-MCI subjects, respectively. ROC curves for different data are shown in and the sensitivity, specificity and effect size summarized in .
Figure 2. Receiver operating characteristic (ROC) curves comparing best oculomotor and neuropsychological variables to differentiate a-MCI from healthy controls. (a) Comparison of the best oculomotor variable from the eye-tracking analysis (overlap AST errors) versus MMSE, (b) Comparison of overlap AST uncorrected saccades versus MMSE for differentiating a-MCI from control group, (c) comparison of overlap AST omission versus MMSE. (d) comparison of gap AST error versus MMSE, (e) comparison of gap AST uncorrected saccades with the MMSE scores and (f) comparison of gap AST omission versus MMSE in detecting a-MCI from control group.
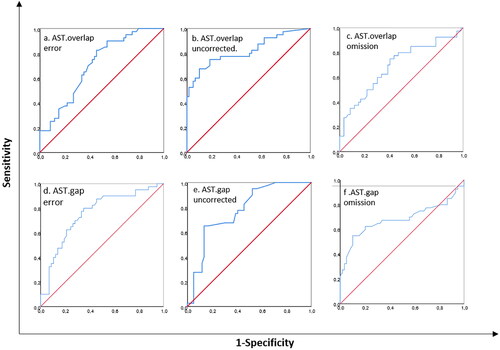
Table 2. Sensitivity and specificity of eye-tracking parameters.
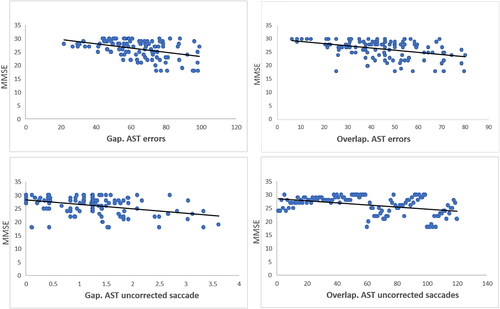
Correlations of performance on the saccade task with MMSE scores
Interestingly we could detect correlation between MMSE scores erroneous and uncorrected saccades (the most sensitive saccade parameters in detecting a-MCI from controls according to ) in a-MCI patients. The Pearson correlation in a-MCI group was significant between MMSE score and saccade errors (r = −0.40 p < 0.05) as well as MMSE score and uncorrected saccades (r = −0.40 p < 0.05) in both gap and overlap tasks in AST which is presented in .
Discussion
In this study, we investigated saccade task as an executive performance and we could indicate a-MCI patients made significantly more saccade errors, more uncorrected errors, and more target omission in anti-saccade tasks, compared to healthy controls.
The first interesting data we observed was saccade reaction time which did not differ in the a-MCI and the controls. This was unexpected since reaction time is normally longer as dementia progresses (Wilcockson et al., Citation2019) and we could show this significance difference between AD patients and healthy controls as well. We suggest that this primary function may not be disturbed at the early phase of cognitive decline (Yang et al., Citation2011). Taking together this may indicate that people with AD gradually lose the efficient control of attention which is related to trigger a saccade (Noiret et al., Citation2018). Our finding related to the gap effect (longer latencies in overlap tasks than in the gap tasks) in a-MCI and AD patients can be attributed to more complexity of saccade triggering in overlap paradigm such as activation of frontal eye field circuits (Alichniewicz et al., Citation2013).
The other interesting finding revealed more anti-saccade error proportion (saccades in the target direction) in a-MCI and AD patients compared to the reference group. This may indicate deficits in inhibitory control in both groups of patients that can be considered as a useful index to determine executive dysfunction (Crawford et al., Citation2013). These errors can be index of (point 34) selective attention deficits in both groups (Peltsch et al., Citation2014). More importantly this might be related to oculomotor dysfunctions which are associated with anatomical brain dysfunctions such as frontal, parietal, and occipital lobe atrophies that result in deficits in frontal eye field and lack of initiation and suppression of unwanted saccades (Rucker, Citation2010).
Inhibition and working memory functions are fundamental for anti-saccade tasks (Kahana Levy, Lavidor, & Vakil, Citation2018). In our data, increased rate of uncorrected errors in a-MCI and AD in anti-saccade tasks might show self-regulatory disorder and degeneration in its neural network and is related to spatial working memory (Alichniewicz et al., Citation2013). The anti-saccade accuracy is associated with dorsolateral prefrontal cortex and frontal eye field which are connected to neural networks of memory. This may reflect frontal dysfunction that had been reported as the early brain deterioration in AD (Alichniewicz et al., Citation2013; Kahana Levy et al., Citation2018; Yun et al., Citation2011). It has been shown that the proportion of uncorrected errors was not impaired in controls and it was not the general consequence of normal aging. So, it was suggested that these inhibition deficits in error rates can discriminate AD patients from healthy controls (Kaiser et al., Citation2018). Correcting errors needs the intact ability of spatial representation of the target and setting of the task that was impaired in a-MCI and AD groups. The participants in both groups showed problems in following and recalling the desired location of the intended visual target that may result from the deterioration in spatial working memory and inhibitory control (Crawford et al., Citation2013). So, the frequency of inhibitory errors and pattern of uncorrected saccades are leading index of inhibitory control and error monitoring (Wilcockson et al., Citation2019).
According to significant differences in the rate of saccades target omission as an index of sustained attention, subjects with a-MCI also revealed deficits in this cognitive domain (Crawford et al., Citation2013). Our finding is in agreement with the previous studies that suggested AD patients show difficulties in frontal lobe functions related to cognitive demanding tasks like working memory and inhibitory control which is found in anti-saccade function (Alichniewicz et al., Citation2013; Crawford et al., Citation2013; Hutton & Ettinger, Citation2006). Besides, attentional capture and decision making were significantly different between a-MCI and healthy controls in AST tasks (Crawford et al., Citation2013). Moreover, poor performance in AST may relate to the difficulty in task comprehension or non-motivated AD patients (Crawford & Higham, Citation2016).
Finally, the significant correlations between MMSE score and AST errors as well as MMSE score and uncorrected saccades in both gap and overlap tasks might be related to dementia severity (Boxer et al., Citation2006; Shafiq-Antonacci, Maruff, Masters, & Currie, Citation2003). The negative correlation between MMSE and AST errors (low MMSE scores correspond with high saccade errors) indicate that the inclusion of more severely demented patients may increase the differences in error rates between cognitively impaired and controls (Chehrehnegar et al., Citation2019).
Taken together, our findings indicate that a-MCI subjects may gradually lose their control of attention. This could be shown by increase in the impairment of both inhibitory control (error proportion) and eye movement error correction. These difficulties may be due to either inhibitory control, working memory, or both (Crawford & Higham, Citation2016; Wilcockson et al., Citation2019). The results confirmed the increase in the AST error proportion and decrease in the frequency of corrected errors (Noiret et al., Citation2018) that cannot be seen in other neurodegenerative disease like schizophrenia (Crawford, Haeger, Kennard, Reveley, & Henderson, Citation1995) or Parkinson disease (Crawford et al., Citation2013). Uncorrected errors are highly related to spatial working memory and inhibitory controls and may not be affected in schizophrenia and Parkinson disease. AD patients show difficulties in attentional disengagement, self-monitoring and error correction network which may cause more difficulties in error correction (Crawford et al., Citation2013).
Our findings support of AST errors with almost 95% sensitivity as an early diagnostic marker of a-MCI, showing executive deficits occur early in disease progression during the pre-clinical stage of AD (Crawford et al., Citation2013; Garbutt et al., Citation2008; Kaufman, Pratt, Levine, & Black, Citation2010, Citation2012). Currently, there is growing consensus on the use of eye-tracking by clinicians as a highly reliable noninvasive device that can be used as an early indicator of cognitive impairment in MCI phase (Wilcockson et al., Citation2019). However, there were no significant differences between a-MCI and cognitively normal controls in saccade reaction time, so this saccade parameters cannot be applied to distinguish between a-MCI and controls.
In our study, age did not show any significant main effect between the groups, while AD group were10 years older than healthy controls .We did not consider age in our analysis due to the fact that age does not have effect on the saccades in individuals more than 45 years without any neurological or psychiatric disorders (Shafiq-Antonacci et al., Citation2003). However, our study had some limitations such as small sample size, single moment of evaluation and no time intervals as well as different levels of disease severity in AD patients. The current study findings have implications for further longitudinal studies investigating the age-related cognitive changes and early diagnosis of cognitive impairment. However, the results of the present study identified saccade movements as a biological marker to detect cognitive dysfunction in early phases.
Conclusion
Our data showed saccadic eye movement paradigms were sensitive indicators of attentional control and working memory deficits in a-MCI participants and could distinguish them from the normal controls. The revealed deficits in a-MCI included increased error rates, excessive uncorrected errors, and the increased proportion of target omission. Patients with a-MCI had great difficulties in correcting eye movements after automatic wrong direction which can be attributed to their impaired spatial working memory. The current study supports the notion that the proportion of errors and uncorrected saccade movements can be considered as the markers for a-MCI early diagnosis and in the mild AD. These indicators can be used as potential biological markers to discriminate between a-MCI and healthy controls and have potentially important implications in terms of expanding the future options for the early detection and monitoring of people in the early stages of AD.
Acknowledgments
We are thankful for Dr. Mohsen Moslem for his help with scientific comments and editing the manuscript. Also, we are very thankful of Prof. Mary Rudner for her scientific comments and her supports.
Disclosure statement
The authors confirm no actual or potential conflict of interest.
References
- Alichniewicz, K. K., Brunner, F., Klunemann, H. H., & Greenlee, M. W. (2013). Neural correlates of saccadic inhibition in healthy elderly and patients with amnestic mild cognitive impairment. Frontiers in Psychology, 4, 467. doi:https://doi.org/10.3389/fpsyg.2013.00467
- Amieva, H., Phillips, L. H., Della Sala, S., & Henry, J. D. (2004). Inhibitory functioning in Alzheimer’s disease. Brain: A Journal of Neurology, 127(Pt 5), 949–964. doi:https://doi.org/10.1093/brain/awh045
- Boxer, A. L., Garbutt, S., Rankin, K. P., Hellmuth, J., Neuhaus, J., Miller, B. L., & Lisberger, S. G. (2006). Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. Journal of Neuroscience, 26(23), 6354–6363. doi:https://doi.org/10.1523/JNEUROSCI.0549-06.2006
- Chehrehnegar, N., Nejati, V., Shati, M., Esmaeili, M., Rezvani, Z., Haghi, M., & Foroughan, M. (2019). Behavioral and cognitive markers of mild cognitive impairment: Diagnostic value of saccadic eye movements and Simon task. Aging Clinical and Experimental Research, 31(11), 1591–1600. doi:https://doi.org/10.1007/s40520-019-01121-w
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences. New York: Academic Press, Elsevier.
- Crawford, T. J., Haeger, B., Kennard, C., Reveley, M. A., & Henderson, L. (1995). Saccadic abnormalities in psychotic patients. I. Neuroleptic-free psychotic patients. Psychological Medicine, 25(3), 461–471. doi:https://doi.org/10.1017/s0033291700033389
- Crawford, T. J., & Higham, S. (2016). Distinguishing between impairments of working memory and inhibitory control in cases of early dementia. Neuropsychologia, 81, 61–67. doi:https://doi.org/10.1016/j.neuropsychologia.2015.12.007
- Crawford, T. J., Higham, S., Mayes, J., Dale, M., Shaunak, S., & Lekwuwa, G. (2013). The role of working memory and attentional disengagement on inhibitory control: Effects of aging and Alzheimer’s disease. Age (Dordrecht, Netherlands), 35(5), 1637–1650. doi:https://doi.org/10.1007/s11357-012-9466-y
- Crawford, T. J., Higham, S., Renvoize, T., Patel, J., Dale, M., Suriya, A., & Tetley, S. (2005). Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer’s disease. Biological Psychiatry, 57(9), 1052–1060. doi:https://doi.org/10.1016/j.biopsych.2005.01.017
- Crawford, T. J., Parker, E., Solis-Trapala, I., & Mayes, J. (2011). Is the relationship of prosaccade reaction times and antisaccade errors mediated by working memory?Experimental Brain Research, 208(3), 385–397. doi:https://doi.org/10.1007/s00221-010-2488-8
- Donaghy, P. C., Taylor, J. P., O’Brien, J. T., Barnett, N., Olsen, K., Colloby, S. J., … Thomas, A. J. (2018). Neuropsychiatric symptoms and cognitive profile in mild cognitive impairment with Lewy bodies. Psychological Medicine, 48(14), 2384–2390. doi:https://doi.org/10.1017/S0033291717003956
- Foroughan, M., Zahra, J., Bayan, P. S., Faraahani, Z. G. M., & Mahdi, R. (2008). Validation of mini-mental state examination (MMSE) in the elderly population of Tehran. Advances in Cognitive Science, 10(238), 29–37.
- Garbutt, S., Matlin, A., Hellmuth, J., Schenk, A. K., Johnson, J. K., Rosen, H., … Boxer, A. L. (2008). Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer’s disease. Brain: A Journal of Neurology, 131(Pt 5), 1268–1281. doi:https://doi.org/10.1093/brain/awn047
- Greenwood, P. M., Parasuraman, R., & Alexander, G. E. (1997). Controlling the focus of spatial attention during visual search: Effects of advanced aging and Alzheimer disease. Neuropsychology, 11(1), 3–12. doi:https://doi.org/10.1037/0894-4105.11.1.3
- Hallett, P. E. (1978). Primary and secondary saccades to goals defined by instructions. Vision Research, 18(10), 1279–1296. doi:https://doi.org/10.1016/0042-6989(78)90218-3
- Hellmuth, J., Mirsky, J., Heuer, H. W., Matlin, A., Jafari, A., Garbutt, S., … Boxer, A. L. (2012). Multicenter validation of a bedside antisaccade task as a measure of executive function. Neurology, 78(23), 1824–1831. doi:https://doi.org/10.1212/WNL.0b013e318258f785
- Heuer, H. W., Mirsky, J. B., Kong, E. L., Dickerson, B. C., Miller, B. L., Kramer, J. H., & Boxer, A. L. (2013). Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology, 81(14), 1235–1243. doi:https://doi.org/10.1212/WNL.0b013e3182a6cbfe
- Hormozi, S., Alizadeh-Khoei, M., Sharifi, F., Taati, F., Aminalroaya, R., Fadaee, S., … Saghebi, H. (2019). Iranian version of barthel index: Validity and reliability in outpatients’ elderly. International Journal of Preventive Medicine, 10, 130. doi:https://doi.org/10.4103/ijpvm.IJPVM_579_18
- Hutton, S. B., & Ettinger, U. (2006). The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology, 43(3), 302–313. doi:https://doi.org/10.1111/j.1469-8986.2006.00403.x
- Jafari, Z., Steffen Moritz, P., Zandi, T., Kamrani, A., & Malyeri, S. (2010). Psychometric properties of Persian version of the Rey Auditory-Verbal Learning Test (RAVLT) among the elderly. Iranian Journal of Psychiatry and Clinical Psychology, 16(1), 56–64.
- Kahana Levy, N., Lavidor, M., & Vakil, E. (2018). Prosaccade and antisaccade paradigms in persons with Alzheimer’s disease: A meta-analytic review. Neuropsychology Review, 28(1), 16–31. doi:https://doi.org/10.1007/s11065-017-9362-4
- Kaiser, A., Kuhlmann, B. G., & Bosnjak, M. (2018). A meta-analysis of inhibitory-control deficits in patients diagnosed with Alzheimer’s dementia. Neuropsychology, 32(5), 615–633. doi:https://doi.org/10.1037/neu0000460
- Kaufman, L. D., Pratt, J., Levine, B., & Black, S. E. (2010). Antisaccades: A probe into the dorsolateral prefrontal cortex in Alzheimer’s disease. A critical review. Journal of Alzheimer’s Disease: JAD, 19(3), 781–793. doi:https://doi.org/10.3233/JAD-2010-1275
- Kaufman, L. D., Pratt, J., Levine, B., & Black, S. E. (2012). Executive deficits detected in mild Alzheimer’s disease using the antisaccade task. Brain and Behavior, 2(1), 15–21. doi:https://doi.org/10.1002/brb3.28
- Kiosses, D. N., & Alexopoulos, G. S. (2005). IADL functions, cognitive deficits, and severity of depression: A preliminary study. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 13(3), 244–249. doi:https://doi.org/10.1176/appi.ajgp.13.3.244
- Lee, M. T., Jang, Y., & Chang, W. Y. (2019). How do impairments in cognitive functions affect activities of daily living functions in older adults?PLoS One, 14(6), e0218112. doi:https://doi.org/10.1371/journal.pone.0218112
- Limongi, F., Noale, M., Bianchetti, A., Ferrara, N., Padovani, A., Scarpini, E., … Group, M. W. (2018). The instruments used by the Italian centres for cognitive disorders and dementia to diagnose mild cognitive impairment (MCI). Aging Clinical and Experimental Research, 31(1), 101–107. doi:https://doi.org/10.1371/journal.pone.0218112
- Luna, B., Velanova, K., & Geier, C. F. (2008). Development of eye-movement control. Brain and Cognition, 68(3), 293–308. . Epub 2018 Sep 3
- Malakouti, S. K., Fatollahi, P., Mirabzadeh, A., Salavati, M., & Zandi, T. (2006). Reliability, validity and factor structure of the GDS-15 in Iranian elderly. International Journal of Geriatric Psychiatry, 21(6), 588–593. doi:https://doi.org/10.1002/gps.1533
- Munoz, D. P., & Everling, S. (2004). Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews. Neuroscience, 5(3), 218–228. doi:https://doi.org/10.1038/nrn1345
- Nakashima, Y., Morita, K., Ishii, Y., Shouji, Y., & Uchimura, N. (2010). Characteristics of exploratory eye movements in elderly people: Possibility of early diagnosis of dementia. Psychogeriatrics, 10(3), 124–130. doi:https://doi.org/10.1111/j.1479-8301.2010.00327.x
- Noiret, N., Carvalho, N., Laurent, E., Chopard, G., Binetruy, M., Nicolier, M., … Vandel, P. (2018). Saccadic eye movements and attentional control in Alzheimer’s disease. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 33(1), 1–13. doi:https://doi.org/10.1093/arclin/acx044
- Oyama, A., Takeda, S., Ito, Y., Nakajima, T., Takami, Y., Takeya, Y., … Morishita, R. (2019). Novel method for rapid assessment of cognitive impairment using high-performance eye-tracking technology. Scientific Reports, 9(1), 12932. doi:https://doi.org/10.1038/s41598-019-49275-x
- Peltsch, A., Hemraj, A., Garcia, A., & Munoz, D. P. (2014). Saccade deficits in amnestic mild cognitive impairment resemble mild Alzheimer’s disease. The European Journal of Neuroscience, 39(11), 2000–2013. doi:https://doi.org/10.1111/ejn.12617
- Pereira, M. L., Camargo, M., Aprahamian, I., & Forlenza, O. V. (2014). Eye movement analysis and cognitive processing: Detecting indicators of conversion to Alzheimer’s disease. Neuropsychiatric Disease and Treatment, 10, 1273–1285. doi:https://doi.org/10.2147/NDT.S55371
- Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., & Kokmen, E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308. doi:https://doi.org/10.1001/archneur.56.3.303
- Pouretemad, H. R., Khatibi, A., Ganjavi, A., Shams, J., & Zarei, M. (2009). Validation of Addenbrooke’s cognitive examination (ACE) in a Persian-speaking population. Dementia and Geriatric Cognitive Disorders, 28(4), 343–347. doi:https://doi.org/10.1159/000252772
- Rucker, J. (2010). Eye movement abnormalities in movement disorders. Encyclopedia of Movement Disorders, 1, 462. eCollection 2018. .
- Sadeghi, N., Noroozian, M., Khalaji, H., & Mokhtari, P. (2012). Validity and reliability of clinical dementia rating scale among the elderly in Iran. Zahedan Journal of Research in Medical Sciences, 14(10), 47–50.
- Shafiq-Antonacci, R., Maruff, P., Masters, C., & Currie, J. (2003). Spectrum of saccade system function in Alzheimer disease. Archives of Neurology, 60(9), 1272–1278. doi:https://doi.org/10.1001/archneur.60.9.1272
- Wilcockson, T. D. W., Mardanbegi, D., Xia, B., Taylor, S., Sawyer, P., Gellersen, H. W., … Crawford, T. J. (2019). Abnormalities of saccadic eye movements in dementia due to Alzheimer’s disease and mild cognitive impairment. Aging, 11(15), 5389–5398. doi:https://doi.org/10.18632/aging.102118
- Yang, Q., Wang, T., Su, N., Liu, Y., Xiao, S., & Kapoula, Z. (2011). Long latency and high variability in accuracy-speed of prosaccades in Alzheimer’s disease at mild to moderate stage. Dementia and Geriatric Cognitive Disorders Extra, 1(1), 318–329. doi:https://doi.org/10.1159/000333080
- Yang, Q., Wang, T., Su, N., Xiao, S., & Kapoula, Z. (2013). Specific saccade deficits in patients with Alzheimer’s disease at mild to moderate stage and in patients with amnestic mild cognitive impairment. Age (Dordrecht, Netherlands), 35(4), 1287–1298. doi:https://doi.org/10.1007/s11357-012-9420-z
- Yun, J. Y., Lee, D. Y., Seo, E. H., Choo, I. H., Park, S. Y., Kim, S. G., & Woo, J. I. (2011). Neural correlates of stroop performance in Alzheimer’s disease: A FDG-PET study. Dementia and Geriatric Cognitive Disorders Extra, 1(1), 190–201. doi:https://doi.org/10.1159/000329517