 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
Previous studies on the interrelationship between sleep and agitation relied on group-aggregates and so results may not be applicable to individuals. This proof-of-concept study presents the single-subject study design with time series analysis as a method to evaluate the association between sleep and agitation in individual nursing home residents using actigraphy.
Method
To record activity, three women and two men (aged 78–89 years) wore the MotionWatch 8© (MW8) for 9 consecutive weeks. Total sleep time and agitation were derived from the MW8 data. We performed time series analysis for each individual separately. To gain insight into the experiences with the actigraphy measurements, care staff filled out an investigator-developed questionnaire on their and participants’ MW8 experiences.
Results
A statistically significant temporal association between sleep and agitation was present in three out of five participants. More agitation was followed by more sleep for participant 1, and by less sleep for participant 4. As for participants 3 and 4, more sleep was followed by more agitation. Two-thirds of the care staff members (16/24) were positive about the use of the MW8. Acceptability of the MW8 was mixed: two residents refused to wear the MW8 thus did not participate, one participant initially experienced the MW8 as somewhat unpleasant, while four participants seemed to experience no substantial problems.
Conclusion
A single-subject approach with time series analysis can be a valuable tool to gain insight into the temporal relationship between sleep and agitation in individual nursing home residents with dementia experiencing sleep disturbance and agitation.
Introduction
Up to 19% of nursing home residents with dementia experience sleep disturbance (van Duinen-van den IJssel et al., Citation2018; Zuidema et al., Citation2007) and up to 85% express some type of agitated behavior (van Duinen-van den IJssel et al., Citation2018; Zuidema et al., Citation2007). Sleep disturbance can compromise quality of life and can lead to impaired daytime functioning (Vitiello & Borson, Citation2001). Agitation is also associated with poor quality of life (Wetzels et al., Citation2010). Furthermore, care staff in nursing homes experienced both sleep disturbance and agitation/aggression in particular as distressing (van Duinen-van den IJssel et al., 2018).
Previous studies with, for the most part, nursing home residents with cognitive impairment generally report a negative (Bliwise et al., Citation1993; Brown et al., Citation2015; Cohen-Mansfield et al., Citation1995; Cohen-Mansfield & Marx, Citation1990; Tanev et al., Citation2017) or no significant (Gehrman et al., Citation2003) interrelationship between sleep (disturbance) and agitation. These studies have yielded valuable information for identifying targets for intervention. However, typical studies in this field applied a nomothetic approach; an approach that aggregates data from a group of individuals and presents results in group averages. This may result in high generalizability to the population, but applicability to specific individuals is often lost, which is a major drawback when making inferences about individual persons (Barlow & Nock, Citation2009; Rosmalen et al., Citation2012).
A small and seemingly irrelevant association at the group level may be large and meaningful at the individual level (Booij et al., Citation2016). Reversibly, a major effect at the group level does not necessarily apply to an individual patient (Rosmalen et al., Citation2012). For instance, the negative correlation of −.48 between nighttime sleep and nonaggressive behavioral agitation identified by Brown et al. (Citation2015), is likely not applicable to each of the studied participants. Instead, the strength and possibly even the direction of the relationship will likely differ between persons.
An alternative for the nomothetic approach is the use of a single-subject (idiographic) approach, for example with time series analysis. The single-subject study is characterized by numerous repeated measures over time, and separate analysis for every individual, that is: every individual is a study on its own. As sleep and agitation can show substantial intraindividual variability, a single-subject approach is suited. With a single-subject study using time series analysis, differences between individuals in the strength and direction of a temporal relationship can be detected (Rosmalen et al., Citation2012). Therefore, results of single-subject studies are applicable to the individual person and consequently, single-subject studies may facilitate personalized care (Molenaar & Campbell, Citation2009; Rosmalen et al., Citation2012).
The applicability of the single-subject approach has been shown in psychology (e.g. Hamaker et al., Citation2005; Molenaar & Campbell, Citation2009), and is increasingly being shown in psychiatry (e.g. Booij et al., Citation2016) and psychosomatics (e.g. Rosmalen et al., Citation2012). However, these studies typically include participants who can complete, for example, a diary on their own (i.e. used ecological momentary assessments), which is impossible for nursing home residents with advanced dementia. Completing a diary by care staff is also not favorable given their limited time. In addition, care staff assessments are less reliable because of their subjective nature (information by someone else) and the fact that different care staff members have to assess one resident. Meanwhile, wrist actigraphy has been used to measure sleep (Ancoli-Israel et al., Citation1997) and agitated behavior (Knuff et al., Citation2019) in patients with dementia. Furthermore, the availability of electronic devices and ambulatory sensors is increasing and sophisticated technologies as well as statistical analysis techniques to measure, integrate, prepare, and analyze time series data with data from sensor devices are rapidly evolving (e.g. Blaauw et al., Citation2016; van der Krieke et al., Citation2015). These recent technological developments increase the feasibility of single-subject studies with dementia patients. In the present study, we used actigraphy data to assess the temporal association between sleep and agitation in individual nursing home residents with dementia experiencing sleep disturbance and agitation.
The aims of the current study, which can be seen as a proof-of-concept, were threefold: we aimed (1) to gain insight into the temporal relationship between sleep and agitation on an individual patient level; (2) to examine whether there were individual differences in the effects (strength and direction of the relationship); and (3) to explore the experiences of nursing home care staff and participants with the actigraphy measurements.
Methods
Study design and participants
We used a single-subject study design to investigate the temporal association between sleep and agitation in individual nursing home residents with dementia experiencing sleep disturbance and agitation. The power of a single-subject study depends on the number of repeated measures per individual (Lütkepohl, Citation2005) instead of the number of participants, as the analyses are done for each individual separately. According to Box et al. (Citation2016) at least 50 observations are needed. We chose a 9-week study period (63 data points) to take into account potential missing data. We limited the amount of participating residents to five, because our study is a proof-of-concept, every individual is a study on its own, and interim analyses demonstrated heterogeneity in the association between sleep and agitation in these five participants.
We recruited participants from a nursing home in the Netherlands. Elderly care physicians judged, also based on care staff observations, which residents were eligible to participate in the study. Residents were required to have (1) a dementia diagnosis (according to DSM-IV criteria), (2) at least 4 weeks sleep disturbance and no response to regular treatment, (3) at least 4 weeks agitation and no response to regular treatment, and (4) a day-to-day varying degree of sleep and agitation. Residents were excluded (1) if their life-expectancy was shorter than 2 months, (2) in case of delirium or a somatic cause for agitation that requested immediate medical treatment, and (3) if the sleep disturbance was a result of an assignable cause previous to the emergence of dementia or by an already known comorbid somatic problem.
For every participant baseline characteristics were gathered concerning age, sex, type of dementia (electronic patient record), dementia severity (Clinical Dementia Rating scale (CDR); where a score of > =1 has a sensitivity of 92% and a specificity: 94% to detect dementia; Hughes et al., Citation1982; Juva et al., 1995), neuropsychiatric symptoms during the past 2 weeks ( Neuropsychiatric Inventory-Nursing Home version [NPI-NH]; internal consistency reliability Cronbach’s alpha = 0.65, Cummings, Citation1997; Lange et al., Citation2004), and agitated behavior during the past 2 weeks ( the Dutch version of the Cohen-Mansfield Agitation Inventory [CMAI-D]; internal consistency reliability Cronbach’s alpha = 0.63–0.91; Cohen-Mansfield et al., Citation1989; De Jonghe & Kat, Citation1996; Finkel et al., Citation1992; Miller et al., Citation1995; Shah et al., Citation1998).
Ethics
The Medical Ethical Committee of the University Medical Center Groningen approved the study protocol (METc2016.389). The (legal) representatives of the participants provided written informed consent.
Procedure and actigraphy
Data collection took place between February 2017 and July 2017. Participants wore a wrist actigraph, also known as an accelerometer (the MotionWatch 8© [MW8], CamNtech; Cambridge, United Kingdom), for 9 consecutive weeks. In patients with dementia, wrist actigraphy has been shown to be a valid method for measuring sleep when using polysomnography as golden standard (Ancoli-Israel et al., Citation1997). Furthermore, activity as captured by actigraphy is highly correlated with informant-based methods for agitated behavior, such as CMAI and NPI scores (Knuff et al., Citation2019; Nagels et al., Citation2006). We recorded activity continuously with the MW8 actigraphy system (CamNtech; Cambridge, United Kingdom), which produces a quantitative measurement of activity every 30 s. We used a disposable hospital-style wrist strap to prevent unintended removal of the MW8 by the participant. All MW8 data were processed with MotionWare software version 1.1.25 (CamNtech; Cambridge, United Kingdom), including algorithms to classify the 30 s epochs as either sleep or awake, as well as to quantify activity. To enable time series analysis, we divided the study period into 24-hour blocks each lasting from 6 pm to 6 pm the next day (to avoid segregation of sleep periods over two different 24-hour blocks).
Sleep duration
Nighttime sleep duration was defined as the actual sleep time in hours during the night. Care staff registered going to bed and rise times by pressing the event marker button; these times were also verified by means of a by care staff recorded logbook. All 30 s sleep epochs recorded during time in bed were summed up to estimate nighttime sleep duration.
Agitation
As a proxy for agitation, we calculated for each 24-hour block the average MW (activity) counts from all 30 s epochs.
Other variables
Changes in psychotropic medication
Because changes in psychotropic medication use might affect an association between sleep and agitation, the elderly care physician registered any changes in psychotropic medication during the study period. However, for none of the residents there were changes in psychotropic medication use during the study.
Experiences with the MW8
After the 9-week study period had ended, care staff members involved in the care of the individual residents filled out an investigator-developed questionnaire. This questionnaire included a mix of multiple choice and open questions about how they experienced the study and the use of the MW8, and how the participating residents experienced wearing the MW8.
Statistical analysis
We used IBM SPSS Version 25 (IBM Corp, Citation2017) for all descriptive statistics as well as to construct data sets for the time series analyses, and R (R Core Team, Citation2019) to perform time series analysis and additional analyses.
For the time series analyses we used vector autoregression (VAR) models. With VAR modeling, individual multivariate time series can be investigated (Brandt & Williams, Citation2007; Lütkepohl, Citation2005). By separating the temporal associations (i.e. lagged effects) from the contemporaneous associations (i.e. same-day effects), the VAR model allows us to make inferences about the temporal order of the effects (Brandt & Williams, Citation2007; Rosmalen et al., Citation2012). Furthermore, VAR modeling allows for bidirectionality and feedback effects, meaning that a variable can both serve as a predictor and as an outcome (Brandt & Williams, Citation2007; Rosmalen et al., Citation2012).
A VAR model consists of a set of regression equations for a system of two or more endogenous variables, which are variables modeled both as predictor and outcome in the model (Brandt & Williams, Citation2007). In the current study, sleep and agitation were included as endogenous variables. For each individual participant, the VAR model can be constructed as
Here and
are respectively sleep measured during the current night and agitation measured during the current day (i.e. the current 24-hour block); and
are the VAR coefficients to be estimated (where
and
are the autocorrelation coefficients and
and
are the cross-correlation coefficients). We chose to include a lag of 1 (one day; the preceding day) to prevent overparameterization (Brandt & Williams, Citation2007). The error terms in the equations are
and
.
In general, the coefficients of VAR equations are difficult to interpret as it is the behavior of the system and all of its coefficients (i.e. the model as a whole) that describe the dynamicity of the model (Brandt & Williams, Citation2007). Therefore, VAR is usually accompanied by the techniques of Granger causality, impulse response function (IRF) analysis, and forecast error variance decomposition (FEVD). These techniques can give a better understanding of the dynamic behavior of the system (Brandt & Williams, Citation2007; Rosmalen et al., Citation2012).
The Granger causality test assesses the direction of the association between two time series. Granger causality is based on the knowledge that a cause can never come after an effect. Therefore, a variable A ‘Granger causes’ variable B if past values of A predict current values of B better than past values of B alone (Brandt & Williams, Citation2007; Granger, Citation1969). We performed Granger causality tests to investigate whether changes in sleep preceded changes in agitation, i.e. whether sleep Granger caused agitation, whether the reverse was true, or both. Given our choice for 1 lag, the VAR model outcomes will not change much from the Granger causality test outcomes. Therefore, we reported the estimates from the VAR models together with the p-values belonging to the Granger causality tests.
IRF analysis can be used to inspect and visualize how a shock (increase) in one endogenous variable influences the other endogenous variable(s) both in terms of duration and magnitude. IRF analysis only considers the temporal associations between the variables. In order to also take the contemporaneous effects into account, we applied Orthogonalized IRFs ([OIRFs]; Brandt & Williams, Citation2007), a variant of the IRF (see Supplementary material for details of this analysis).
The final tool for assessing a VAR model is FEVD, which is useful for estimating how much of the variation of each of the endogenous variables is due to changes in each of the other endogenous variables over a specific period of time. It thus shows the relative contribution of each variable in the system (Brandt & Williams, Citation2007).
Before performing the VAR analyses, we imputed missing values (introduced due to not wearing the MW8 or because the MW8 ran out of battery) with the R-package Amelia II, an appropriate method for imputation of time series data that takes potential lagged effects into account (Honaker et al., Citation2011). The variables time (1, 2, 3, 4, …t), sleept, agitationt, sleept–1, and agitationt–1 were used in the imputation model. We used the R-package Autovar (Emerencia et al., Citation2016; van der Krieke et al., Citation2015) to perform the VAR analyses and Granger causality tests, and the vars R-package (Pfaff, Citation2008) to perform the IRF and FEVD analyses (see Supplementary material for details).
We used a two-tailed alpha level of 0.05 to determine statistical significance.
Results
Descriptives
Nine residents met inclusion criteria. For two of them, no consent was given by their legal representative. Two other residents refused to wear the MW8; one of the reasons was the thought that we could follow the resident with the MW8. Therefore, five participants remained (see ); three women and two men, aged between 78 and 89, diagnosed with Alzheimer’s disease; mixed dementia (a combination of Alzheimer’s disease and vascular dementia); or alcohol-related dementia.
Table 1. Characteristics of the study participants and descriptive statistics regarding sleep and agitation.
At baseline, the experienced neuropsychiatric symptoms varied across participants (). Concerning agitated behavior, some of the frequently occurring types of behavior were general restlessness (all participants), pacing/aimless wandering (participants 1, 2, 3, and 4), cursing/verbal aggression (participants 1, 3, 4, and 5), constant unwarranted request for attention/help (participants 1, 2, 4, and 5), and repetitive sentences or questions (participants 1, 2, 4, and 5). These types of agitated behavior occurred at least once a week or more (a frequency score of ≥ 3 for the CMAI-D) and are therefore labeled as clinically relevant (Cohen-Mansfield et al., Citation1989; Zuidema et al., Citation2007).
Time series data
The time series of the participants’ daily levels of sleep and agitation are depicted in (see for more descriptive details on the time series). For participant 3, the study period ended after 60 days instead of 63 days because the MW8 ran out of battery. Participants 2 and 5 had a significant time trend in sleep and in agitation over the course of the 9-week long study period (as estimated in the VAR model, see ). Over time the two participants slept more and were on average less agitated.
Figure 1. Time series of the participants (9-week study period).
Note. Sleep is actual sleep time in hours and (the average degree of) agitation was estimated by averaging the motionwatch (activity) counts for each 24-hour block. Imputed data included.
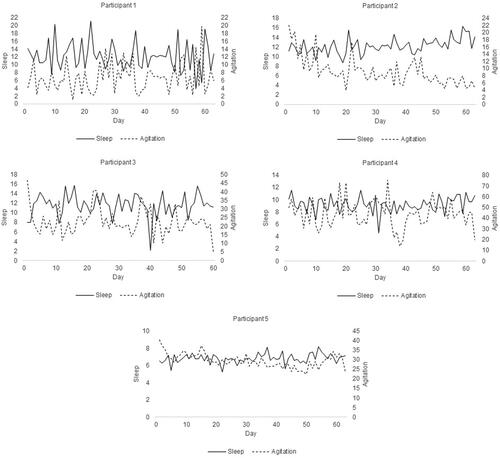
Table 2. Granger causality tests and unstandardized estimates from the one-lag VAR model separate for all study participants.
Temporal association between sleep and agitation
The results of the VAR models and Granger causality tests are presented in . For participant 1, a higher average level of agitation at t–1 significantly predicted more sleep at t. Regarding participant 3, more sleep at t–1 significantly predicted a higher average level of agitation at t. As for participant 4, more sleep at t–1 significantly predicted a higher average level of agitation at t, and a higher average level of agitation at t–1 significantly predicted less sleep at t.
Contemporaneous association between sleep and agitation
For participant 1, the significant contemporaneous association was negative. That is, on days that participant 1 was on average less agitated, the participant slept more and vice versa (r = −.64, p < .001). No significant contemporaneous association was found for participants 2, 3, 4, and 5.
OIRF and FEVD analyses
In the Supplementary material, we present the OIRFs for participant 1 as an example; they showed support for the finding from the Granger causality test that a higher average level of agitation at t–1 was significantly associated with more sleep at t.
Considering the found temporal effects (see ), including a trend (.05 ≤ p < .10), we present in the Supplementary material the FEVDs for participants 1 and 4 as an example. The FEVDs showed that over 10 days the explained variance in agitation by a shock of 1 standard deviation (SD) in sleep was 5.8% or 38.2% for participant 1 (depending on the chosen order regarding the direction of a contemporaneous association) and 6.0% or 6.7% for participant 4. The explained variance in sleep by a shock of 1 SD in agitation was over 10 days 7.5% or 49.2% for participant 1 and 6.7% or 7.0% for participant 4.
Experiences with the MW8
During the study, the MW8 has been removed at least three times by a participant and once by a care staff member as the wrist strap was considered too loose. Furthermore, several care staff members unjustly thought that the event marker button on the MW8 was an on/off switch. The MW8 battery had to be changed once in the case of participants 1, 2, and 4. Changing the battery entailed that the MW8 needed to be removed and that the researcher had to reactivate the MW8.
Of the 24 care staff members who filled out a questionnaire, the majority (N = 16) used positive words when they were asked about their experience with the use of the MW8. Four care staff members stated that they had to carefully remember to press the event marker button on the MW8 and two other members mentioned they sometimes forgot to press it. Five care staff members found it difficult to determine if the button was pressed correctly. The red indicator light, which lit up when the button was pressed, was difficult to see. On a scale of 0 (not at all) to 10 (totally) care staff members could state how user-friendly they considered the MW8 to be. Answers ranged from 5 to 10; mean: 7.71, SD: 1.30. Nineteen of the 24 care staff members said that the chosen wrist strap was good. The others said it was easily broken, or became too loose after a while.
Every participating resident noticed the MW8. Participant 1 experienced the MW8 sometimes as unpleasant, but repeatedly explaining the function was helpful. The other four participants had no substantial problems with wearing the MW8. Participant 4 found it strange to wear the MW8 and asked a lot during the first week about the reason for wearing it. Later on, participant 4 was not occupied with the MW8 anymore and regarded the watch as its own. Participant 5 seemed proud to wear the MW8 for research and also asked care staff members about the button they needed to press. Pressing the button was sometimes experienced as annoying by participants 1, 2, and 3. Participant 4 pressed the button a number of times during the study period, this because of discovering that by pressing the button the red indicator light lit up. The MW8 generally stayed stable on the wrist in the case of participants 3 and 5.
Discussion
This proof-of-concept study examined the temporal association between sleep and agitation in five nursing home residents with dementia experiencing sleep disturbance and agitation, and explored the experiences of care staff and participants with the actigraphy measurements. While previous nomothetic studies generally reported a negative (e.g. Brown et al., Citation2015; Cohen-Mansfield & Marx, Citation1990) or no association (Gehrman et al., Citation2003) between sleep and agitation, we found that the temporal association between sleep and agitation differs in presence, strength, sign and direction between individuals (). A difference in sign was shown for participants 1 and 4: on average more agitation during the day was followed by more sleep the during the night for participant 1, but by less sleep for participant 4. A difference in direction was also found: while for participants 1 and 4 a change in agitation was followed by a change in sleep, for participants 3 and 4 a change in sleep was followed by a change in agitation (so, for participant 4 both directions were found). A difference in strength was found for participants 1 and 4: for participant 1 agitation accounted for much more of the explained variance in sleep over a period of 10 days than for participant 4. Finally, a difference in presence of the association was also found, as for participants 2 and 5, we found no statistically significant temporal relationship between sleep and agitation
Furthermore, overall, care staff members’ experiences with the MW8 were positive and the participating residents were able to wear the MW8 the vast majority of the 9-week study period. To the best of our knowledge, only one study with one patient with dementia reported a longer measurement period with an actigraph (Werth et al., Citation2002).
Implications
With the use of a single-subject approach, more tailored advice for each person could be possible; it yields, for example, indications for treatment focus. For participant 1, the advice could be to focus on increasing sleep duration because more sleep the preceding night was associated with on average less agitation during the current day. However, this association did not reach significance (). Additionally, the OIRF for participant 1 shows that the effect of more sleep was only present on the first day, but did not persist the days thereafter (see ). Furthermore, only around 6% of the total variance of agitation was explained by sleep and agitation itself (see ), whereby sleep merely accounted for almost 6% or 40% of the explained variance in agitation, depending on the chosen order (see ). For participant 4, by contrast, the advice could be to focus on restricting sleep duration because more sleep the preceding night was associated with on average more agitation during the current day. However, only around 9% of the total variance of agitation was explained by sleep and agitation itself (), whereby sleep only accounted for 6% or almost 7% of the explained variance in agitation, depending on the chosen order (see ). So for both participants chances are that an intervention directed at sleep contributes only in a small way. Probably more predictors play a role in agitation for these participants. Hence, intervening on other predictors potentially has more effect. But taken together, this study does illustrate heterogeneity between participants and its results can lead to personalized advice. In the future, it is important to evaluate which interventions can be used according to the results.
Furthermore, our study supports the use of actigraphy regarding nursing home residents with dementia experiencing sleep disturbance and agitation. Thus far, studies on the relationship between sleep and agitation in the nursing home setting have used clinical observations and/or questionnaires to measure agitation (e.g. Brown et al., Citation2015). These measures can be complemented by actigraphy or even replaced to lessen the burden for care staff.
Strengths and limitations
A strength of the present study resides in that the ecological validity of findings is enhanced, as the behavior of residents is continuously monitored in daily life (Trull & Ebner-Priemer, Citation2013).
The validity of actigraphy to assess sleep in nursing home residents with dementia has been established (Ancoli-Israel et al., Citation1997). In addition, earlier studies have used actigraphy to measure agitation in persons with dementia (Khan et al., Citation2018; Knuff et al., Citation2019; Nagels et al., Citation2006). Nonetheless, a limitation of the current study is that we did not differentiate normal movement from agitated movement. Measuring activity instead of pure agitation may have diluted our effects as previous studies have shown for instance a negative relationship between sleep and agitation (e.g. Brown et al., Citation2015), but a positive relationship between sleep and activity (e.g. Eggermont & Scherder, Citation2006; Volicer et al., Citation2006).
A second limitation is that we did not take naps during the day into account. Generally speaking, nursing home residents with dementia can be quite inactive. So it would be rather difficult to distinguish naps from just sitting still during the day. However, it seems plausible that including naps during the day can have an effect on agitation.
Also, there is room for improvement in terms of experiences with the actigraphy measurements. Every participant noticed the MW8 and three out of five participants sometimes experienced pressing the button on the MW8 as annoying. The wrist strap could also loosen. Care staff can give valuable input as to assessing if a resident would be a suitable candidate for actigraphy. Additionally, we believe that, to a certain extent, engaging the participant in the study can be beneficial. For example, giving a simple explanation to the participant when carrying out a MW8 action. As for the wrist strap, more attention should be paid to the possible loosening of the strap during the study, for example when pressing the MW8 button.
Furthermore, the number of measurements necessary for time series analysis (at least 50; Box et al., Citation2016) can be a disadvantage in routine clinical care, especially if the level of agitation needs direct action to prevent harm to other residents or care staff. Since we measured nighttime sleep, only one measurement per day was possible. Other (neuropsychiatric) symptoms, like agitation; mood; or pain, however, allow smaller measurement intervals, resulting in a shorter study period to obtain the same amount of measurements.
Future directions
In the absence of a gold standard, future studies could be done to validate actigraphy for agitation in nursing home residents with dementia against other instruments, such as the Body Sensor Network ([BSN]; Bankole et al., Citation2012) or the multi-modal sensor platform Detection of Agitation and Aggression in people with Dementia ( [DAAD]; Khan et al., 2017; Ye et al., Citation2019). Also, one could consider employing machine learning algorithms (e.g. Khan et al., 2017; Ye et al., Citation2019) to detect and predict the occurrence of agitation in nursing home residents with dementia.
Moreover, further research should explore the effect of variables that might influence the association of sleep and agitation within persons, such as light (CitationFigueiro et al., 2019; Münch et al., Citation2017); the use of psychotropics (Brimelow et al., Citation2019) and pain (Husebo et al., Citation2014). Also, a single-subject study with time series analysis might be used to gain insight into the relationship of agitation with other highly fluctuating variables in individuals with dementia, such as mood and pain; to the best of our knowledge no such research with a single-subject design has been done before.
In addition, when generalizability (i.e. exploring if certain associations can be found in groups of people) is desired then systematic replication of the single-subject study might be a way to identify prototypic individuals (Molenaar & Campbell, Citation2009; Rosmalen et al., Citation2012). Also, it is possible for a nomothetic randomized controlled trial on treatment of, for instance, agitation to incorporate a single-subject study design to identify personal causes of agitation; which would be in line with the increasing demand for personalized care.
Conclusion
This proof-of-concept study clearly demonstrates the potential of a single-subject study with time series analysis for identifying individualized etiological models that may help in developing individualized treatment advice for nursing home residents with dementia. Because this study methodology deals with differences between individuals, it may benefit future research on (relationships between) neuropsychiatric symptoms in dementia, such as sleep disturbance and agitation.
Supplemental Material
Download MS Word (515.1 KB)Acknowledgments
We would like to thank the participants and care staff for their participation in the study. Furthermore, the Motionwatches were kindly provided by the iLab of the department of Psychiatry of the University Medical Center Groningen (UMCG, www.ilab-psychiatry.nl).
Disclosure statement
The authors report no conflict of interest.
Funding
This work was supported in part by the Verenso grant.
Additional information
Funding
References
- Ancoli-Israel, S., Clopton, P., Klauber, M. R., Fell, R., & Mason, W. (1997). Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep, 20(1), 24–27. https://doi.org/https://doi.org/10.1093/sleep/20.1.24
- Bankole, A., Anderson, M., Smith-Jackson, T., Knight, A., Oh, K., Brantley, J., Barth, A., & Lach, J. (2012). Validation of noninvasive body sensor network technology in the detection of agitation in dementia. American Journal of Alzheimer’s Disease & Other Dementias®, 27(5), 346–354. https://doi.org/https://doi.org/10.1177/1533317512452036
- Barlow, D. H., & Nock, M. K. (2009). Why can’t we be more idiographic in our research? Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 4(1), 19–21. https://doi.org/https://doi.org/10.1111/j.1745-6924.2009.01088.x
- Blaauw, F. J., Schenk, H. M., Jeronimus, B. F., van der Krieke, L., de Jonge, P., Aiello, M., & Emerencia, A. C. (2016). Let’s get physiqual - An intuitive and generic method to combine sensor technology with ecological momentary assessments. Journal of Biomedical Informatics, 63, 141–149. https://doi.org/https://doi.org/10.1016/j.jbi.2016.08.001
- Bliwise, D. L., Carroll, J. S., Lee, K. A., Nekich, J. C., & Dement, W. C. (1993). Sleep and “sundowning” in nursing home patients with dementia. Psychiatry Research, 48(3), 277–292. https://doi.org/https://doi.org/10.1016/0165-1781(93)90078-U
- Booij, S. H., Bos, E. H., de Jonge, P., & Oldehinkel, A. J. (2016). The temporal dynamics of cortisol and affective states in depressed and non-depressed individuals. Psychoneuroendocrinology, 69, 16–25. https://doi.org/https://doi.org/10.1016/j.psyneuen.2016.03.012
- Box, G. E. P., Jenkins, G. M., Reinsel, G. C., & Ljung, G. M. (2016). Time series analysis: Forecasting and control (5th ed.). John Wiley & Sons.
- Brandt, P. T., & Williams, J. T. (2007). Multiple time series models. Sage Publications.
- Brimelow, R. E., Wollin, J. A., Byrne, G. J., & Dissanayaka, N. N. (2019). Prescribing of psychotropic drugs and indicators for use in residential aged care and residents with dementia. International Psychogeriatrics, 31(06), 837–847. https://doi.org/https://doi.org/10.1017/S1041610218001229
- Brown, D. T., Westbury, J. L., & Schüz, B. (2015). Sleep and agitation in nursing home residents with and without dementia. International Psychogeriatrics, 27(12), 1945–1955. https://doi.org/https://doi.org/10.1017/S1041610215001568
- Cohen-Mansfield, J., & Marx, M. S. (1990). The relationship between sleep disturbances and agitation in a nursing home. Journal of Aging and Health, 2(1), 42–57. https://doi.org/https://doi.org/10.1177/089826439000200104
- Cohen-Mansfield, J., Marx, M. S., & Rosenthal, A. S. (1989). A description of agitation in a nursing home. Journal of Gerontology, 44(3), M77–M84. https://doi.org/https://doi.org/10.1093/geronj/44.3.M77
- Cohen-Mansfield, J., Werner, P., & Freedman, L. (1995). Sleep and agitation in agitated nursing home residents: An observational study. Sleep, 18(8), 674–680. https://doi.org/https://doi.org/10.1093/sleep/18.8.674
- Cummings, J. L. (1997). The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology, 48(5 Suppl 6), S10–S16. https://doi.org/https://doi.org/10.1212/wnl.48.5_suppl_6.10s
- De Jonghe, J. F., & Kat, M. G. (1996). Factor structure and validity of the Dutch version of the Cohen-Mansfield Agitation Inventory (CMAI-D). Journal of the American Geriatrics Society, 44(7), 888–889. https://doi.org/https://doi.org/10.1111/j.1532-5415.1996.tb03762.x
- Eggermont, L. H. P., & Scherder, E. J. A. (2006). Physical activity and behaviour in dementia: A review of the literature and implications for psychosocial intervention in primary care. Dementia, 5(3), 411–428. https://doi.org/https://doi.org/10.1177/1471301206067115
- Emerencia, A. C., van der Krieke, L., Bos, E. H., de Jonge, P., Petkov, N., & Aiello, M. (2016). Automating vector autoregression on electronic patient diary data. IEEE Journal of Biomedical and Health Informatics, 20(2), 631–643. https://doi.org/https://doi.org/10.1109/JBHI.2015.2402280
- Figueiro, M. G., Plitnick, B., Roohan, C., Sahin, L., Kalsher, M., & Rea, M. S. (2019). Effects of a tailored lighting intervention on sleep quality, rest-activity, mood, and behavior in older adults with Alzheimer disease and related dementias: A randomized clinical trial. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 15(12), 1757–1767. https://doi.org/https://doi.org/10.5664/jcsm.8078
- Finkel, S. I., Lyons, J. S., & Anderson, R. L. (1992). Reliability and validity of the Cohen-Mansfield agitation inventory in institutionalized elderly. International Journal of Geriatric Psychiatry, 7(7), 487–490. https://doi.org/https://doi.org/10.1002/gps.930070706
- Gehrman, P. R., Martin, J. L., Shochat, T., Nolan, S., Corey-Bloom, J., & Ancoli-Israel, S. (2003). Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 11(4), 426–433. https://doi.org/https://doi.org/10.1097/00019442-200307000-00005
- Granger, C. W. J. (1969). Investigating causal relations by econometric models and cross-spectral methods. Econometrica, 37(3), 424–438. https://doi.org/https://doi.org/10.2307/1912791
- Hamaker, E. L., Dolan, C. V., & Molenaar, P. C. M. (2005). Statistical modeling of the individual: Rationale and application of multivariate stationary time series analysis. Multivariate Behavioral Research, 40(2), 207–233. https://doi.org/https://doi.org/10.1207/s15327906mbr4002_3
- Honaker, J., King, G., & Blackwell, M. (2011). Amelia II: A program for missing data. Journal of Statistical Software, 45(7), 1–47. https://doi.org/https://doi.org/10.18637/jss.v045.i07
- Hughes, C. P., Berg, L., Danziger, W. L., Coben, L. A., & Martin, R. L. (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry: The Journal of Mental Science, 140(6), 566–572. https://doi.org/https://doi.org/10.1192/bjp.140.6.566
- Husebo, B. S., Ballard, C., Fritze, F., Sandvik, R. K., & Aarsland, D. (2014). Efficacy of pain treatment on mood syndrome in patients with dementia: A randomized clinical trial. International Journal of Geriatric Psychiatry, 29(8), 828–836. https://doi.org/https://doi.org/10.1002/gps.4063
- IBM Corp. (2017). IBM SPSS statistics for Windows (Version 25) [Computer software]. IBM Corp.
- Juva, K., Sulkava, R., Erkinjuntti, T., Ylikoski, R., Valvanne, J., & Tilvis, R. (1995). Usefulness of the clinical dementia rating scale in screening for dementia. International Psychogeriatrics, 7(1), 17–24. https://doi.org/https://doi.org/10.1017/s1041610295001815
- Khan, S. S., Ye, B., Taati, B., & Mihailidis, A. (2018). Detecting agitation and aggression in people with dementia using sensors – A systematic review. Alzheimer’s & Dementia, 14(6), 824–832. https://doi.org/https://doi.org/10.1016/j.jalz.2018.02.004
- Khan, S. S., Zhu, T., Ye, B., Mihailidis, A., Iaboni, A., Newman, K., Wang, A. H. & Martin, S. L. (2017). DAAD: A framework for detecting agitation and aggression in people living with dementia using a novel multi-modal sensor network [Paper presentation]. In R. Gottumukkala, X. Ning, G. Dong, V. Raghavan, S. Aluru, G. Karypis, L. Miele, & X. Wu (Eds.), Proceedings of the 2017 IEEE International Conference on Data Mining Workshops (ICDMW) (pp. 703–710). The Institute of Electrical and Electronics Engineers. https://doi.org/https://doi.org/10.1109/ICDMW.2017.98
- Knuff, A., Leung, R. H., Seitz, D. P., Pallaveshi, L., & Burhan, A. M. (2019). Use of actigraphy to measure symptoms of agitation in dementia. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 27(8), 865–869. https://doi.org/https://doi.org/10.1016/j.jagp.2019.02.013
- Lange, R. T., Hopp, G. A., & Kang, N. (2004). Psychometric properties and factor structure of the Neuropsychiatric Inventory Nursing Home version in an elderly neuropsychiatric population. International Journal of Geriatric Psychiatry, 19(5), 440–448. https://doi.org/https://doi.org/10.1002/gps.1108
- Lütkepohl, H. (2005). New introduction to multiple time series analysis. Springer-Verlag.
- Miller, R. J., Snowdon, R., & Vaughan, R. (1995). The use of the Cohen-Mansfield Agitation Inventory in the assessment of behavioral disorders in nursing homes. Journal of the American Geriatrics Society, 43(5), 546–549. https://doi.org/https://doi.org/10.1111/j.1532-5415.1995.tb06104.x
- Molenaar, P. C. M., & Campbell, C. G. (2009). The new person-specific paradigm in psychology. Current Directions in Psychological Science, 18(2), 112–117. https://doi.org/https://doi.org/10.1111/j.1467-8721.2009.01619.x
- Münch, M., Schmieder, M., Bieler, K., Goldbach, R., Fuhrmann, T., Zumstein, N., Vonmoos, P., Scartezzini, J.-L., Wirz-Justice, A., & Cajochen, C. (2017). Bright light delights: Effects of daily light exposure on emotions, restactivity cycles, sleep and melatonin secretion in severely demented patients. Current Alzheimer Research, 14(10), 1063–1075. https://doi.org/https://doi.org/10.2174/1567205014666170523092858
- Nagels, G., Engelborghs, S., Vloeberghs, E., van Dam, D., Pickut, B. A., & de Deyn, P. P. (2006). Actigraphic measurement of agitated behaviour in dementia. International Journal of Geriatric Psychiatry, 21(4), 388–393. https://doi.org/https://doi.org/10.1002/gps.1483
- Pfaff, B. (2008). VAR, SVAR and SVEC models: Implementation within R package vars. Journal of Statistical Software, 27(4), 1–32. https://doi.org/https://doi.org/10.18637/jss.v027.i04
- R Core Team. (2019). R: A language and environment for statistical computing [Computer software]. https://www.R-project.org/
- Rosmalen, J. G. M., Wenting, A. M. G., Roest, A. M., de Jonge, P., & Bos, E. H. (2012). Revealing causal heterogeneity using time series analysis of ambulatory assessments: Application to the association between depression and physical activity after myocardial infarction. Psychosomatic Medicine, 74(4), 377–386. https://doi.org/https://doi.org/10.1097/PSY.0b013e3182545d47
- Shah, A., Evans, H., & Parkash, N. (1998). Evaluation of three aggression/agitation behavior rating scales for use on an acute admission and assessment psychogeriatric ward. International Journal of Geriatric Psychiatry, 13(6), 415–420. https://doi.org/https://doi.org/10.1002/(sici)1099-1166(199806)13:6 < 415::aid-gps788 > 3.0.co;2-a
- Tanev, K. S., Winokur, A., & Pitman, R. K. (2017). Sleep patterns and neuropsychiatric symptoms in hospitalized patients with dementia. The Journal of Neuropsychiatry and Clinical Neurosciences, 29(3), 248–253. https://doi.org/https://doi.org/10.1176/appi.neuropsych.16090166
- Trull, T. J., & Ebner-Priemer, U. (2013). Ambulatory assessment. Annual Review of Clinical Psychology, 9, 151–176. https://doi.org/https://doi.org/10.1146/annurev-clinpsy-050212-185510
- van der Krieke, L., Emerencia, A. C., Bos, E. H., Rosmalen, J. G. M., Riese, H., Aiello, M., Sytema, S., & de Jonge, P. (2015). Ecological momentary assessments and automated time series analysis to promote tailored health care: A proof-of-principle study. JMIR Research Protocols, 4(3), e100. https://doi.org/https://doi.org/10.2196/resprot.4000
- van Duinen-van den IJssel, J. C. L., Mulders, A. J. M. J., Smalbrugge, M., Zwijsen, S. A., Appelhof, B., Zuidema, S. U., de Vugt, M. E., Verhey, F. R. J., Bakker, C., & Koopmans, R. T. C. M. (2018). Nursing staff distress associated with neuropsychiatric symptoms in young-onset dementia and late-onset dementia. Journal of the American Medical Directors Association, 19(7), 627–632. https://doi.org/https://doi.org/10.1016/j.jamda.2017.10.004
- Vitiello, M. V., & Borson, S. (2001). Sleep disturbances in patients with Alzheimer’s disease: Epidemiology, pathophysiology and treatment. CNS Drugs, 15(10), 777–796. https://doi.org/https://doi.org/10.2165/00023210-200115100-00004
- Volicer, L., Simard, J., Heartquist Pupa, J., Medrek, R., & Riordan, M. E. (2006). Effects of continuous activity programming on behavioral symptoms of dementia. Journal of the American Medical Directors Association, 7(7), 426–431. https://doi.org/https://doi.org/10.1016/j.jamda.2006.02.003
- Werth, E., Savaskan, E., Knoblauch, V., Fontana Gasio, P., van Someren, E. J. W., Hock, C., & Wirz-Justice, A. (2002). Decline in long-term circadian rest-activity cycle organization in a patient with dementia. Journal of Geriatric Psychiatry and Neurology, 15(1), 55–59. https://doi.org/https://doi.org/10.1177/089198870201500111
- Wetzels, R. B., Zuidema, S. U., de Jonghe, J. F. M., Verhey, F. R. J., & Koopmans, R. T. C. M. (2010). Determinants of quality of life in nursing home residents with dementia. Dementia and Geriatric Cognitive Disorders, 29(3), 189–197. https://doi.org/https://doi.org/10.1159/000280437
- Ye, B., Khan, S. S., Chikhaoui, B., Iaboni, A., Schindel Martin, L., Newman, K., Wang, A., & Mihailidis, A. (2019). Challenges in collecting big data in a clinical environment with vulnerable population: Lessons learned from a study using a multi-modal sensors platform. Science and Engineering Ethics, 25(5), 1447–1466. https://doi.org/https://doi.org/10.1007/s11948-018-0072-y
- Zuidema, S. U., de Jonghe, J. F. M., Verhey, F. R. J., & Koopmans, R. T. C. M. (2007). Neuropsychiatric symptoms in nursing home patients: Factor structure invariance of the dutch nursing home version of the neuropsychiatric inventory in different stages of dementia. Dementia and Geriatric Cognitive Disorders, 24(3), 169–176. https://doi.org/https://doi.org/10.1159/000105603
- Zuidema, S. U., Derksen, E., Verhey, F. R. J., & Koopmans, R. T. C. M. (2007). Prevalence of neuropsychiatric symptoms in a large sample of Dutch nursing home patients with dementia. International Journal of Geriatric Psychiatry, 22(7), 632–638. https://doi.org/https://doi.org/10.1002/gps.1722
