Abstract
Objectives
To explore alcohol consumption among older Norwegian adults with symptoms of cognitive decline, assess the agreement between the reports of older adults and their next of kin regarding a person’s alcohol consumption, and explore clinical and sociodemographic variables associated with agreement.
Method
Alcohol consumption was measured among 3608 older adults consulting specialist health care for symptoms of cognitive decline. Agreement between the participant and their next of kin regarding the participant’s alcohol consumption was assessed with a weighted kappa (κ). A logistic regression analysis for hierarchical data was used to explore variables associated with agreement.
Results
Both the participants and their next of kin reported that more than 20% of the participants consumed alcohol 1–3 times a week, and that approximately 10% consumed alcohol four or more times a week. The agreement between the participant’s and their next of kin’s report regarding the participant’s alcohol consumption was high (κ = .852), and variables associated with agreement were no cognitive decline, not drinking alcohol during the last year or ever as reported by the participant, and low agitation scores on a psychiatric assessment.
Conclusion
This paper found alcohol consumption among older adults with symptoms of cognitive decline that was above the national average in Norway. This is also the first paper to demonstrate that a next of kin can be a reliable source of information regarding older adults’ alcohol consumption. Health personnel should consider these findings when performing medical assessments or developing interventions for older adults.
Introduction
Dementia is a syndrome characterized by cognitive decline and impairment in psychological, behavioral, social, and daily function, and is caused by a variety of diseases. It affects up to 50 million people worldwide (Patterson, Citation2018), and despite decreasing incidence rates (Wolters et al., 2020), the total number of cases is projected to increase threefold by 2050 due to a rapidly aging population (Patterson, Citation2018). A recent study by the Norwegian National Advisory Unit on Ageing and Health has estimated that 101,000 Norwegians currently suffer from dementia, and that this number is likely to be more than double by 2050 (Gjøra et al., 2021).
The association between dementia and alcohol has been discussed for many years. However, while the short-term neurotoxic effects of alcohol are well-documented (Bates et al., Citation2002; Cargiulo, Citation2007; Harper, Citation2009; Ridley et al., Citation2013; Sullivan et al., Citation2003), the exact role of alcohol in the development of dementia is complicated. The relationship between heavy drinking and dementia has been more clear, however, and a recent high-quality review conducted by the 2020 Lancet Commission lends further support to this association (Livingston et al., Citation2020). Their meta-analysis found that consuming >21 units of alcohol per week increased the risk of developing dementia, with a relative risk of 1.18 (95% CI: 1.06; 1.31) compared to lighter drinking.
In Norway, older adults consume almost twice as much alcohol compared to what they did a few decades ago (Bratberg et al., Citation2016; Bye & Østhus, Citation2008; Ministry of Health and Care Services, 2011–2012), mirroring the trend seen elsewhere in the West (Grant et al., Citation2017; Han et al., Citation2017; Moore et al., Citation2005). This development is worrying given alcohol’s role in dementia, the adverse physical health effects it may have (Caputo et al., Citation2012; World Health Organization, Citation2018), especially in older adults (NIH National Institute on Aging, Citation2017), and because alcohol may aggravate existing cognitive decline (Dufour et al., Citation1992). Furthermore, while both heavy drinking and dementia are independently associated with poorer health and cognitive decline, the interaction between them could possibly exacerbate symptoms of both. Consequently, growing rates of consumption will likely increase the need for care and treatment, which will in turn increase the burden on both the health care system and next of kin. It is a problem then that knowledge about alcohol consumption among older adults with cognitive decline specifically is sparse (Frydenlund, Citation2011; Støver et al., Citation2012), despite the fact that the Norwegian government has pledged to prioritize people with problems due to alcohol (Ministry of Health and Care Services, 2011–2012).
What we do know about older adults with cognitive decline is that they tend to underreport their alcohol consumption, and reasons include memory problems, the use of alcohol as self-medication, and the stigma associated with drinking (Aira et al., Citation2005, Citation2008; Graham, Citation1986; Johannessen et al., Citation2016; Merrick et al., Citation2008). Generally speaking, people engaged in heavy drinking may also be more likely to underreport (Boniface et al., Citation2014). Health personnel are therefore at risk of underestimating the impact alcohol may have on their patients’ health. Some primary care physicians avoid asking their older patients about alcohol (Jensen et al., Citation2012; Johannesen et al., Citation2015; Lid et al., Citation2015; Mules et al., Citation2012), and even when they do ask, their accuracy in assessing alcohol use may be less than ideal (Mant et al., Citation2000). Asking a next of kin in addition to the patient about the patient’s alcohol use could improve the assessment of alcohol consumption. Little is known about the degree of agreement between these two information sources, but such knowledge could provide important information for clinicians and policy makers alike. An accurate assessment is important not only for its own sake, but also because alcohol use is essential information when diagnosing dementia and forming treatment plans.
The present paper has two main aims. Firstly, we aim to outline the alcohol consumption patterns of older adults consulting specialist health care for symptoms of cognitive decline. Secondly, we will assess the agreement between the reports of participants and their next of kin regarding the participant’s alcohol consumption, and explore clinical and sociodemographic variables associated with whether or not these two information sources agree.
Method
Participants
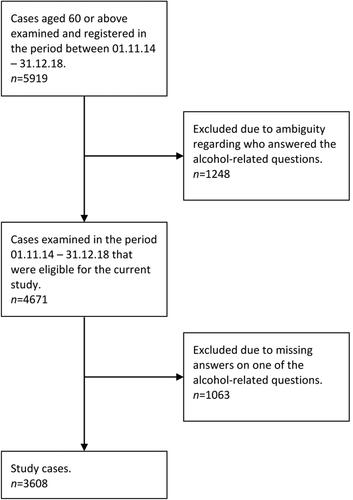
People aged 60 years or older being examined for symptoms of cognitive decline and registered in the Norwegian Registry of Persons Assessed for Cognitive Symptoms in Specialist Health Care Services (NorCog) between November 2014 and December 2018 were included in this study. In Norway, people seeking medical assistance typically visit their primary care physician. The physician may complete the necessary assessments in their office, or refer the patient to the specialist health care system (Norwegian Directorate of Health, Citation2015).
NorCog is a comprehensive, national registry developed to improve the quality of assessment and treatment of dementia at outpatient hospital clinics in Norway. These clinics consist of old age psychiatry clinics, general geriatric clinics, and memory clinics focused on older adults with cognitive decline. The registry covers a large variety of sociodemographic information, physical-, psychiatric-, and cognitive measures, as well as medical diagnoses. It also includes a question about the participant’s alcohol consumption, given to both the participant and their next of kin. Participants were eligible for study inclusion only if both the participant and their next of kin had reported the participant’s alcohol consumption.
The sample selection process is illustrated in . People registered before November 2014 were ineligible in our study because a person’s alcohol consumption was reported only by either the participant or their next of kin. An estimated 2995 participants were never registered in the NorCog database between November 2014 and December 2018, for a variety of reasons (Norwegian National Advisory Unit on Ageing and Health, 2014–2018). Thus, out of the 5919 cases registered between 2014 and 2018, a total of 3608 cases were included in the present study.
Measures
The primary NorCog measurements of interest in our study are listed in . Demographic information used in the analyses were the patient’s age, sex, number of years of education, current employment status, marital status, use of medications, and the type of relationship to next of kin.
Table 1. NorCog measurements included in study analyses.
Statistical analysis
The data was analyzed with SPSS versions 25/26 and STATA version 16. Imputation of missing values was done with SPSS 26 and Excel. Depending on the specific hypotheses being tested, between-group differences were tested with χ2-tests, one-way ANOVA, or independent samples t-tests.
Weighted kappa (κ) with quadratic weights was used to assess agreement between participants and their next of kin regarding the participant’s alcohol consumption. In addition, weighted κ stratified by gender, age (60–74 vs. 75+ years), and marital status were calculated. We also explored whether weighted κ differed depending on if the participant’s spouse/cohabitant was female vs. male. Thus, a weighted κ was calculated separately for female participants accompanied to the assessment by a male spouse/cohabitant and vice versa, assuming for all cases that each spouse/cohabitant was the opposite gender of the participant. Traditional cut-offs for interpreting values of Cohen’s kappa are as follows: ‘Poor’ (<.00); ‘Slight’ (.00–.20); ‘Fair’ (.21–.40); ‘Moderate’ (.41–.60); ‘Substantial’ (.61–.80); and ‘Almost Perfect’ (.81–1.00) (Landis & Koch, Citation1977). A logistic regression model was estimated to assess association between a number of clinical and sociodemographic variables and the dichotomous outcome: agreement vs. disagreement. The data likely exhibited a hierarchical structure due to participants being examined at different health institutions. The degree of clustering at the institution level was assessed by an intra-class correlation coefficient (ICC), which was found to be 10.6%. Random effects for institution were therefore included in the model to correctly adjust the estimates for within-institution correlations. A Box-Tidwell test was used to assess the functional relationship between the continuous variables and the logit. In the case of non-linear relationships, the continuous variables were categorized.
Missing values for the measures PADL and NPI-Q were imputed in the following way. For each item, an empirical distribution was generated, and a random number drawn from this distribution was used to substitute missing values. This procedure was repeated until all imputable cases were imputed. Only cases with at least 50% non-missing values per variable were imputed. Missing values for marital status and medication use were logically imputed when other information about the participants was sufficient to replace missing values. Regression analysis was performed on cases with no missing values on covariates.
Ethical considerations
NorCog data collection and storage was approved by the Norwegian Data Inspectorate. All participants in NorCog have signed an informed consent form for later use of their data. The present study was approved by the South-Eastern Regional Committee for Medical Research Ethics (REK sør-øst B: 21490) and the NorCog publication board.
Results
In total, 3608 persons with a mean (SD) age of 75.9 (7.4) years participated (). Of those, 1906 (52.8%) were women. According to the attending physician’s overall assessment, 1559 (52.7%) met criteria for a dementia diagnosis, while 961 (32.5%) and 138 (4.7%) suffered from mild cognitive decline and subjective cognitive decline, respectively.
Table 2. Demographic and clinical characteristics of study sample (n = 3608).
A comparison of the study sample (n = 3608) and the sample of participants who were excluded due to not answering both alcohol-related questions (n = 1063) indicated that the excluded participants were significantly younger (mean 74.9, SD 8.0 years, p < .001 for independent samples t-test) than the study participants (mean 75.9, SD 7.4 years), and had more years of education (mean 11.8, SD 3.9 years, p < .001 for independent samples t-test) than the study participants (mean 11.0, SD 3.6 years).
provides information about alcohol consumption and its relationship with the participant’s MMSE score. As illustrated, 11.2% of the participants self-reported alcohol consumption 4–7 times a week, while 12.7% of the next of kin reported this frequency among the participants. More than 20% reported that the participants consumed alcohol 1–3 times a week. Male participants consumed alcohol more often than female participants, according to both the participants themselves (p < .001 for χ2-test), and their next of kin (p < .001 for χ2-test). In addition, participants consuming alcohol more often were likely to score higher on the MMSE, according to both the participants and their next of kin (both p < .001 for ANOVA).
Table 3. Participants’ alcohol consumption stratified for cognitive status (n = 3556), %.
The agreement between the next of kin and the participant regarding the participant’s alcohol consumption was high, as demonstrated by a weighted κ = .852 (95% CI: .850; .859). shows that 64.5% were in complete agreement about the participant’s alcohol consumption, while 25.4% disagreed by only one category. In other words, 89.9% answered the same category or the closest adjacent category. Weighted κ for female participants = .860 (95% CI: .844; .874), male participants = .837 (95% CI: .817; .855), participants aged 60–74 years = .849 (95% CI: .832; .872), participants aged 75+ years = .849 (95% CI: .831; .865), married/cohabited participants = .856 (95% CI: .839; .869), single participants = .833 (95% CI: .804; .852). Finally, weighted κ for female participants accompanied to the assessment by a male spouse/cohabitant was κ = .859 (95% CI: .825; .878), and for male participants accompanied by a female spouse/cohabitant, weighted κ = .853 (95% CI: .829; .873).
Table 4. Cross-table of agreement between participant and next of kin regarding participant’s alcohol use (n = 3608), n.
presents the results of the logistic regression analysis. In the adjusted model, participants reporting ‘Not [drinking alcohol] at all the last year or ever’ compared to those consuming alcohol, and participants with MMSE scores of 28–30 compared to those with scores ≤ 24, had significantly higher odds of agreeing with their next of kin regarding alcohol use. Participants reporting drinking ‘4–7 times a week’ also had significantly higher odds of agreement than participants consuming less alcohol, as the confidence interval for this category (95% CI: .39; .83) did not overlap with any other categories. Finally, participants with an NPI-Q score > 2 on the agitation subsyndrome had significantly lower odds of agreeing with their next of kin regarding alcohol use compared to those with agitation scores of 0.
Table 5. Results of logistic regression analysis for hierarchical data (ICC = 8.2% among included cases) on agreement regarding participant’s alcohol use (n = 2603).
Discussion
In this study of older Norwegian adults consulting specialist health care for symptoms of cognitive decline, both the participants and their next of kin reported that more than 20% of the participants consumed alcohol 1–3 times a week, and that approximately 10% of the participants consumed alcohol 4–7 times a week. The agreement between the participant and their next of kin regarding the participant’s alcohol consumption was high, and variables associated with agreement were no cognitive decline, not drinking alcohol last year or ever as reported by the participant, and low scores on the agitation subsyndrome of the NPI-Q.
The proportion of our sample reporting consumption of alcohol 1–3 times a week (>20%, see ) was comparable to the percentage among community-dwelling older adults aged 65 or older in Norway, which is estimated to be 25.0% based on HUNT3 data (Tevik et al., Citation2017). However, while only 3% of the HUNT3 participants reported alcohol consumption 4–7 times a week, 10% of our study sample reported this frequency. This frequency borders on the cut-off for ‘elevated consumption’ as defined by the US National Institute on Alcohol Abuse and Alcoholism (Dufour et al., Citation1992) and the American Geriatrics Society (Lang et al., Citation2007), which define elevated alcohol consumption among people older than 65 as more than one alcoholic drink per day or seven drinks per week. Importantly, there was no true upper bound for the category ‘4–7 times a week’ in our study, as participants consuming alcohol more often would still be forced to choose this response. Therefore, we do not know the true extent of their drinking and a number of participants could possibly qualify for elevated consumption. Why our participants reported more frequent consumption than HUNT3 participants is not clear, but the HUNT3 study was conducted almost 10 years prior to when data for our study was collected, and one possible explanation may be the rapidly growing rates of alcohol consumption among older adults. Another reason may be that our sample had all been referred for examination of symptoms of cognitive decline, which most HUNT3 participants had not.
Interestingly, participants drinking more often were more likely to score higher on the MMSE. Although this could be interpreted as a certain amount of alcohol protecting against cognitive decline (Ilomaki et al., Citation2015; Koch et al., Citation2019; Piumatti et al., Citation2018), this finding may in reality be due to the tendency of older adults with poor physical or cognitive health to avoid alcohol (Fillmore et al., Citation2007; Hassing, Citation2018; Stockwell et al., Citation2016). Taken together, therefore, our findings lend some credence to the idea that elevated alcohol consumption increases the risk of cognitive decline, although testing this association was beyond the scope of the current study.
We found exceptionally high agreement between the participant and their next of kin regarding the participant’s alcohol use. The κ-value for this agreement was considerably larger than what was found in a study measuring agreement between primary care physicians and their patients (κ = .85 and κ = .52, respectively) (Mant et al., Citation2000). This finding was somewhat surprising given how people with cognitive decline often underreport their alcohol consumption (Aira et al., Citation2005, Citation2008; Graham, Citation1986; Johannessen et al., Citation2016; Merrick et al., Citation2008). One possible explanation for this apparent contradiction is that older adults may be more disposed to report their consumption accurately when asked about it directly. As mentioned, some physicians avoid asking their older patients about alcohol, and patients may not disclose this information of their own accord (Johannessen et al., Citation2016, p. 3). It is also likely easier to recall consumption frequency than exact amount, which in turn probably increases the odds of agreement between independent raters.
Another potential explanation for the high κ-value may be the quality of the relationship between the participant and the next of kin. Although NorCog does not measure relationship quality directly, descriptive statistics did show that 91.1% of all next of kin were either a cohabitant/spouse or a child/-in-law. Furthermore, 88.2% of all next of kin had weekly face-to-face contact with the participant. Thus, it is probably safe to assume the next of kin had some insight into the participant’s personal life, which would probably increase the odds of agreement. On the other hand, we found that high scores on the agitation subsyndrome of the NPI-Q were related to lower odds of agreement. Besides possibly influencing how a participant responds to queries about their alcohol consumption, agitation may affect the quality of the relationship between the person in question and their next of kin. For instance, agitation among older adults has been linked to distress in their caregivers, and some next of kin respond to this situation with impatience, irritation, or anger (de Vugt et al., Citation2004; Gitlin et al., Citation2014; Kaufer et al., Citation1998; Matsumoto et al., Citation2007; Petrovic et al., Citation2007; Tan et al., Citation2005). One might expect that such a troubled relationship could make it more difficult to agree about alcohol consumption, although this remains speculation until these hypotheses can be tested empirically.
As mentioned, the logistic regression analysis also found that participants drinking alcohol had lower odds of agreeing with their next of kin regarding the participant’s alcohol consumption, compared to those not drinking alcohol the last year or ever. Apart from the mere ease of remembering less consumption, there may be other explanations for why agreement is higher when there is little consumption. For instance, restraint in alcohol consumption can be traced to a host of cultural (Dawson et al., Citation1995; Galvan & Caetano, Citation2003; Li et al., Citation2019; Livingston et al., Citation2020, p. 11; Vedøy & Amundsen, Citation2008) and health-related factors (Fillmore et al., Citation2007; Hassing, Citation2018; Stockwell et al., Citation2016; Tevik et al., Citation2019a, Citation2019b), which a next of kin is likely aware of. Another noteworthy finding is that agreement was higher for the category ‘4–7 times a week’ than for all other categories except the category ‘Not at all last year or ever’. This could be important because as many as two thirds of all participants reported drinking between a few times a year and 2–3 times a week, i.e. the frequencies associated with the lowest odds of agreement.
The regression analysis also found that MMSE scores of at least 28 was associated with higher odds of agreement. Lower scores of the MMSE have been shown to indicate poorer cognitive function than what is normal for a given age group (John & Montgomery, Citation2003), including scores below 28 (Crum et al., Citation1993), so it is reasonable to assume that people with scores below 28 are less able to accurately recall their alcohol consumption. The finding that memory issues is one reason why older adults with cognitive decline are prone to underreport their alcohol consumption (Graham, Citation1986) supports this interpretation.
Whatever the explanation, there seems to be no immediately apparent reason for why the high rate of agreement uncovered in this study should be called into doubt. Rather, this may be an important first step in spreading awareness that the next of kin can be a reliable source of information regarding an older person’s alcohol consumption, even if the person in question is cognitively impaired. However, some caution is warranted when interpreting the reports of patients and their next of kin even if they seem to agree. While self-report measures of alcohol consumption seem to be reasonably accurate (Del Boca & Darkes, Citation2003; Del Boca & Noll, Citation2000), the possibility that both the participant and their next of kin underreported the participant’s alcohol consumption to an equal degree could not be ruled out. Therefore, overreliance on self-reports or reports from a next of kin should be avoided.
Strengths and limitations
This study has a number of strengths. We had access to a large sample of people participating in a comprehensive database covering a range of demographic, medical, and psychiatric measures, including the alcohol-related question from the highly regarded HUNT3 study (HUNT Research Centre, 2006–2008). This study also includes measurements from both the participants and their next of kin, which not only provides us with the opportunity to examine agreement between these information sources but also increases the validity of the overall assessment.
Despite a large sample recruited from diverse locations in Norway, there are some limitations to representativity. Firstly, people assessed in the specialist health care system in Norway, like our participants, are generally healthier than people assessed in primary health care (Michelet et al., Citation2020). Future studies on related topics should make an effort to include a broader category of participants in order to maximize representativity. In addition, an estimated 2995 eligible participants were not registered in the NorCog database during 2014–2018 for reasons such as capacity limitations and poor physical condition of the participants. Future endeavors to study agreement and its covariates might be better served by using a less extensive battery of tests, which could increase completion rates and be less demanding for participants with poorer physical condition.
Other limitations include the use of frequency to assess alcohol consumption, which may obfuscate actual consumption because the amount consumed on any given occasion can vary. That said, our frequency item may have yielded more accurate reports because frequency is probably easier to remember and may be less stigmatizing than asking about exact amount. Nevertheless, future use of a question measuring exact amount would be an important step in assessing both the reliability and validity of our findings. Another thing of note is that the NorCog register lacked a means of evaluating the relationship quality between the person being assessed and their next of kin, and a high-quality relationship may be associated with higher odds of agreement regarding alcohol consumption. Unfortunately, such a variable could not be included in our regression analysis. That said, despite the likelihood that our large sample included relationships of many different qualities, the κ-value was still very high overall. In addition, relationship type (e.g., spouse, child, friend, etc.) was tested, but was not associated with agreement.
Furthermore, the ICC suggests there were systematic differences in regard to agreement depending on which institution the participants were assessed at. Such variability is difficult to correct for retrospectively, so strict adherence to NorCog interview protocols should be maintained at all times. Such protocols should also be periodically revised to ensure assessment uniformity within and between institutions. Finally, we are unable to draw causal links between the clinical and sociodemographic variables and agreement, due to the cross-sectional design of this study. Longitudinal studies would strengthen the predictive power of any covariates of interest.
Clinical implications
Despite the limitations, some implications can be drawn from this study. Although health personnel should form a habit of asking patients about their alcohol use directly, our findings have demonstrated that the next of kin can be a reliable, alternative source of information. In fact, there are indications that the next of kin may be the most reliable respondent out of the two when there is disagreement; we have discussed research showing that patients may underreport, and our participants did report slightly lower consumption than their next of kin, and underreporting has been associated with memory problems, which in turn is supported by our finding that lower scores on the MMSE were associated with lower odds of agreement. Thus, although there was high overall agreement, there are reasons to assume the next of kin could be the most reliable respondent when there is doubt, with the caveat that this could not be concluded definitively in this study. The implications of these findings extend beyond mere practicality; if, as is often the case, the patient’s cognitive status is so severe that the reliability of their own report is compromised, this study provides evidence that a next of kin can provide reasonably accurate information about alcohol consumption.
Conclusion
This paper has explored the drinking patterns of older adults with cognitive decline, and found that more than 20% of participants consumed alcohol 1–3 times a week while approximately 10% consumed alcohol four or more times a week. This is also the first paper to indicate that a next of kin can be a reliable source of information regarding older adults’ alcohol consumption, and agreement between the two sources is highest when the older adult reports not drinking or drinking 4–7 times a week, and shows no signs of cognitive decline or agitation. Health personnel should consider these findings when performing medical assessments or developing interventions for older adults. Hopefully, these findings can strengthen the assessment and treatment of older adults with cognitive decline, and improve the situation of their caregivers and next of kin.
Disclosure statement
The authors report no conflict of interest.
Acknowledgements
The authors would like to thank all participants in the NorCog registry, colleagues working on the registry, and the Norwegian National Advisory Unit on Ageing and Health.
Additional information
Funding
References
- Aira, M., Hartikainen, S., & Sulkava, R. (2005). Community prevalence of alcohol use and concomitant use of medication—a source of possible risk in the elderly aged 75 and older?International Journal of Geriatric Psychiatry, 20(7), 680–685. https://doi.org/10.1002/gps.1340
- Aira, M., Hartikainen, S., & Sulkava, R. (2008). Drinking alcohol for medicinal purposes by people aged over 75: A community-based interview study. Family Practice, 25(6), 445–449. https://doi.org/10.1093/fampra/cmn065
- Bates, M. E., Bowden, S. C., & Barry, D. (2002). Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Experimental and Clinical Psychopharmacology, 10(3), 193–212. https://doi.org/10.1037//1064-1297.10.3.193
- Boniface, S., Kneale, J., & Shelton, N. (2014). Drinking pattern is more strongly associated with under-reporting of alcohol consumption than socio-demographic factors: Evidence from a mixed-methods study. BMC Public Health, 14(1), 1–9. https://doi.org/10.1186/1471-2458-14-1297
- Bratberg, G. H., Wilsnack, S. C., Wilsnack, R., Haugland, S. H., Krokstad, S., Sund, E. R., & Bjørngaard, J. H. (2016). Gender differences and gender convergence in alcohol use over the past three decades (1984–2008), The HUNT Study, Norway. BMC Public Health, 16(1), 1–12. https://doi.org/10.1186/s12889-016-3384-3
- Bye, E. K., & Østhus, S. (2008). Alkoholkonsum blant eldre: Hovedfunn fra spørreundersøkelser 1985–2008. Statens institutt for rusmiddelforskning (SIRUS). https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2009-og-eldre/alkoholkonsumblanteldre1985_2008.pdf
- Caputo, F., Vignoli, T., Leggio, L., Addolorato, G., Zoli, G., & Bernardi, M. (2012). Alcohol use disorders in the elderly: A brief overview from epidemiology to treatment options. Experimental Gerontology, 47(6), 411–416. https://doi.org/10.1016/j.exger.2012.03.019
- Cargiulo, T. (2007). Understanding the health impact of alcohol dependence. American Journal of Health-System Pharmacy, 64(5 Suppl 3), S5–S11. https://doi.org/10.2146/ajhp060647
- Crum, R. M., Anthony, J. C., Bassett, S. S., & Folstein, M. F. (1993). Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA, 269(18), 2386–2391.
- Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., & Gornbein, J. (1994). The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44(12), 2308–2308. https://doi.org/10.1212/WNL.44.12.2308
- Dawson, D. A., Grant, B. F., Chou, S. P., & Pickering, R. P. (1995). Subgroup variation in US drinking patterns: Results of the 1992 national longitudinal alcohol epidemiologic study. Journal of Substance Abuse, 7(3), 331–344. https://doi.org/10.1016/0899-3289(95)90026-8
- de Vugt, M. E., Stevens, F., Aalten, P., Lousberg, R., Jaspers, N., Winkens, I., Jolles, J., & Verhey, F. R. (2004). Do caregiver management strategies influence patient behaviour in dementia?International Journal of Geriatric Psychiatry, 19(1), 85–92. https://doi.org/10.1002/gps.1044
- Del Boca, F. K., & Darkes, J. (2003). The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction, 98, 1–12. https://doi.org/10.1046/j.1359-6357.2003.00586.x
- Del Boca, F. K., & Noll, J. A. (2000). Truth or consequences: The validity of self-report data in health services research on addictions. Addiction, 95(11), S347–S360. https://doi.org/10.1080/09652140020004278
- Dufour, M. C., Archer, L., & Gordis, E. (1992). Alcohol and the elderly. Clinics in Geriatric Medicine, 8(1), 127–142.
- Fillmore, K. M., Stockwell, T., Chikritzhs, T., Bostrom, A., & Kerr, W. (2007). Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies and new hypotheses. Ann Epidemiol, 17(5 Suppl), S16–S23. https://doi.org/10.1016/j.annepidem.2007.01.005
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
- Frydenlund, R. (2011). Eldre, alkohol og legemiddelbruk. En kunnskapsoppsummering. Kompetansesenter rus – Oslohttp://www.forebygging.no/Global/eldre_kunnskapsoppsummering%20_web.pdf
- Galvan, F. H., & Caetano, R. (2003). Alcohol use and related problems among ethnic minorities in the United States. Alcohol Research & Health, 27(1), 87–94.
- Gitlin, L. N., Marx, K. A., Stanley, I. H., Hansen, B. R., & Van Haitsma, K. S. (2014). Assessing neuropsychiatric symptoms in people with dementia: A systematic review of measures. International Psychogeriatrics, 26(11), 1805–1848. https://doi.org/10.1017/S1041610214001537
- Gjøra, L., Strand, Børn H., Bergh, S., Borza, T., Braekhus, A., Engedal, K., Johannessen, A., Kvello-Alme, M., Krokstad, S., Livingston, G., Matthews, F. E., Myrstad, C., Skjellegrind, H., Thingstad, P., Aakhus, E., Aam, S., & Selbaek, G. (2021). Current and future prevalence estimates of mild cognitive impairment, dementia, and its subtypes in a population-based sample of people 70 years and older in Norway: The HUNT study. Journal of Alzheimer’s Disease, 79(3), 1213–1226. https://doi.org/10.3233/JAD-201275
- Graham, K. (1986). Identifying and measuring alcohol abuse among the elderly: Serious problems with existing instrumentation. Journal of Studies on Alcohol, 47(4), 322–326. https://doi.org/10.15288/jsa.1986.47.322
- Grant, B. F., Chou, S. P., Saha, T. D., Pickering, R. P., Kerridge, B. T., Ruan, W. J., Huang, B., Jung, J., Zhang, H., Fan, A., & Hasin, D. S. (2017). Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74(9), 911–923. https://doi.org/10.1001/jamapsychiatry.2017.2161
- Han, B. H., Moore, A. A., Sherman, S., Keyes, K. M., & Palamar, J. J. (2017). Demographic trends of binge alcohol use and alcohol use disorders among older adults in the United States, 2005–2014. Drug and Alcohol Dependence, 170, 198–207. https://doi.org/10.1016/j.drugalcdep.2016.11.003
- Harper, C. (2009). The neuropathology of alcohol-related brain damage. Alcohol and Alcoholism (Oxford, Oxfordshire), 44(2), 136–140. https://doi.org/10.1093/alcalc/agn102
- Hassing, L. B. (2018). Light alcohol consumption does not protect cognitive function: A longitudinal prospective study. Frontiers in Aging Neuroscience, 10, 81. https://doi.org/10.3389/fnagi.2018.00081
- HUNT Research Centre. (2006–2008). Alcohol Frequency Last 12 months. https://hunt-db.medisin.ntnu.no/hunt-db/variable/4122
- Ilomaki, J., Jokanovic, N., Tan, E. C. K., & Lonnroos, E. (2015). Alcohol consumption, dementia and cognitive decline: An overview of systematic reviews. Current Clinical Pharmacology, 10(3), 204–212. https://doi.org/10.2174/157488471003150820145539
- Jensen, C. J., Lukow, H. R., & Heck, A. L. (2012). Identifying barriers to care for older adults with substance use disorders and cognitive impairments. Alcoholism Treatment Quarterly, 30(2), 211–223. https://doi.org/10.1080/07347324.2012.663302
- Johannesen, A., Helvik, A.-S., Engedal, K., Ulstein, I., & Sørlie, V. (2015). Prescribers-of-psychotropic-drugs-experiencesand-reflections-on-use-and-misuse-of-alcoholand-psychotropic-drugs-among-older-people. Quality in Primary Care, 23(3), 134–140.
- Johannessen, A., Helvik, A. S., Engedal, K., & Sorlie, V. M. (2016). Older peoples’ narratives of use and misuse of alcohol and psychotropic drugs. Scandinavian Journal of Caring Sciences, 30(3), 586–593. https://doi.org/10.1111/scs.12282
- John, P. S., & Montgomery, P. (2003). Is subjective memory loss correlated with MMSE scores or dementia?Journal of Geriatric Psychiatry and Neurology, 16(2), 80–83. https://doi.org/10.1177/0891988703016002003
- Kaufer, D. I., Cummings, J. L., Christine, D., Bray, T., Castellon, S., Masterman, D., MacMillan, A., Ketchel, P., & DeKosky, S. T. (1998). Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: The Neuropsychiatric Inventory Caregiver Distress Scale. Journal of the American Geriatrics Society, 46(2), 210–215. https://doi.org/10.1111/j.1532-5415.1998.tb02542.x
- Kaufer, D. I., Cummings, J. L., Ketchel, P., Smith, V., MacMillan, A., Shelley, T., Lopez, O. L., & DeKosky, S. T. (2000). Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of Neuropsychiatry and Clinical Neurosciences, 12(2), 233–239. https://doi.org/10.1176/jnp.12.2.233
- Koch, M., Fitzpatrick, A. L., Rapp, S. R., Nahin, R. L., Williamson, J. D., Lopez, O. L., DeKosky, S. T., Kuller, L. H., Mackey, R. H., Mukamal, K. J., Jensen, M. K., & Sink, K. M. (2019). Alcohol Consumption and risk of dementia and cognitive decline among older adults with or without mild cognitive impairment. JAMA Network Open, 2(9), e1910319. https://doi.org/10.1001/jamanetworkopen.2019.10319
- Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174.
- Lang, I.,Guralnik, J.,Wallace, R. B., &Melzer, D. (2007). What level of alcohol consumption is hazardous for older people? Functioning and mortality in U.S. and English national cohorts. Journal of the American Geriatrics Society, 55(1), 49–57. https://doi.org/10.1111/j.1532-5415.2006.01007.x
- Lawton, M. P., & Brody, E. M. (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9(3), 179–186. https://doi.org/10.1093/geront/9.3_Part_1.179
- Li, J., Wu, B., Tevik, K., Krokstad, S., & Helvik, A. S. (2019). Factors associated with elevated consumption of alcohol in older adults-comparison between China and Norway: The CLHLS and the HUNT Study. BMJ Open, 9(8), e028646. https://doi.org/10.1136/bmjopen-2018-028646
- Lid, T. G., Nesvåg, S., & Meland, E. (2015). When general practitioners talk about alcohol: Exploring facilitating and hampering factors for pragmatic case finding. Scandinavian Journal of Public Health, 43(2), 153–158. https://doi.org/10.1177/1403494814565129
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
- Mant, J., Murphy, M., Rose, P., & Vessey, M. (2000). The accuracy of general practitioner records of smoking and alcohol use: Comparison with patient questionnaires. Journal of Public Health, 22(2), 198–201. https://doi.org/10.1093/pubmed/22.2.198
- Matsumoto, N., Ikeda, M., Fukuhara, R., Shinagawa, S., Ishikawa, T., Mori, T., Toyota, Y., Matsumoto, T., Adachi, H., Hirono, N., & Tanabe, H. (2007). Caregiver burden associated with behavioral and psychological symptoms of dementia in elderly people in the local community. Dementia and Geriatric Cognitive Disorders, 23(4), 219–224. https://doi.org/10.1159/000099472
- Merrick, E. L., Horgan, C. M., Hodgkin, D., Garnick, D. W., Houghton, S. F., Panas, L., Saitz, R., & Blow, F. C. (2008). Unhealthy drinking patterns in older adults: Prevalence and associated characteristics. Journal of the American Geriatrics Society, 56(2), 214–223. https://doi.org/10.1111/j.1532-5415.2007.01539.x
- Michelet, M., Lund, A., Strand, B. H., Engedal, K., Selbaek, G., & Bergh, S. (2020). Characteristics of patients assessed for cognitive decline in primary healthcare, compared to patients assessed in specialist healthcare. Scandinavian Journal of Primary Health Care, 38(2), 107–116. https://doi.org/10.1080/02813432.2020.1753334
- Ministry of Health and Care Services. (2011–2012). Se meg! En helhetlig rusmiddelpolitikk (Meld. St. 30). https://www.regjeringen.no/contentassets/bba17f176efc40269984ef0de3dc48e5/no/pdfs/stm201120120030000dddpdfs.pdf
- Moore, A. A., Gould, R., Reuben, D. B., Greendale, G. A., Carter, M. K., Zhou, K., & Karlamangla, A. (2005). Longitudinal patterns and predictors of alcohol consumption in the United States. American Journal of Public Health, 95(3), 458–464. https://doi.org/10.2105/AJPH.2003.019471
- Mules, T., Taylor, J., Price, R., Walker, L., Singh, B., Newsam, P., Palaniyappan, T., Snook, T., Ruselan, M., Ryan, J., Santhirasegaran, J., Shearman, P., Watson, P., Zino, R., Signal, L., Fougere, G., Moriarty, H., & Jenkin, G. (2012). Addressing patient alcohol use: A view from general practice. Journal of Primary Health Care, 4(3), 217–222.
- NIH National Institute on Aging (2017, May 16). Facts About Aging and Alcohol. Retrieved February 22, 2021, from https://www.nia.nih.gov/health/facts-about-aging-and-alcohol
- Norwegian Directorate of Health. (2015, November 1). Nasjonal veileder for henvisninger til spesialisthelsetjenesten [nettdokument]. Retrieved February 22, 2021, from https://www.helsedirektoratet.no/veiledere/henvisningsveileder#referere
- Norwegian National Advisory Unit on Ageing and Health. (2014–2018). Resultater. Retrieved June 3, 2020, from https://www.aldringoghelse.no/forskning/norkog/resultater/
- Norwegian National Advisory Unit on Ageing and Health. (2019a). BASAL DEMENSUTREDNING. Anbefalte tester og skjemaer, samlet. Retrieved February 1, 2021, from https://www.aldringoghelse.no/wp-content/uploads/2020/09/basal-d-skjemaersamlet-leger-092020-web.pdf
- Norwegian National Advisory Unit on Ageing and Health. (2019b). BASAL DEMENSUTREDNING. Beskrivelse og tolkning av tester og skjemaer. Retrieved February 1, 2021, from https://www.aldringoghelse.no/ah-archive/documents/Basal_D_BESKRIVELSE_TOLKNING_11.2019_pr02.12.pdf
- Patterson, C. (2018). World alzheimer report 2018. The state of the art of dementia research: New frontiers. Alzheimer’s Disease International. https://apo.org.au/node/260056
- Petrovic, M., Hurt, C., Collins, D., Burns, A., Camus, V., Liperoti, R., Marriott, A., Nobili, F., Robert, P., Tsolaki, M., Vellas, B., Verhey, F., & Byrne, E. J. (2007). Clustering of behavioural and psychological symptoms in dementia (BPSD): A European Alzheimer’s disease consortium (EADC) study. Acta Clinica Belgica, 62(6), 426–432. https://doi.org/10.1179/acb.2007.062
- Piumatti, G., Moore, S. C., Berridge, D. M., Sarkar, C., & Gallacher, J. (2018). The relationship between alcohol use and long-term cognitive decline in middle and late life: A longitudinal analysis using UK Biobank. Journal of Public Health (Oxford, England), 40(2), 304–311. https://doi.org/10.1093/pubmed/fdx186
- Ridley, N. J., Draper, B., & Withall, A. (2013). Alcohol-related dementia: An update of the evidence. Alzheimer’s Research & Therapy, 5(1), 3–8. https://doi.org/10.1186/alzrt157
- Siafarikas, N., Selbaek, G., Fladby, T., Šaltytė Benth, J., Auning, E., & Aarsland, D. (2018). Frequency and subgroups of neuropsychiatric symptoms in mild cognitive impairment and different stages of dementia in Alzheimer’s disease. International Psychogeriatrics, 30(1), 103–113. https://doi.org/10.1017/S1041610217001879
- Stockwell, T., Zhao, J., Panwar, S., Roemer, A., Naimi, T., & Chikritzhs, T. (2016). Do “moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. Journal of Studies on Alcohol and Drugs, 77(2), 185–198. https://doi.org/10.15288/jsad.2016.77.185
- Støver, M., Bratberg, G., Nordfjaern, T., & Krokstad, S. (2012). Use of alcohol and prescription drugs among elderly (60+) in Norway. The HUNT study.
- Sullivan, E. V., Harding, A. J., Pentney, R., Dlugos, C., Martin, P. R., Parks, M. H., Desmond, J. E., Chen, S. A., Pryor, M. R., & De Rosa, E. (2003). Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism: Clinical and Experimental Research, 27(2), 301–309.
- Tan, L. L., Wong, H. B., & Allen, H. (2005). The impact of neuropsychiatric symptoms of dementia on distress in family and professional caregivers in Singapore. International Psychogeriatrics, 17(2), 253–263. https://doi.org/10.1017/s1041610205001523
- Tevik, K., Selbaek, G., Engedal, K., Seim, A., Krokstad, S., & Helvik, A.-S. (2017). Use of alcohol and drugs with addiction potential among older women and men in a population-based study. The Nord-Trondelag Health Study 2006–2008 (HUNT3). PLoS One, 12(9), e0184428. https://doi.org/10.1371/journal.pone.0184428
- Tevik, K., Selbaek, G., Engedal, K., Seim, A., Krokstad, S., & Helvik, A.-S. (2019a). Factors associated with alcohol consumption and prescribed drugs with addiction potential among older women and men - the Nord-Trøndelag health study (HUNT2 and HUNT3), Norway, a population-based longitudinal study. BMC Geriatrics, 19(1), 113. https://doi.org/10.1186/s12877-019-1114-2
- Tevik, K., Selbaek, G., Engedal, K., Seim, A., Krokstad, S., & Helvik, A.-S. (2019b). Mortality in older adults with frequent alcohol consumption and use of drugs with addiction potential - The Nord Trøndelag Health Study 2006–2008 (HUNT3), Norway, a population-based study. PLoS One, 14(4), e0214813. https://doi.org/10.1371/journal.pone.0214813
- Vedøy, T. F., & Amundsen, E. J. (2008). Rusmiddelbruk blant personer med innvandrerbakgrunn. Oversikter fra befolkningsundersøkelser. (SIRUS rapport nr. 1/2008). Statens institutt for rusmiddelforskning (SIRUS). https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2009-og-eldre/sirusrap.1.08.pdf
- Wolters, F. J., Chibnik, L. B., Waziry, R., Anderson, R., Berr, C., Beiser, A., Bis, J. C., Blacker, D., Bos, D., Brayne, C., Dartigues, J.-F., Darweesh, S. K. L., Davis-Plourde, K. L., de Wolf, F., Debette, S., Dufouil, C., Fornage, M., Goudsmit, J., Grasset, L., … Hofman, A. (2020). Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology, 95(5), e519–e531. https://doi.org/10.1212/WNL.0000000000010022
- World Health Organization. (2018, September 27). Global status report on alcohol and health 2018. https://www.who.int/publications/i/item/9789241565639