Abstract
Objectives
Alcohol misuse is known to be a risk factor for dementia. This study aimed to explore the association between risky drinking and cognitive impairment in a cohort study of middle aged and older people at risk of dementia.
Method
The sample comprised 15,582 people aged 50 and over drawn from the PROTECT study. Risky drinking was defined according to a score of 4 or above on the Alcohol Use Disorders Identification Test (AUDIT). Cognitive function was assessed on visual episodic memory, spatial working memory, verbal working memory and verbal reasoning.
Results
Risky drinkers at baseline were more likely to be younger, male, white British, married, of higher educational status, current or past tobacco smokers and to have moderate to severe depression than non-risky drinkers. Risky drinkers were also more likely to be impaired on self-reported instrumental activities of daily living and subjective cognitive decline. At baseline, risky drinkers were less likely than non-risky drinkers to show impairment on verbal reasoning and spatial working memory but not on visual episodic memory or verbal working memory. Risky drinking at baseline predicted decline in cognitive function on visual episodic memory, verbal reasoning and spatial working memory at 2 year follow-up, but only verbal working memory and spatial working memory remained significant outcomes after controlling for possible confounders.
Conclusion
Although of small effect size, the association between risky drinking and impairment on measures of working memory and visuospatial function warrants further examination; particularly given the possibility of partial reversibility in alcohol related cognitive impairment.
Introduction
The relationship between alcohol consumption and cognitive function has been noted as far back as the mid-twentieth century (Maletz & Gardner, Citation1943), but its role as an independent risk factor for cognitive decline has only become apparent over the past 15 years, with a recognised association between heavy drinking and the development of dementia (Harper, Citation2009; Ridley et al., Citation2013). Previous studies have reported that heavy drinkers, defined as consuming more than the equivalent of 24 UK units per week, are more likely to show impairment in memory, executive function, and processing speed tasks compared with non-drinkers (Downer et al., Citation2015; Reid et al., Citation2006). A more recent nationwide retrospective cohort study of hospital admissions over a 5 year time frame, (Schwarzinger et al., Citation2018) found that patients with an alcohol use disorder, were 3 times more likely to develop any type of dementia than those without.
However, the relationship between lower levels of alcohol consumption and cognitive impairment remains unclear. Early studies explored the impact of drinking assessed cognitive impairment without validation against clinical presentation or diagnostic schedules (Zuccalà et al., Citation2006). Some prospective studies found alcohol intake to be associated with a reduced risk of mild cognitive impairment in moderate drinkers (Ganguli et al., Citation2005). A 23 year follow-up study of drinkers aged between 35 and 55 (Sabia et al., Citation2018) found that the highest risk of dementia was found in abstainers and in those drinking more than 14 units of alcohol per week, compared with those drinking 1 to 14 units per week. Other studies have found either no protective effect (Lipnicki et al., Citation2019) or even a higher risk of progression from mild cognitive impairment [MCI] to dementia in people drinking fewer than two drinks (3.5 UK units) a day for at least 6 months, compared with abstainers (Xu et al., Citation2017). Lao et al. (Citation2021) found that risky alcohol intake of more than 27.5 grammes of alcohol (3.5 UK units of alcohol) per day was associated with a greater likelihood of progression to dementia than abstention.
The authors found a linear association was found between alcohol consumption and MCI, with a one-drink increment per week in alcohol intake associated with an increased risk of 3.8% for the development of MCI.
An observational cohort study with weekly alcohol intake and cognitive performance measured repeatedly over 30 years in 550 middle-aged men and women enrolled in the Whitehall II study found that people drinking moderately (14–21 units/week) had three times the odds of right sided hippocampal atrophy compared to non-drinkers. There was also no protective effect from light drinking (1–7 units/week) over abstinence (Topiwala et al., Citation2017). Chen et al. (Citation2018) highlighted the dose dependent effects of vascular risk factors (VRF) on bilateral dorsolateral prefrontal cortex (DLPFC) in MCI individuals. They postulated that dynamic compensatory neural processes that fluctuated along with variations of VRF loading could play a key role in the progression of MCI in drinkers. These findings indicated that complex neurophysiological mechanisms could explaining progression to dementia in MCI.
The Alcohol Use Disorders Identification Test [AUDIT] (Saunders et al., Citation1993) is a widely used screening tool to detect alcohol misuse and the risk of alcohol related harm, but there has so far been no known published research that has used the AUDIT to examine the association between alcohol use and cognitive impairment in older people. Previous studies have used the CAGE screening instrument (Kuźma et al., Citation2014; Lopes et al., Citation2010; Sabia et al., Citation2018), which is validated against alcohol dependence rather than moderate drinking. This study aimed to examine the relationship between risky drinking and cognitive impairment in a large prospective online cohort aimed at the identification of risk factors for dementia.
Materials and methods
Sample
Participants were drawn from the PROTECT Study (www.protectstudy.org.uk), a cohort of adults aged 50 and over. This cohort was recruited online and all procedures are performed remotely on the participants’ computer. First started in 2015, it included UK residents with an adequate understanding of English who were able to use a computer and had internet access. Participants were recruited from publicising the study in the national media and via existing older adult cohorts, but with no information on refusal rates. Any potential participants with an established diagnosis of dementia were not included in the study. The study had ethical approval from the London Bridge NHS Research Ethics Committee (Ref: 13/LO/1578). Potential participants registered on the PROTECT website, from where they could download the study information sheet. All participants were required to sign an online consent form before their participation in the study.
Data collection
Following enrolment into the study, participants completed baseline questionnaires covering socio-demographic information that included age, gender, education, ethnicity, medical and mental health history, as well as a lifestyle questionnaire. Data analysed in the current study included all the above socio-demographic data, as well as smoking status, body mass index, assessment of mood and alcohol consumption at baseline and data on cognitive function and functional impairment at baseline and at 2 year follow-up.
Screening tools
Alcohol consumption was measured using the AUDIT-C, a screening tool for alcohol use and misuse that has been validated against alcohol use disorders in older populations (Gomez et al., Citation2006), with a cut-off point of 3/4 used to detect risky drinking with a sensitivity of 94% and specificity of 80% (Aalto et al., Citation2011). This includes people at increasing, higher risk and probably dependent drinking.
The tool comprises the first 3 questions of the full 10-item Alcohol Use Disorders Identification Test (AUDIT), covering drinking frequency, amount of alcohol consumed on a typical drinking day and the frequency of binge drinking – defined as the consumption of 6 or more units (48 grammes of pure alcohol) on a single drinking occasion. Each item is scored between 0 and 4, with a maximum total of 12 points. Mood was assessed using the PHQ-9, a 9-item Patient Health Questionnaire used as a screen for depression (Kroenke, 2021).
Anxiety was assessed with the GAD-7, a 7-item screening questionnaire for generalised anxiety disorder (Spitzer et al., 2006). A cut-off point of 9/10 on both scales is used to identify moderate to severe, and severe depression or anxiety. Each item is rated between 0 and 4, based on frequency of symptoms, from ‘not at all’ to ‘nearly every day’; with a maximum score of 36 for the PHQ-9 and 28 for the GAD-7. Smoking status was assessed by a yes or no answer to the question ‘Have you ever smoked tobacco?’ and Body Mass Index defined by a score of 25 and over as being overweight. Cognitive function was assessed using a validated online cognitive test package consisting of 4 tasks: a Paired Associate Learning Test (PALT) for visual episodic memory (Owen et al., Citation1999); a Self-Ordered-Search (SOS) test for spatial working memory (Owen et al., Citation1990); a digit span test (DS) for verbal working memory (Blackburn & Benton, Citation1957) and a verbal reasoning (VR) test for grammatical reasoning (Baddeley, 1968). Participants were asked to complete each task on 3 occasions over 1 week to provide a baseline assessment. An average of these 3 readings was used for analysis in this study. Subjective cognitive decline was assessed using the subject and informant versions of the short version of the Informant Questionnaire on Cognitive Decline in the Elderly – IQCODE (Jorm, Citation1994). The IQCODE is a 16-item questionnaire uses information to assess changes in functional abilities over the previous 10 years, with both a subject and informant rated version. Each question is rated on a 5-point scale from 1 (‘much improved’) to 5 (‘much worse’), with a score of 3 representing ‘no change’ and a maximum score of 80. This was supplemented by a self-rated scale for instrumental activities of daily living (IADLs), derived from the Older Americans Resource and Service (OARS) multidimensional functional assessment questionnaire (Fillenbaum & Smyer, Citation1981). In addition to the self-performance rating in the original OARS scale from 0 (‘on my own’) to 3 (‘with full help’), a difficulty rating was included from 0 (‘no difficulty’) to 2 (‘great difficulty’); with a maximum score of 35.
Statistical analysis
Data were analysed for both categorical and continuous variables using IBM SPSS Statistics package, Version 27 (IBM Corp, Citation2020) . The chi-squared test was used to compare categorical variables and the t-test for ordinal and scale data. The Bonferroni correction was applied for multiple univariate comparisons of categorical data in the adjustment of p values. Tests of cognitive function and functional decline were analysed as dependent variables; sociodemographic, alcohol and mood variables were analysed as independent variables. Sociodemographic data comprised age, sex, ethnicity, educational status and living arrangements. Other data independent variables included AUDIT score, smoking status, PHQ-9 and GAD-7 scores and BMI. Measures of cognitive function were scores on PALT, DS, VR and SOS tests and measures of functional status were subject and informant-rated IQCODE scores, as well as IADL score. For univariate analyses, categories for impairment were defined according to whether participants scored above or below the mean score for age, IADL, PHQ and GAD-7 scores. A cut-off point of 25 and above was used to define participants as overweight. The cut-off point for IQCODE scores was a mean score of 3.3, taken from existing norm-referenced data validated against a clinical diagnosis of dementia (Jorm, Citation1994). Given the high level of educational status within the sample, scores that were more than 1 standard deviation below mean PALT, DS, VR and SOS scores were used as cut-off points for cognitive impairment. For univariate analyses, risky and non-risky drinking was compared against the above categorical variables. For categorical variables with more than one category, the following were compared: White British with other ethnic groups for ethnicity, married status compared with other relationship status and schooling below or including secondary education for educational status.
For multivariate analysis, binary logistic regression was used to calculate odds ratios with the forward LR procedure used to examine the impact of confounders of age, sex, marital and educational status, smoking status, BMI, depression and anxiety. A change in the regression coefficient of less than 10% indicated the absence of the entered variable as a confounder. Variables were entered into the regression equation if they had a p value < 0.1 on univariate analysis.
Results
Socio-demographic characteristics for the 15,582 participants at baseline are detailed in .
Table 1. Baseline characteristics (N = 15,582).
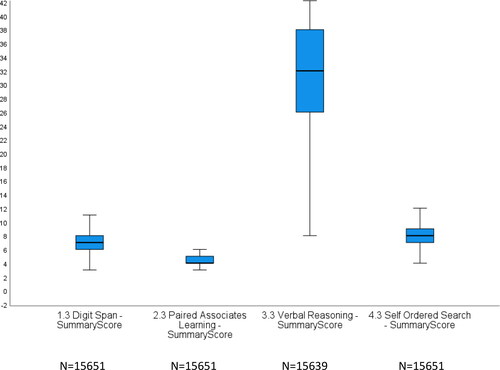
also shows scores on domains of cognitive and functional impairment, with a more detailed boxplot in showing distribution of scores of tests of cognitive function. DS scores ranged from 3 to 11 (median 7, skew 0.59), PALT from 3 to 7 (median 4. skew 0.35), VR from 9 to 43 (median 32, skew −0.05) and SOS from 5 to 13 (median 8, skew −1.12).
Univariate analyses for risky drinking are detailed in . Participants drinking at risky levels compared with non-risky drinkers were more likely to be younger (t = 3.49, df = 1650, p < 0.001), male (χ2 = 12.5, df = 1, p < 0.001), white British (χ2 = 101.2, df = 1, p < 0.001), married (χ2 = 97, df = 1, p < 0.001), of higher educational status (χ2 = 17.4, df 1, p < 0.001) and current or past tobacco smokers (χ2 = 20.4, df = 1, p < 0.001). They were also more likely to be impaired in their everyday functional abilities, as measured by mean IADL (t = −2.9, df = 1796, p = 0.004) and subjective IQCODE (t = −12.2, df = 1534, p < 0.001) scores. Risky drinkers were also more likely to show moderate to severe or severe depression (t = −11.6, df 1578, p < 0.001) and anxiety (t = −12.4, df 1597, p < 0.001). Effect sizes for differences in mean IQCODE, PHQ-9 and GAD-7 were moderate or large (Cohen’s d > 0.5). On measures of cognitive function, those drinking at risky levels were less likely to show impairment on verbal reasoning (t = −3.3, df 1808, p = 0.001) and self-ordered search tasks (t = −3.0, df 1764, p = 0.003). Effect sizes for these differences were very small (Cohen’s d < 0.1).
Table 2. Univariate analysis for drinking risk at baseline.
In order to test for the possibility of confounding, multivariate binary logistic regression analyses were performed using baseline drinking status as the independent and IADL, subject IQCODE, verbal reasoning and self-ordered search, as dependent variables; controlling for age, sex, ethnicity, marital status, educational level, smoking status, depression and anxiety. These results are detailed in , which shows that subjects with risky drinking were less likely to show cognitive impairment on tests of verbal reasoning and self-ordered search tasks and more likely to show cognitive decline on IADL and subjective IQ-CODE scores than non-risky drinkers.
Table 3. Drinking status at baseline as predictor of cognitive function and functional decline at year 2.
Given the possibility of reverse causality with risky drinking as an independent predictor of cognitive impairment rather than vice versa, analysis was then carried out for combined baseline and follow-up data on the same sample at year 2. There were a total of 5316 participants from the original sample at year 2. Further univariate analysis was performed for baseline drinking status to predict cognitive function at year 2. The results from this analysis are detailed in , which shows that risky drinking at baseline predicted decline in cognitive function on digit span, verbal reasoning and self-ordered search tasks at 2 year follow-up. All these results had small effects sizes of <0.2. In order to test for the possibility of confounding, binary logistic regression analyses were performed for digit span, verbal reasoning and self-ordered search, controlling for age, sex, ethnicity, marital status, educational level, smoking status, depression and anxiety. These results are detailed in . Risky drinking at baseline was an independent predictor of decline only in digit span and self-ordered search tasks, with risky drinkers at baseline 1.6 times more likely to be impaired on both tasks at year 2 follow-up, compared with baseline non-risky drinkers.
Table 4. Baseline drinking status and cognitive function at Year 2.
Table 5. Baseline risky drinking status as a predictor of cognitive function at Year 2.
In order to further examine the association between risky drinking and these markers of cognitive impairment, simple linear regression was carried out, with score on self-ordered search and digit span test score at year 2 as the dependent and AUDIT score at baseline as the independent variable. Simple linear regression showed a significant relationship only between AUDIT score and digit span score (p < 0.001). For every increase in 1 point increase in AUDIT score, there was a 0.03 (95% Confidence Intervals 0.08–0.44) point decrease in digit span score. The scatterplot of standardised predicted values against standardised residuals for this associations, showed that the data met the assumptions of homogeneity of variance and linearity and the residuals were approximately normally distributed.
Discussion
This study found that compared to non-drinkers and those drinking at lower risk drinking levels, participants drinking at risky levels were more likely to be younger, male, of white British ethnicity, married, of higher educational status and current or past tobacco smokers. Risky drinkers were also more likely to be impaired on self-reported instrumental activities of daily living and subjective cognitive decline and more likely to show moderate to severe or severe depression. The finding of greater impairment on self-reported instrumental activities of daily living may reflect changes other than those directly related to cognitive impairment, particularly given that such deterioration is more commonly seen in people with established dementia, who would have been excluded from the study.
On measures of cognitive function, those drinking at risky levels at baseline were less likely than non-risky drinkers to show impairment on verbal reasoning and self-ordered search tests but not on paired association learning and digit span tests. Risky drinking at baseline predicted decline in cognitive function on digit span, verbal reasoning and self-ordered search tasks at 2 year follow-up, but only digit span and self-ordered speech remained significant outcomes after controlling for possible confounders. There was also a significant linear association between risky drinking at baseline and DS score at 2 year follow-up.
A number of studies have reported data on drinking and performance using the digit span test to assess the association between alcohol intake and verbal working memory in older people. The Baltimore Longitudinal Study of Aging found an inverse association between greater alcohol consumption and memory impairment, but this finding was not replicated on longitudinal analyses (Beydoun et al., Citation2014). Similar findings were reported in the Aging, Demographics, and Memory Study (Herring & Paulson, Citation2018), in which cross-sectional analyses found moderate drinkers performed significantly better than abstainers, but no association was found on longitudinal analyses. Cross sectional analysis from other studies have been inconclusive. The Established Populations for Epidemiologic Studies of the Elderly study found that those drinking up to 14 grammes of pure alcohol per day showed higher scores compared with non-drinkers (Herbert et al., Citation1993). The Women’s Health Initiative study of randomised clinical trials of hormone therapy found no association between mean daily volume of alcohol consumed and digit span score (Espeland et al., Citation2006). In a further study of 206 first-ever ischaemic stroke patients, no differences were observed in digit span score when people with alcohol use disorder were compared to those without (Laari et al., Citation2020). Our finding of greater impairment in working memory at 2 year follow-up is supported by studies alcohol use disorders to be associated with frontal impairment on functional magnetic resonance imaging (Desmond et al., Citation2003; Tapert et al., Citation2001).
Few studies have examined the association between alcohol consumption and visuospatial memory in older people. In the cross-sectional Hordaland Health Study that compared middle-aged drinkers with non-drinkers, reduced risk of impairment on the Kendrick Object Learning Test was associated with consumption of up to half a glass of wine per day compared with non-drinkers (Nurk et al., Citation2009). In contrast, a cohort study to identity risk factors for Alzheimer’s Disease used the Lifetime Consumption to compare 3 groups of participants: never/minimal, former and current drinkers, using a test of visuospatial memory. No significant differences were observed between these 3 groups (Kalapatapu et al., Citation2017). The most conclusive study examining the association between spatial working memory came from the UK Biobank study of 8312 participants with a mean age 62. Drinking daily or on most days of the week was associated with a greater likelihood of spatial memory impairment, mediated by differences in posterior cingulate cortex volume (Suzuki et al., 2019). This is supported by the finding of cortical parietal atrophy in people with alcohol use disorder on functional magnetic resonance imaging (Harris et al., Citation2008).
Previous studies exploring the relationship between alcohol consumption and activities of daily living have focussed on personal care, rather than IADLs. These studies have produced conflicting findings of both a greater likelihood (Abbott et al., , Citation2011) or no association (Lee et al., Citation2019; Rist et al., Citation2015) between alcohol consumption and cognitive decline. Studies examining IADLs have been inconclusive, either an association (León-Muñoz et al., Citation2017) or no association (Lang et al., Citation2007) between lower risk drinking and impairment in IADLs. There have been no previous studies that have examined the association between alcohol consumption the performance in IADLs using either the IQCODE or OARS IADL scale.
Strengths and limitations
The strengths of this study are that it contains a sample that was recruited online to produce large enough numbers within data analysis to improve the specificity of results.
However, the sample represents a selection bias, with 75% female and over 50% with an undergraduate or postgraduate degree. However, there was no noticeable skew in the distribution of cognitive scores.
There is also the possibility of self-report bias in underestimation of alcohol use and overestimation of abilities within instrumental activities of daily living, particularly given the marginally higher self, versus informant-reported rating of cognitive decline. Although small, this is shown in the difference in mean IQCODE scores between self and informant-reported versions of the scale. A higher representation of females and those of higher educational status may also be associated with improved performance on cognitive function and slower functional decline through better general health status. Similarly, there is a risk of both self-report and recall bias in completion of information about mood. These findings are all known to influence the validity of epidemiological studies that recruit older people (Golomb et al., Citation2012).
There is also the possibility of low sensitivity and Type 1 statistical error, but this was controlled for using the appropriate correction. The possibility of confounding and reverse causality were also taken into account through the use of appropriate analysis from the longitudinal study design. Lastly, although there were moderate effects sizes for the association between risky drinking and measures of subjective cognitive decline and mood, significant associations between risky drinking and measures of cognitive function have small effect sizes which may have arisen from the large sample size. There was also no coding of individual physical disorders or medication and no distinction between current and former smokers, all of which may been confounders in the association between alcohol use and cognitive impairment.
Despite its limitations, this study shows novel findings of an association between drinking at levels suggestive of hazardous, harmful and dependent use and impaired memory on verbal and visuospatial tasks, in addition to impairment on functional indicators of cognitive decline. This finding is all the more striking, given the 2 year follow time frame across which cognitive decline was assessed. Frontal and parietal dysfunction is known to have a strong association with alcohol use disorder, as measured by impaired glucose metabolism seen on functional brain imaging (Gilman et al., Citation1990; Wik et al., Citation1988). Although frontal lobe impairment is also an early manifestation of Alzheimer’s Disease, there is considerable scope for further scope for future studies to explore the role of early parietal dysfunction in people at risk of alcohol related cognitive decline using tests of visuospatial memory. This also has implications for clinical practice, as everyday activities involving visuospatial speed and focus are dependent on the intact parietal function (Turken et al., Citation2008). In addition, there is now emerging evidence the finding of partial recovery of grey matter volume in the inferior parietal lobule following abstinence from alcohol (van Eijk et al., Citation2013).
The unique contribution of this study is in the use of standardised cognitive tests as markers of both cognitive impairment and cognitive decline in middle aged and older people at risk of alcohol misuse. It also adds to the existing literature in the addition of cognitive testing for visuospatial impairment in routine clinical practice in the detection of alcohol related cognitive impairment.
Acknowledgements
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aalto, M., Alho, H., Halme, J. T., & Seppä, K. (2011). The alcohol use disorders identification test (AUDIT) and its derivatives in screening for heavy drinking among the elderly. International Journal of Geriatric Psychiatry, 26(9), 881–885. https://doi.org/10.1002/gps.2498
- Abbott, R. D., Kadota, A., Miura, K., Hayakawa, T., Kadowaki, T., Okamura, T., Okayama, A., Masaki, K. H., & Ueshima, H. (2011). Impairments in activities of daily living in older Japanese men in Hawaii and Japan. Journal of Aging Research, 2011, 1–8. https://doi.org/10.4061/2011/324592
- Baddeley, A. D. (1968). Closure and response bias in short-term memory for form. British Journal of Psychology (London, England: 1953), 59(2), 139–145. https://doi.org/10.1111/j.2044-8295.1968.tb01126.x
- Beydoun, M. A., Gamaldo, A. A., Beydoun, H. A., Tanaka, T., Tucker, K. L., Talegawkar, S. A., Ferrucci, L., & Zonderman, A. B. (2014). Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. The Journal of Nutrition, 144(6), 890–901. https://doi.org/10.3945/jn.113.189027
- Blackburn, H. L., & Benton, A. L. (1957). Revised administration and scoring of the digit span test. Journal of Consulting Psychology, 21(2), 139–143. https://doi.org/10.1037/h0047235
- Chen, H., Su, F., Ye, Q., Wang, Z., Shu, H., & Bai, F. (2018). The dose-dependent effects of vascular risk factors on dynamic compensatory neural processes in mild cognitive impairment. Frontiers in Aging Neuroscience, 10, 131. https://doi.org/10.3389/fnagi.2018.00131
- Desmond, J. E., Chen, S. H., DeRosa, E., Pryor, M. R., Pfefferbaum, A., & Sullivan, E. V. (2003). Increased frontocerebellar activation in alcoholics during verbal working memory: An fMRI study. NeuroImage, 19(4), 1510–1520. https://doi.org/10.1016/S1053-8119(03)00102-2
- Downer, B., Jiang, Y., Zanjani, F., & Fardo, D. (2015). Effects of alcohol consumption on cognition and regional brain volumes among older adults. American Journal of Alzheimer’s Disease and Other Dementias, 30(4), 364–374. https://doi.org/10.1177/1533317514549411
- Espeland, M. A., Coker, L. H., Wallace, R., Rapp, S. R., Resnick, S. M., Limacher, M., Powell, L. H., Messina, C. R., … Women’s Health Initiative Study of Cognitive, A. (2006). Association between alcohol intake and domain-specific cognitive function in older women. Neuroepidemiology, 27(1), 1–12. https://doi.org/10.1159/000093532
- Fillenbaum, G. G., & Smyer, M. A. (1981). The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. Journal of Gerontology, 36(4), 428–434. https://doi.org/10.1093/geronj/36.4.428
- Ganguli, M., Vander Bilt, J., Saxton, J. A., Shen, C., & Dodge, H. H. (2005). Alcohol consumption and cognitive function in late life: A longitudinal community study. Neurology, 65(8), 1210–1217. https://doi.org/10.1212/01.wnl.0000180520.35181.24
- Gilman, S., Adams, K., Koeppe, R. A., Berent, S., Kluin, K. J., Modell, J. G., Kroll, P., & Brunberg, J. A. (1990). Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Annals of Neurology, 28(6), 775–785. https://doi.org/10.1002/ana.410280608
- Golomb, B. A., Chan, V. T., Evans, M. A., Koperski, S., White, H. L., & Criqui, M. H. (2012). The older the better: Are elderly study participants more non-representative? A cross-sectional analysis of clinical trial and observational study samples. BMJ Open, 2(6), e000833. https://doi.org/10.1136/bmjopen-2012-000833
- Gomez, A., Conde, A., Santana, J. M., Jorrin, A., Serrano, I. M., & Medina, R. (2006). The diagnostic usefulness of AUDIT and AUDIT-C for detecting hazardous drinkers in the elderly. Aging & Mental Health, 10(5), 558–561. https://doi.org/10.1080/13607860600637729
- Harper, C. (2009). The neuropathology of alcohol-related brain damage. Alcohol and Alcoholism (Oxford, Oxfordshire), 44(2), 136–140. https://doi.org/10.1093/alcalc/agn102
- Harris, G. J., Jaffin, S. K., Hodge, S. M., Kennedy, D., Caviness, V. S., Marinkovic, K., Papadimitriou, G. M., Makris, N., & Oscar-Berman, M. (2008). Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcoholism, Clinical and Experimental Research, 32(6), 1001–1013. https://doi.org/10.1111/j.1530-0277.2008.00661.x
- Herbert, L. E., Scherr, P. A., Beckett, L. A., Albert, M. S., Rosner, B., Taylor, J. O., & Evans, D. A. (1993). Relation of smoking and low-to-moderate alcohol consumption to change in cognitive function: A longitudinal study in a defined community of older persons. American Journal of Epidemiology, 137(8), 881–891. https://doi.org/10.1093/oxfordjournals.aje.a116749
- Herring, D., & Paulson, D. (2018). Moderate alcohol use and apolipoprotein E-4 (ApoE-4): Independent effects on cognitive outcomes in later life. Journal of Clinical and Experimental Neuropsychology, 40(4), 326–337. https://doi.org/10.1080/13803395.2017.1343803
- IBM Corp. (2020). IBM SPSS statistics for windows, Version 27.0. IBM Corp.
- Jorm, A. F. (1994). A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychological Medicine, 24(1), 145–153. https://doi.org/10.1017/s003329170002691x
- Kalapatapu, R. K., Ventura, M. I., & Barnes, D. E. (2017). Lifetime alcohol use and cognitive performance in older adults. Journal of Addictive Diseases, 36(1), 38–47. https://doi.org/10.1080/10550887.2016.1245029
- Kroenke, K. (2021). PHQ-9: Global uptake of a depression scale. World Psychiatry: official Journal of the World Psychiatric Association (WPA), 20(1), 135–136. https://doi.org/10.1002/wps.20821
- Kuźma, E., Llewellyn, D. J., Langa, K. M., Wallace, R. B., & Lang, I. A. (2014). History of alcohol use disorders and risk of severe cognitive impairment: A 19-year prospective cohort study. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 22(10), 1047–1054. https://doi.org/10.1016/j.jagp.2014.06.001
- Laari, S. P. K., Kauranen, T. V., Turunen, K. E. A., Mustanoja, S. M., Tatlisumak, T., & Poutiainen, E. T. (2020). Executive dysfunction related to binge drinking in ischemic stroke. Cognitive and Behavioural Neurology, 33(1), 23–32.
- Lang, I., Guralnik, J., Wallace, R. B., & Melzer, D. (2007). What level of alcohol consumption is hazardous for older people? Functioning and mortality in U.S. and English national cohorts. Journal of the American Geriatrics Society, 55(1), 49–57. https://doi.org/10.1111/j.1532-5415.2006.01007.x
- Lao, Y., Hou, L., Li, J., Hui, X., Yan, P., & Yang, K. (2021). Association between alcohol intake, mild cognitive impairment and progression to dementia: A dose-response meta-analysis. Aging Clinical and Experimental Research, 33(5), 1175–1185. https://doi.org/10.1007/s40520-020-01605-0
- Lee, Y. H., Lu, P., Chang, Y. C., Shelley, M., Lee, Y. T., & Liu, C. T. (2019). Associations of alcohol consumption status with activities of daily living among older adults in China. Journal of Ethnicity and Substance Abuse, 20(3), 1–16.
- León-Muñoz, L. M., Guallar-Castillon, P., Garcia-Esquinas, E., Galan, I., & Rodriguez-Artalejo, F. (2017). Alcohol drinking patterns and risk of functional limitations in two cohorts of older adults. Clinical Nutrition, 36(3), 831–838.
- Lipnicki, D. M., Makkar, S. R., Crawford, J. D., Thalamuthu, A., Kochan, N. A., Lima-Costa, M. F., Castro-Costa, E., Ferri, C. P., Brayne, C., Stephan, B., Llibre-Rodriguez, J. J., Llibre-Guerra, J. J., Valhuerdi-Cepero, A. J., Lipton, R. B., Katz, M. J., Derby, C. A., Ritchie, K., Ancelin, M.-L., Carrière, I., … Sachdev, P. S., for Cohort Studies of Memory in an International Consortium (COSMIC) (2019). Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: A COSMIC collaboration cohort study. PLOS Medicine, 16(7), e1002853. https://doi.org/10.1371/journal.pmed.1002853
- Lopes, M. A., Furtado, E. F., Ferrioli, E., Litvoc, J., & Bottino, C. M. (2010). Prevalence of alcohol-related problems in an elderly population and their association with cognitive impairment and dementia. Alcoholism: Clinical and Experimental Research, 34(4), 726–733. https://doi.org/10.1111/j.1530-0277.2009.01142.x
- Maletz, L., & Gardner, A. (1943). A study of alcoholic deterioration. Quarterly Journal of Studies on Alcohol, 3(4), 546–553. https://doi.org/10.15288/qjsa.1943.3.546
- Nurk, E., Refsum, H., Drevon, C. A., Tell, G. S., Nygaard, H. A., Engedal, K., & Smith, A. D. (2009). Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. The Journal of Nutrition, 139(1), 120–127. https://doi.org/10.3945/jn.108.095182
- Owen, A. M., Downes, J. J., Sahakian, B. J., Polkey, C. E., & Robbins, T. W. (1990). Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia, 28(10), 1021–1034. https://doi.org/10.1016/0028-3932(90)90137-D
- Owen, A. M., Herrod, N. J., Menon, D. K., Clark, J. C., Downey, S. P., Carpenter, T. A., Minhas, P. S., Turkheimer, F. E., Williams, E. J., Robbins, T. W., Sahakian, B. J., Petrides, M., & Pickard, J. D. (1999). Redefining the functional organization of working memory processes within human lateral prefrontal cortex. The European Journal of Neuroscience, 11(2), 567–574. https://doi.org/10.1046/j.1460-9568.1999.00449.x
- Reid, M. C., Van Ness, P. H., Hawkins, K. A., Towle, V., Concato, J., & Guo, Z. (2006). Light to moderate alcohol consumption is associated with better cognitive function among older male veterans receiving primary care. Journal of Geriatric Psychiatry and Neurology, 19(2), 98–105. https://doi.org/10.1177/0891988706286513
- Ridley, N. J., Draper, B., & Withall, A. (2013). Alcohol-related dementia: An update of the evidence. Alzheimer’s Research & Therapy, 5(1), 3. https://doi.org/10.1186/alzrt157
- Rist, P. M., Marden, J. R., Capistrant, B. D., Wu, Q., & Glymour, M. M. (2015). Do physical activity, smoking, drinking, or depression modify transitions from cognitive impairment to functional disability? Journal of Alzheimer’s Disease, 44(4), 1171–1180. https://doi.org/10.3233/JAD-141866
- Sabia, S., Fayosse, A., Dumurgier, J., Dugravot, A., Akbaraly, T., Britton, A., Kivimäki, M., & Singh-Manoux, A. (2018). Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ (Clinical Research ed.), 362, k2927. https://doi.org/10.1136/bmj.k2927
- Saunders, J. B., Aasland, O. G., Babor, T. F., de la Fuente, J. R., & Grant, M. (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction (Abingdon, England), 88(6), 791–804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x
- Schwarzinger, M., Pollock, B. G., Hasan, O. S. M., Dufouil, C., Rehm, J., Baillot, S., Guibert, Q., Planchet, F., & Luchini, S. (2018). Contribution of alcohol use disorders to the burden of dementia in France 2008-13: A nationwide retrospective cohort study. The Lancet Public Health, 3(3), e124–e132. https://doi.org/10.1016/S2468-2667(18)30022-7
- Spitzer, R. L., Kroenke, K., Williams, J. B., & Lowe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. https://doi.org/10.1001/archinte.166.10.1092
- Suzuki, H., Venkataraman, A. V., Bai, W., Guitton, F., Guo, Y., Dehghan, A., & Matthews, P. M., Alzheimer’s Disease Neuroimaging Initiative (2019). Associations of regional brain structural differences with aging, modifiable risk factors for dementia, and cognitive performance. JAMA Network Open, 2(12), e1917257. https://doi.org/10.1001/jamanetworkopen.2019.17257
- Tapert, S. F., Brown, G. G., Kindermann, S. S., Cheung, E. H., Frank, L. R., & Brown, S. A. (2001). fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research, 25(2), 236–245. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11236838
- Topiwala, A., Allan, C. L., Valkanova, V., Zsoldos, E., Filippini, N., Sexton, C., Mahmood, A., Fooks, P., Singh-Manoux, A., Mackay, C. E., Kivimäki, M., & Ebmeier, K. P. (2017). Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ, 357, j2353. https://doi.org/10.1136/bmj.j2353
- Turken, A., Whitfield-Gabrieli, S., Bammer, R., Baldo, J. V., Dronkers, N. F., & Gabrieli, J. D. (2008). Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. NeuroImage, 42(2), 1032–1044. https://doi.org/10.1016/j.neuroimage.2008.03.057
- van Eijk, J., Demirakca, T., Frischknecht, U., Hermann, D., Mann, K., & Ende, G. (2013). Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcoholism, Clinical and Experimental Research, 37(1), 67–74. https://doi.org/10.1111/j.1530-0277.2012.01853.x
- Wik, G., Borg, S., Sjögren, I., Wiesel, F. A., Blomqvist, G., Borg, J., Greitz, T., Nybäck, H., Sedvall, G., & Stone-Elander, S. (1988). PET determination of regional cerebral glucose metabolism in alcohol-dependent men and healthy controls using 11C-glucose. Acta Psychiatrica Scandinavica, 78(2), 234–241. https://doi.org/10.1111/j.1600-0447.1988.tb06330.x
- Xu, W., Wang, H., Wan, Y., Tan, C., Li, J., Tan, L., & Yu, J. T. (2017). Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. European Journal of Epidemiology, 32(1), 31–42. https://doi.org/10.1007/s10654-017-0225-3
- Zuccalà, G., Onder, G., Pedone, C., Cesari, M., Landi, F., Bernabei, R., & Cocchi, A. (2001). Dose-related impact of alcohol consumption on cognitive function in advanced age: results of a multicenter survey. Alcoholism: Clinical and Experimental Research. 25(12), 1743–1748.