 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
Late-life depression (LLD) is a common and debilitating disorder. Previously, resting-state studies have revealed abnormal functional connectivity (FC) of brain networks in LLD. Since LLD is associated with emotional-cognitive control deficits, the aim of this study was to compare FC of large-scale brain networks in older adults with and without a history of LLD during a cognitive control task with emotional stimuli.
Methods
Cross-sectional case-control study. Twenty participants diagnosed with LLD and 37 never-depressed adults 60–88 years of age underwent functional magnetic resonance imaging during an emotional Stroop task. Network-region-to-region FC was assessed with seed regions in the default mode, the frontoparietal, the dorsal attention, and the salience networks.
Results
FC between salience and sensorimotor network regions and between salience and dorsal attention network regions were reduced in LLD patients compared to controls during the processing of incongruent emotional stimuli. The normally positive FC between these networks were negative in LLD patients and inversely correlated with vascular risk and white matter hyperintensities.
Conclusions
Emotional-cognitive control in LLD is associated with aberrant functional coupling between salience and other networks. This expands on the network-based LLD model and proposes the salience network as a target for future interventions.
Introduction
Late-life depression (LLD) is common but not a normal part of aging. Several recognized risk factors for depression become more prevalent in older adulthood, such as spousal bereavement, social isolation, and deterioration of physical performance that restricts activities. Nevertheless, several studies have found that mental well-being appears to be maintained or even increase with advancing adult age (Steptoe et al., Citation2015), and in certain attention and memory tasks, an age-related positivity effect is observed with bias toward emotionally positive information (Reed & Carstensen, Citation2012). For those who do develop LLD, this is associated with reduced quality of life, risk of subsequent frailty with loss of independence (Dapp et al., Citation2021), a twofold increased dementia risk (Cherbuin et al., Citation2015), and premature death (Wei et al., Citation2019). To prevent the severe consequences of depression in older adulthood, it is important to understand the alterations in the brain underlying LLD and how these are different from the brain changes seen in healthy aging.
Several studies have compared neuroimaging findings in older adults with and without depression. A recent coordinate-based meta-analysis attempting to summarize both structural voxel-based morphometry and functional neuroimaging comparing LLD and healthy aging controls failed to localize a significant convergent regional abnormality (Saberi et al., Citation2022). The lack of convergence on any distinct brain areas as abnormal in LLD has shifted the pathological models of LLD from focusing on single brain regions to considering the disruption of brain networks (Tadayonnejad & Ajilore, Citation2014). Large-scale brain networks are sets of spatially separate brain areas displaying temporal dependence in their patterns of neuronal activity, that is, they are functionally connected. Functional connectivity (FC) can be measured as the time-series correlation of blood oxygen level-dependent signals obtained by functional magnetic resonance imaging (fMRI). Some of the most well-known large-scale brain networks have been implicated in depression. The default mode network (DMN) is a network easily identifiable in resting-state fMRI (rs-fMRI) and typically becomes deactivated during the performance of attention-demanding external tasks (Raichle, Citation2015). Its primary components are the medial prefrontal cortex (PFC), the posterior cingulate cortex with the adjacent precuneus, and the angular gyri. The DMN is known to be involved when thinking about oneself, personal past or future, and about the mental state of others (Yeshurun et al., Citation2021). In depression, aberrant DMN activation has been linked to rumination (Zhou et al., Citation2020). In rs-fMRI, DMN activity tends to be anticorrelated with the activity of the frontoparietal network (FPN) (Raichle, Citation2015). In the literature, several other names are used for versions of the FPN, including the extrinsic mode network, the central executive network, the executive control network, the cognitive control network, and the lateral FPN (Uddin et al., Citation2019). For clarity, the term FPN is used consistently throughout this paper. The main hubs of the FPN are located in the dorsolateral PFC and the parietal cortex adjacent to the intraparietal sulcus (Gratton et al., Citation2018). The FPN is involved in executive control and solving demanding goal-driven tasks. When the FPN is engaged during external tasks, the DMN becomes relatively disengaged. Conversely, when the DMN is engaged during internal mental processes, the FPN is comparatively disengaged (Hugdahl et al., Citation2019). A third network, the salience network, is proposed to have a key role in detecting salient stimuli and responding by shifting the balance between recruitment of the DMN and the FPN accordingly (Goulden et al., Citation2014). The primary components of the salience network are the anterior insula and the dorsal anterior cingulate cortex (dACC). Resting-state studies have additionally identified regions in the rostral PFC and the supramarginal gyrus/angular gyrus as part of the salience network (Raichle, Citation2011). Subcortical structures associated with the salience network include the amygdala, substantia nigra, and ventral tegmental area. The current use of the term salience network subsumes the ventral attention network and the cingulo-opercular network (Uddin et al., Citation2019). The salience network also interacts with the dorsal attention network (DAN), whose core regions include the frontal eye fields (FEF) and the intraparietal sulcus. Disrupted FC within and between the DMN, FPN, and salience networks has been proposed as a possible network-based model of LLD (Gunning et al., Citation2021), although the detailed nature of the network disruptions is unknown.
Previous studies of the large-scale brain networks in LLD have mainly used rs-fMRI. Individuals with LLD commonly have reduced performance on cognitive tasks in addition to the pathognomonic aberrant regulation of emotions. Assessment of brain network connectivity during processing of a task that is both cognitively demanding and contains emotional stimuli thus appears particularly apt for revealing underlying mechanisms in LLD. In younger adults with a history of depression, the tendency to be biased by emotionally negative information has recently been linked to changes in the FC between the DMN and the salience network during processing of an emotional interference task (Guha et al., Citation2021). In older adults, such negativity bias is inconsistently observed perhaps due to counteraction by the age-related positivity effect (Gray et al., Citation2020). Changes in FC between the DMN and the salience network during emotional interference processing are, however, also a feature of healthy aging (Almdahl et al., Citation2021). Since normal aging does not predispose individuals to depression, a relevant research question is whether the FC of these large-scale networks during emotional interference processing differs between LLD and healthy aging. Correspondingly, the primary aim of this study was to compare within- and between-network FC of the DMN, FPN, DAN, and salience networks during processing of an emotional interference task in older adults (60–90 years of age) with and without a history of LLD. A secondary aim was to investigate how these associations are modulated by known biological risk factors for LLD, such as vascular disease risk and white matter hyperintensities (WMHs).
Materials and methods
Participants and screening assessments
Two groups of participants were included in the study: An LLD group and a never-depressed control group. The LLD group was recruited from specialist psychiatric health care units in and around Oslo. The main inclusion criterium for the LLD group was having a unipolar depressive episode according to the ICD-10 and/or DSM-V criteria occurring after the age of 60. For ethical reasons the patients in the LLD group had received treatment as prescribed by their therapist/physician without delay and consequently some of the participants would have experienced improvement of their depressive symptoms by the time of the study. The control group was recruited from the community through advertisements for volunteers >60 years of age, with no current or previous depression, no particular concerns regarding memory or other cognitive functions, no concerns about the current development of dementia, and no known heightened risk of depression or dementia based on family history. The control participants were screened for psychiatric symptoms by a medical physician and/or psychologist and none fulfilled the diagnostic criteria for a current or a previous depressive episode. The following inclusion criteria applied to both groups: fluency in the Norwegian language, no severe hearing or visual impairment, no known contraindications for MRI, no dementia diagnosis at inclusion, and no other major psychiatric or somatic disease or medication/substance use capable of significantly reducing cognitive functions. Based on these criteria, 70 individuals were included: 30 in the LLD group and 40 in the control group. Exclusion criteria after inclusion were the inability to perform the cognitive tests or complete the MRI with sufficient image quality or more than ±2.5 mm translational or ±2.5° rotational movement during the MRI task sequence. In total, 13 individuals were excluded; two were found to have significantly reduced visual functions interfering with cognitive testing, two did not fit into the 32-channel head coil used in the MRI scanner, three were unable to complete the full MRI session, one had a large tumor with a mass effect revealed on the MRI, one was excluded due to a technical error during the MRI, and four were excluded due to excessive in-scanner head motion. The resulting final sample consisted of 20 participants with LLD and 37 healthy older adults.
All participants were interviewed about their current and previous medical history and performed a battery of neuropsychological tests. Depressive symptoms were measured with the short form of the Geriatric Depression Scale (Sheikh & Yesavage, Citation1986). A vascular risk score was calculated based on the Framingham study’s prediction of 10-year cardiovascular disease risk using the method with body-mass index rather than blood lipids (the calculator is available at https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/). All participants provided written informed consent and the study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki. This study is a part of the DEPDEM project at Oslo University Hospital and was approved by the South East Regional Committee for Medical and Health Research Ethics in Norway [Reference No. 2016/1938].
Emotional Stroop task paradigm and fMRI acquisition
The protocol for the event-related emotional Stroop task (eStroop) and the fMRI protocol used in the study have been described previously (Almdahl et al., Citation2021). Briefly, the participants viewed photographs of faces expressing happiness or fear with the word happy or fear written in capital red letters across the face. The presented word in each trial was either congruent or incongruent with the emotional facial expression. The participants were asked to identify the facial expression while ignoring the word. For incongruent trials, there was a direct semantic conflict between the task-relevant facial emotion and the task-irrelevant emotional word. For congruent trials, the word and the face expressed the same emotion. There were 148 face-word trials; half were incongruent and half were congruent. Each trial lasted 1 s and was separated by a varying intertrial interval of 3–5 s, resulting in a total duration of 12 min and 27 s. Trials with registered response time (RT) < 300 ms were removed (two of 8436 trials).
MRI preprocessing and analysis
The FC analyses were performed using the CONN toolbox version 18.b (Whitfield-Gabrieli & Nieto-Castanon, Citation2012). The functional images were realigned, unwarped, and slice-time corrected. Outlier scans for scrubbing were identified with a scan-to-scan motion threshold of 0.9 mm and a global signal z value threshold of 5. The functional and structural images were segmented and normalized to MNI space before the functional images were smoothed with an 8 mm FWHM kernel. Component-based noise correction was performed with five components each from white matter and cerebrospinal fluid, the six regressors from the realignment with their first-order temporal derivatives, the scrubbing components, and the baseline, congruent, incongruent, and error conditions with first-order derivatives. The fMRI signal was linear detrended and high-pass filtered (>0.008 Hz). The aim was to study FC during emotional interference processing and thus the incongruent trials were selected in the network analysis. An alternative would be to incorporate a between-condition contrast, constricting the connectivity analysis to the difference between the incongruent and congruent trials, but based on our previous experience with the task paradigm this was considered to be a too stringent approach. Seed-based FC analyses were performed as general linear models with hemodynamic response function weighting. Bivariate correlations were the outcome measures. From the CONN functional network atlas, the network regions of interest (ROIs) of the DMN (four), the FPN (four), the DAN (four), and the salience network (seven) were selected as seeds. (All the seeds and targets are listed in Supplementary Table S1). The targets were all the 32 canonical network ROIs in the atlas. Significance was set at <0.05 with a false discovery rate (FDR) correction at the seed level.
Table 1. Demographic information, clinical, and vascular scores.
The total burden of WMHs in the brain was quantified visually by a neuroradiologist based on a fluid-attenuated inversion-recovery MRI sequence (FLAIR) using Fazekas’ scale (Fazekas et al., Citation1987). In addition, total volume of hyperintense lesions in the FLAIR images were automatically estimated with use of the lesion prediction algorithm (Schmidt, Citation2017) as implemented in the LST toolbox version 3.0.0 (www.statistical-modelling.de/lst.html) for SPM.
Although the main focus of the study was network FC, potential differences between LLD and healthy older adults in the brain regions activated during emotional interference were assessed as additional analyses and the methods are described in the supplementary material.
Statistical analyses
The descriptive and cognitive data were compared between the groups using the Mann-Whitney U test (MWU) for continuous variables and the χ2-test for categorical variables. In addition, to adjust for age, sex and years of education as potentially confounding variable, multiple linear regression models were estimated with the specified descriptive or cognitive variable as the dependent variable and group, age, sex, and education as the independent variables. The analyses were performed using SPSS version 26 (SPSS Statistics, IBM). To address the problem of multiple testing for the analysis of 15 cognitive scores, FDR correction was performed following the Benjamini-Hochberg procedure. For eStroop task performance, a 2 × 2 mixed analysis of variance (ANOVA) with congruency as the “within-subject” factor (repeated measure for the incongruent and the congruent trials) and group as the “between-subjects” factor (LLD/controls) was performed twice; one model with the mean RTs of correct trials as the dependent variable and another model with accuracy as the dependent variable. The key purpose of the mixed ANOVA was to assess whether there was an interaction between the two factors on the dependent variable, i.e. whether the incongruency effect differs between the LLD group and the control group. The RTs were log-transformed to achieve an approximately normal distribution. Accuracy data were skewed after log-transformation, and ANOVA-type statistics for accuracy were calculated with the Brunner & Langer nonparametric model using the nparLD package in R version 3.6.3 (R Core Team, https://www.R-project.org/). The effect size Cohen’s d (d) was calculated from the MWU/χ2-value for the descriptive and cognitive scores and from the t-statistics for the FC results using Psychometrica calculators (Lenhard & Lenhard, Citation2016). Power calculations performed during planning of the project based on previous fMRI studies of brain activation during eStroop-like tasks suggested that 20 subjects in each group would yield a power >80%. For FC of the connections that were significantly different between groups, explorative analyses were performed within the LLD group with calculation of Spearman’s rank correlation coefficients with the depression score, completion time (in seconds) on the inhibition trial of the D-KEFS Color-Word Interference Test (Delis et al., Citation2000) as a measure of executive function, the vascular risk score, and the measures of WMH burden.
Results
Demographic, clinical and cognitive scores
Descriptive characteristics are shown in . There was a small but not statistically significant group difference in age, sex, and years of education; therefore, the subsequent between-group analyses were performed both with and without adjustment for these three demographic factors. Fourteen (70%) participants in the LLD group reported having experienced depression also earlier in life and sixteen (80%) were currently using antidepressants. The LLD group also had higher use of non-psychotropic drugs including lipid-lowering drugs both before and after demographic correction. WMH burden was higher in the LLD group, but the group difference was not statistically significant after correction for the demographic factors. The LLD group performed worse than the controls on tests of executive functions, including completion time of Part B of the Trail-making test and the inhibition and task-switching parts of the D-KEFS Color-Word Interference Test both before and after correction for the demographic factors (Supplementary table S2).
Table 2. Network ROI-to-ROI functional connectivity: significant group differences.
Performance on the eStroop task
The mean RT was longer in the LLD group (915.7 ms, standard deviation (SD) 156.3) than in the control group (822.7 ms, SD 138.6; F(df1)=5.9, p = 0.018 for the main effect of group in the ANOVA). Accuracy was not significantly different between the groups (LLD 91.6%, SD 8.7; controls 94.9%, SD 4.1; F(df1)=2.6, p = 0.108). There was no significant interaction (congruency x group interaction F(df1)=1.7, p = 0.204 for RT and F(df1)=0.02, p = 0.891 for accuracy) signifying that there was no significant difference in the incongruency effect between the groups. There was a significant main effect of congruency indicating that the face-word incongruency produced an effect both on RT and accuracy overall (F(df1)=86.3, p < 0.001 for RT and F(df1)=24.8, p < 0.001 for accuracy).
Functional network connectivity
Comparing the groups on FC between network ROIs during the incongruent task condition, there were significant differences for a number of the salience network seeds and one of the DAN seeds as shown in . After adjustment for age, sex, and education, the functional connections between the ACC region of the salience network and the left lateral region of the sensorimotor network, between the right rostral PFC of the salience network and the left lateral region of the sensorimotor network, and between the right rostral PFC of the salience network and the left FEF of the DAN were significantly weaker in the LLD group, with large effect sizes ( and ).
Figure 1. Functional connectivity differences between the groups. The functional connections that had significantly weaker strength in the LLD group than in the control group are shown in blue with the corresponding box plots. All the possible functional connections (19 x 32 correlations) that were assessed in the analysis are shown in the background as gray lines.
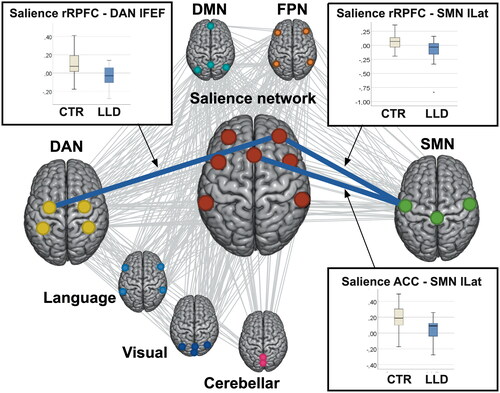
These connections were further explored within the LLD group. None of the connections correlated with depressive symptom score or with executive function assessed by completion time on the inhibition trial of the D-KEFS Color-Word Interference Test. A higher vascular risk score correlated with decreased FC between the ACC region of the salience network and the left lateral region of the sensorimotor network, while a higher WMH burden correlated with decreased FC between the right rostral PFC of the salience network and the left FEF of the DAN, as shown in .
Table 3. Correlation within the LLD group between functional connectivity and measures of executive function, depressive symptoms, vascular risk, and white matter hyperintensity burden
The supplementary whole-brain analysis of task-related brain activation for the incongruent versus congruent contrast revealed a cluster in the left visual association cortex/angular gyrus with reduced activation in the LLD group both before and after demographic correction (Supplementary table S3).
Discussion
Previously, FC of large-scale brain networks in LLD has mainly been assessed in resting-state studies. Many of these studies have focused on the DMN and/or the FPN and reported that relative to healthy controls, older adults with depression appear to have increased within-network connectivity of the DMN (Andreescu et al., Citation2013; Eyre et al., Citation2016; Wu et al., Citation2011), decreased within-network connectivity of the FPN (Alexopoulos et al., Citation2012), and reduced (Andreescu et al., Citation2013; Chen et al., Citation2016; Gandelman et al., Citation2019) or altered (Li et al., Citation2017) connectivity between the DMN and FPN at rest. The current study assessed network FC during an emotional interference task and found that older adults with and without a history of LLD had comparable FC of the DMN and FPN. The LLD group did, however, have aberrant connectivity of the salience network. The salience network is the network driving the shifts in the dynamic balance between the DMN and the FPN (Goulden et al., Citation2014). It is therefore conceivable that disruptions of the salience network could produce aberrations in FC between the DMN and the FPN. The salience network has a key position in the triple network model proposed as a unifying framework for understanding affective and cognitive dysfunction in psychiatric and neurological disorders (Menon, Citation2011). The model draws on the salience network being an integrating hub that detects and maps saliency based on external stimuli from sensory networks, internal self-referential information from the DMN, reward and motivational input from limbic circuits, and top-down control from the FPN (and the DAN). After saliency assignment, the salience network then initiates control signals to other large-scale networks to enable access to attention and other resources (Menon, Citation2011). This framework has recently been adopted in a network-based model for LLD, which proposes that disruptions within and between the salience network, the DMN, and the FPN give rise to maladaptive regulation of emotions and cognitive deficits (Gunning et al., Citation2021). A few resting-state studies have reported an altered FC of the salience network in LLD patients relative to healthy older adults, but with divergent results. For instance, one resting-state study assessing synchronization of networks identified by individual component analysis (ICA) found that a network corresponding to the salience network was positively correlated with the FPN in healthy older adults, but the correlation was significantly less positive in actively depressed older adults (Wang et al., Citation2015). Another ICA-based resting-state LLD study found abnormally increased positive connectivity between the dACC of the salience network and the left FPN, while the anterior insula of the salience network had abnormally decreased negative connectivity with the left FPN (Li et al., Citation2017). A seed-based connectivity study using the posterior cingulate cortex of the DMN as the seed found decreased FC with the dACC of the salience network in late-onset depression (Yin et al., Citation2016). Although there are divergences in the specific FC patterns reported in these previous resting-state studies, their results, together with those of the current task-based study, support that the interactions of the salience network with other networks are disrupted in LLD in line with the proposed network-based model.
Brain network characteristics are different during task performance compared to the resting state. Resting-state FC correlated strongly with task FC in a meta-analysis, but more efficient global information transmission and greater integration between brain systems was found during task performance (Di et al., Citation2013). While the FPN and the DMN tend to be anticorrelated in the resting state, this can change to greater context-dependent integration during tasks (Vatansever et al., Citation2015). Cognitive control has been suggested to rely on such task-dependent changes in coupling between the FPN, the DMN, and the salience network (Cocchi et al., Citation2013). Impairment of cognitive control is an important feature of LLD, and it is thus not unlikely that alterations in task-evoked FC, as well as in intrinsic FC, are involved in the pathophysiology of LLD. Large-scale network interactions during tasks have scarcely been studied before, but one study using an executive-control task found reduced FC between the dACC and the dorsal PFC in LLD patients relative to nondepressed older adults (Aizenstein et al., Citation2009). The study only assessed these two regions and did not address large-scale brain networks as such, but the two ROIs correspond to prominent hubs of the salience network and the FPN. The finding in the current study of reduced FC of the rostral PFC of the salience network with regions of the DAN and the sensorimotor network is noteworthy because the rostral PFC has been hypothesized to play a strategic role in the context-dependent integration of the salience network and the FPN/DAN during cognitive control (Cocchi et al., Citation2013). In the control group, the FC of the rostral PFC with the DAN and the sensorimotor network was positive, but in the LLD group, it was negative, suggesting a disconnection between these systems.
Our results point to deficits in the salience networks’ interactions with the sensorimotor network in LLD. The salience network has strong and somatotopically distinct functional connections with the sensorimotor cortex in healthy individuals and is believed to integrate sensory inputs with other information to map what is most salient and then facilitate behavioral responses, including immediate motor responses (Hegarty et al., Citation2020). The observed aberrations in the coupling between the salience and sensorimotor networks could thus potentially lead to weak salience mapping and slow motor responses, as posited by the triple network model (Menon, Citation2011). The LLD group did, on average, have slower responses with longer task RTs. Successful performance on the eStroop task also relies on the control of motor inhibition. The LLD group had diminished FC between the right rostral PFC of the salience network and the left FEF of the DAN. The between-network FC of the salience network and the DAN was also the FC measure that best explained variance in motor inhibition in a graph theory-based study of healthy adults (Hsu et al., Citation2020). Emerging evidence implicates sensory and motor processing in psychopathology (Harrison et al., Citation2019). Our supplementary analysis of brain activation patterns revealed reduced activity in the left visual association cortex in the LLD group relative to the controls. Reduced activation in the visual association areas has been previously observed in major depressive disorder during the performance of a visual working memory task (Le et al., Citation2017), suggesting that visual information processing can be implicated in depression.
Exploratory correlation analyses showed that FC between the right rostral PFC of the salience network and the left FEF of the DAN correlated with WMH burden within the LLD group. This echoes the results of studies linking LLD with white matter lesions. WMH burden increases with aging but has been reported to be higher in LLD than in age-matched controls (Respino et al., Citation2019) and is associated with an increased risk of incident LLD (Almdahl et al., Citation2022; van Agtmaal et al., Citation2017). WMHs mark areas of various degrees of microstructural aberrations, including edema, demyelination, and axonal loss (Wardlaw et al., Citation2015), that are believed to impair communication in fiber tracts, resulting in disturbance of networks involved in emotion and cognitive processing (Alexopoulos, Citation2019). WMHs in LLD patients have previously been found to correspond with disrupted structural connectivity in cognitive control and sensorimotor systems, which again correspond with executive dysfunction (Respino et al., Citation2019). In the current study, there was no observed association between FC and executive function in the LLD group, but this was a subgroup analysis with a low sample size, which discourages strong inferences. We did find a negative correlation with the vascular risk score within the LLD group, which is consistent with previous reports of an association between some vascular risk factors and LLD (Valkanova & Ebmeier, Citation2013). Vascular risk factor burden has even been found to increase the risk of new-onset depression independent of WMHs, suggesting that it may be mediated by other microvascular changes (Adams et al., Citation2018). This could explain why the FC of one connection appeared to be correlated with vascular risk, while the FC of another was correlated with the WMH burden. Collectively, these findings further motivate efforts to improve vascular health, as it might also prevent LLD. The current results may have implications for future new interventions in LLD, as it appears that network FC patterns can be targeted by cognitive and physical training. It has been shown that working memory task training can increase the functional integration of the salience network with the FPN and the DAN (Finc et al., Citation2020), and aerobic exercise can reportedly increase the within-network functional integrity of the salience network in older adults (Voss et al., Citation2019).
LLD is a heterogeneous disorder both in terms of clinical presentation and underlying pathologies, which has probably contributed to the inconsistencies in the LLD literature (Alexopoulos, Citation2019). Our study is limited by the small sample size, and it prevented us from comparing subgroups based on severity of depressive symptoms, medication use, sex, or age at onset. Similarly, we did not assess subgroups based on treatment response. Some of the participants will have experienced improvement of their depressive symptoms possibly resulting in changes of their functional connectivity. Task-based fMRI studies are more difficult to perform than rs-fMRI. In our study, 14% of the original sample was unable to complete the task-fMRI protocol with good quality data and had to be excluded. This is also reflected in the literature, where sample sizes are usually much lower for task-based relative to resting-state studies. Nevertheless, because network interactions appear to reconfigure when the brain is actively processing external stimuli, it is crucial to also include task-based studies to gauge the full breadth of network dysfunction in LLD. To the best of our knowledge, the results of direct assessment of FC within and between multiple large-scale brain networks during performance of a cognitive task in LLD relative to healthy older adults have not been reported before. The results are explorative and need to be confirmed in other datasets. Another design limitation was the slight demographic differences between the groups (the control group consisted of more men than women, the opposite was true for the LLD group, and on average the control group was younger and marginally more educated). To address this, the demographical variables were included as additional covariates in the analyses of the clinical scores and the fMRI data. Many studies that include a version of the Stroop task use a standardized interference score. We chose to assess performance on the eStroop task with a congruency x group ANOVA and thus we did not calculate a separate interference score. One strength of the study is that it contains a cognitively well-characterized sample through the use of a battery of cognitive tests. There are different approaches to assess FC, and results derived from seed-based ROI-to-ROI connectivity methods cannot be directly compared with those from ICA- or graph theory-based methods. Network FC may also depend on specific task features. This study used an emotional task; thus, a pure cognitive task could lead to different FC states. Another limitation of the current approach is that it only assesses the interaction between two network regions at a time. Future task-based studies should thus explore the more complex simultaneous interaction of several networks.
In conclusion, during the processing of a cognitive control task with emotional stimuli, the salience network had reduced FC with the sensorimotor network and the DAN in LLD patients, even to the degree that normally positive functional connections between these networks had become negative. This reduced between-network FC of the salience network correlated with a higher vascular risk score and greater WMH burden within the LLD group. The results support and extend the proposed network model for LLD.
Supplemental Material
Download MS Word (562.4 KB)Acknowledgements
The authors thank all of the participants in the DEPDEM project for their time and effort in completing assessments for the benefit of research. This study was funded by the Department of Old Age Psychiatry, Oslo University Hospital. The project has received internal research grants from the Division of Mental Health and Addiction at Oslo University Hospital annually since 2017. Recruitment of patients were done at the Department of Old Age Psychiatry and the District Psychiatric Centers at Oslo University Hospital, at the Old Age Psychiatry Unit of the Norwegian Women’s Public Health Association Institution Grefsenlia, and at the private practice of specialist psychologist in old age psychiatry, Mirka Kraus. We thank all staff members that were involved in recruitment of participants at these institutions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adams, S., Conner, S., Himali, J. J., Beiser, A., Vasan, R. S., Seshadri, S., & Pase, M. P. (2018). Vascular risk factor burden and new-onset depression in the community. Preventive Medicine, 111, 348–350. https://doi.org/10.1016/j.ypmed.2017.11.022
- Aizenstein, H. J., Butters, M. A., Wu, M., Mazurkewicz, L. M., Stenger, V. A., Gianaros, P. J., Becker, J. T., Reynolds, C. F., & Carter, C. S. (2009). Altered functioning of the executive control circuit in late-life depression: Episodic and persistent phenomena. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 17(1), 30–42. https://doi.org/10.1097/JGP.0b013e31817b60af
- Alexopoulos, G. S. (2019). Mechanisms and treatment of late-life depression. Translational Psychiatry, 9(1), 188. https://doi.org/10.1038/s41398-019-0514-6
- Alexopoulos, G. S., Hoptman, M. J., Kanellopoulos, D., Murphy, C. F., Lim, K. O., & Gunning, F. M. (2012). Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders, 139(1), 56–65. https://doi.org/10.1016/j.jad.2011.12.002
- Almdahl, I. S., Agartz, I., Hugdahl, K., & Korsnes, M. (2022). Brain pathology and cognitive scores prior to onset of late-life depression. International Journal of Geriatric Psychiatry, 37(3), 1–15. https://doi.org/10.1002/gps.5686
- Almdahl, I. S., Martinussen, L. J., Agartz, I., Hugdahl, K., & Korsnes, M. S. (2021). Inhibition of emotions in healthy aging: Age-related differences in brain network connectivity. Brain and Behavior, 11(5), e02052. https://doi.org/10.1002/brb3.2052
- Andreescu, C., Tudorascu, D. L., Butters, M. A., Tamburo, E., Patel, M., Price, J., Karp, J. F., Reynolds, C. F., & Aizenstein, H. (2013). Resting state functional connectivity and treatment response in late-life depression. Psychiatry Research, 214(3), 313–321. https://doi.org/10.1016/j.pscychresns.2013.08.007
- Chen, J., Shu, H., Wang, Z., Zhan, Y., Liu, D., Liao, W., Xu, L., Liu, Y., & Zhang, Z. (2016). Convergent and divergent intranetwork and internetwork connectivity patterns in patients with remitted late-life depression and amnestic mild cognitive impairment. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 83, 194–211. https://doi.org/10.1016/j.cortex.2016.08.001
- Cherbuin, N., Kim, S., & Anstey, K. J. (2015). Dementia risk estimates associated with measures of depression: A systematic review and meta-analysis. BMJ Open, 5(12), e008853. https://doi.org/10.1136/bmjopen-2015-008853
- Cocchi, L., Zalesky, A., Fornito, A., & Mattingley, J. B. (2013). Dynamic cooperation and competition between brain systems during cognitive control. Trends in Cognitive Sciences, 17(10), 493–501. https://doi.org/10.1016/j.tics.2013.08.006
- Dapp, U., Minder, C. E., Golgert, S., Klugmann, B., Neumann, L., & von Renteln-Kruse, W. (2021). The inter-relationship between depressed mood, functional decline and disability over a 10-year observational period within the Longitudinal Urban Cohort Ageing Study (LUCAS). Journal of Epidemiology and Community Health, 75(5), 450–457. https://doi.org/10.1136/jech-2020-214168
- Delis, D. C., Kramer, J. H., Kaplan, E., & Ober, B. A. (2000). California verbal learning test. 2nd ed. (Adult version). San Antonio, TX: Psychological Corporation.
- Di, X., Gohel, S., Kim, E. H., & Biswal, B. B. (2013). Task vs. rest-different network configurations between the coactivation and the resting-state brain networks. Frontiers in Human Neuroscience. 7, 493. https://doi.org/10.3389/fnhum.2013.00493
- Eyre, H. A., Yang, H., Leaver, A. M., Van Dyk, K., Siddarth, P., Cyr, N. S., Narr, K., Ercoli, L., Baune, B. T., & Lavretsky, H. (2016). Altered resting-state functional connectivity in late-life depression: A cross-sectional study. Journal of Affective Disorders, 189, 126–133. https://doi.org/10.1016/j.jad.2015.09.011
- Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., Zimmerman,., & R., A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR. American Journal of Roentgenology, 149(2), 351–356. https://doi.org/10.2214/ajr.149.2.351
- Finc, K., Bonna, K., He, X., Lydon-Staley, D. M., Kuhn, S., Duch, W., & Bassett, D. S. (2020). Dynamic reconfiguration of functional brain networks during working memory training. Nature Communications, 11(1), 2435. https://doi.org/10.1038/s41467-020-15631-z
- Gandelman, J. A., Albert, K., Boyd, B. D., Park, J. W., Riddle, M., Woodward, N. D., Kang, H., Landman, B. A., & Taylor, W. D. (2019). Intrinsic Functional Network Connectivity Is Associated With Clinical Symptoms and Cognition in Late-Life Depression. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 4(2), 160–170. https://doi.org/10.1016/j.bpsc.2018.09.003
- Goulden, N., Khusnulina, A., Davis, N. J., Bracewell, R. M., Bokde, A. L., McNulty, J. P., & Mullins, P. G. (2014). The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. NeuroImage, 99, 180–190. https://doi.org/10.1016/j.neuroimage.2014.05.052
- Gratton, C., Sun, H., & Petersen, S. E. (2018). Control networks and hubs. Psychophysiology, 55(3), e13032. https://doi.org/10.1111/psyp.13032
- Gray, V., Douglas, K. M., & Porter, R. J. (2020). Emotion processing in depression and anxiety disorders in older adults: Systematic review. BJPsych Open, 7(1), e7. https://doi.org/10.1192/bjo.2020.143
- Guha, A., Yee, C. M., Heller, W., & Miller, G. A. (2021). Alterations in the default mode-salience network circuit provide a potential mechanism supporting negativity bias in depression. Psychophysiology, 58(12), e13918. https://doi.org/10.1111/psyp.13918
- Gunning, F. M., Oberlin, L. E., Schier, M., & Victoria, L. W. (2021). Brain-based mechanisms of late-life depression: Implications for novel interventions. Seminars in Cell & Developmental Biology, 116, 169–179. https://doi.org/10.1016/j.semcdb.2021.05.002
- Harrison, L. A., Kats, A., Williams, M. E., & Aziz-Zadeh, L. (2019). The Importance of Sensory Processing in Mental Health: A Proposed Addition to the Research Domain Criteria (RDoC) and Suggestions for RDoC 2.0. Frontiers in Psychology, 10, 103. https://doi.org/10.3389/fpsyg.2019.00103
- Hegarty, A. K., Yani, M. S., Albishi, A., Michener, L. A., & Kutch, J. J. (2020). Salience network functional connectivity is spatially heterogeneous across sensorimotor cortex in healthy humans. NeuroImage, 221, 117177. https://doi.org/10.1016/j.neuroimage.2020.117177
- Hsu, H. M., Yao, Z. F., Hwang, K., & Hsieh, S. (2020). Between-module functional connectivity of the salient ventral attention network and dorsal attention network is associated with motor inhibition. PloS One, 15(12), e0242985. https://doi.org/10.1371/journal.pone.0242985
- Hugdahl, K., Kazimierczak, K., Beresniewicz, J., Kompus, K., Westerhausen, R., Ersland, L., Gruner, R., & Specht, K. (2019). Dynamic up- and down-regulation of the default (DMN) and extrinsic (EMN) mode networks during alternating task-on and task-off periods. PloS One, 14(9), e0218358. https://doi.org/10.1371/journal.pone.0218358
- Le, T. M., Borghi, J. A., Kujawa, A. J., Klein, D. N., & Leung, H. C. (2017). Alterations in visual cortical activation and connectivity with prefrontal cortex during working memory updating in major depressive disorder. NeuroImage. Clinical, 14, 43–53. https://doi.org/10.1016/j.nicl.2017.01.004
- Lenhard, W., & Lenhard, A. (2016). Calculation of effect sizes. Retrieved from: https://www.psychometrica.de/effect_size.html. Psychometrica. DOI: 10.13140/RG.2.2.17823.92329
- Li, W., Wang, Y., Ward, B. D., Antuono, P. G., Li, S. J., & Goveas, J. S. (2017). Intrinsic inter-network brain dysfunction correlates with symptom dimensions in late-life depression. Journal of Psychiatric Research, 87, 71–80. https://doi.org/10.1016/j.jpsychires.2016.12.011
- Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. https://doi.org/10.1016/j.tics.2011.08.003
- Raichle, M. E. (2011). The restless brain. Brain Connect 1(1), 3–12. https://doi.org/10.1089/brain.2011.0019
- Raichle, M. E. (2015). The brain’s default mode network. Annual Review of Neuroscience, 38, 433–447. https://doi.org/10.1146/annurev-neuro-071013-014030
- Reed, A. E., & Carstensen, L. L. (2012). The theory behind the age-related positivity effect. Frontiers in Psychology, 3, 339. https://doi.org/10.3389/fpsyg.2012.00339
- Respino, M., Jaywant, A., Kuceyeski, A., Victoria, L. W., Hoptman, M. J., Scult, M. A., Sankin, L., Pimontel, M., Liston, C., Belvederi Murri, M., Alexopoulos, G. S., & Gunning, F. M. (2019). The impact of white matter hyperintensities on the structural connectome in late-life depression: Relationship to executive functions. NeuroImage Clinical, 23, 101852. https://doi.org/10.1016/j.nicl.2019.101852
- Saberi, A., Mohammadi, E., Zarei, M., Eickhoff, S. B., & Tahmasian, M. (2022). Structural and functional neuroimaging of late-life depression: A coordinate-based meta-analysis. Brain Imaging and Behavior, 16(1), 518–531. https://doi.org/10.1007/s11682-021-00494-9
- Schmidt, P. (2017). Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging. PhD diss., Chapter 6.1, Ludwig-Maximilians-Universität]. http://nbn-resolving.de/urn:nbn:de:bvb:19-203731.
- Sheikh, J. I., & Yesavage, J. A. (1986). Geriatric depression scale (GDS) Recent evidence and development of a shorter version. Clinical Gerontologist, 5(1-2), 165–173. https://doi.org/10.1300/J018v05n01_09
- Steptoe, A., Deaton, A., & Stone, A. A. (2015). Subjective wellbeing, health, and ageing. Lancet (London, England), 385(9968), 640–648. https://doi.org/10.1016/S0140-6736(13)61489-0
- Tadayonnejad, R., & Ajilore, O. (2014). Brain network dysfunction in late-life depression: A literature review. Journal of Geriatric Psychiatry and Neurology, 27(1), 5–12. https://doi.org/10.1177/0891988713516539
- Uddin, L. Q., Yeo, B., T., T., & Spreng, R. N. (2019). Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topography, 32(6), 926–942. https://doi.org/10.1007/s10548-019-00744-6
- Valkanova, V., & Ebmeier, K. P. (2013). Vascular risk factors and depression in later life: A systematic review and meta-analysis. Biological Psychiatry, 73(5), 406–413. https://doi.org/10.1016/j.biopsych.2012.10.028
- van Agtmaal, M., J., M., Houben, A., Pouwer, F., Stehouwer, C., D., A., & Schram, M. T. (2017). Association of microvascular dysfunction with late-life depression: A systematic review and meta-analysis. JAMA Psychiatry, 74(7), 729–739. https://doi.org/10.1001/jamapsychiatry.2017.0984
- Vatansever, D., Menon, D. K., Manktelow, A. E., Sahakian, B. J., & Stamatakis, E. A. (2015). Default mode network connectivity during task execution. NeuroImage, 122, 96–104. https://doi.org/10.1016/j.neuroimage.2015.07.053
- Voss, M. W., Sutterer, M., Weng, T. B., Burzynska, A. Z., Fanning, J., Salerno, E., Gothe, N. P., Ehlers, D. K., McAuley, E., & Kramer, A. F. (2019). Nutritional supplementation boosts aerobic exercise effects on functional brain systems. Journal of Applied Physiology (Bethesda, Md. : 1985), 126(1), 77–87. https://doi.org/10.1152/japplphysiol.00917.2017
- Wang, L., Chou, Y. H., Potter, G. G., & Steffens, D. C. (2015). Altered synchronizations among neural networks in geriatric depression. BioMed Research International, 2015, 343720. https://doi.org/10.1155/2015/343720
- Wardlaw, J. M., Valdes Hernandez, M. C., & Munoz-Maniega, S. (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. Journal of the American Heart Association, 4(6), 001140. https://doi.org/10.1161/JAHA.114.001140
- Wei, J., Hou, R., Zhang, X., Xu, H., Xie, L., Chandrasekar, E. K., Ying, M., & Goodman, M. (2019). The association of late-life depression with all-cause and cardiovascular mortality among community-dwelling older adults: Systematic review and meta-analysis. The British Journal of Psychiatry : The Journal of Mental Science, 215(2), 449–455. https://doi.org/10.1192/bjp.2019.74
- Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. https://doi.org/10.1089/brain.2012.0073
- Wu, M., Andreescu, C., Butters, M. A., Tamburo, R., Reynolds, C. F., & Aizenstein, H. (2011). Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Research, 194(1), 39–46. https://doi.org/10.1016/j.pscychresns.2011.04.003
- Yeshurun, Y., Nguyen, M., & Hasson, U. (2021). The default mode network: Where the idiosyncratic self meets the shared social world. Nature Reviews. Neuroscience, 22(3), 181–192. https://doi.org/10.1038/s41583-020-00420-w
- Yin, Y., He, X., Xu, M., Hou, Z., Song, X., Sui, Y., Liu, Z., Jiang, W., Yue, Y., Zhang, Y., Liu, Y., & Yuan, Y. (2016). Structural and functional connectivity of default mode network underlying the cognitive impairment in late-onset depression. Scientific Reports, 6, 37617. https://doi.org/10.1038/srep37617
- Zhou, H. X., Chen, X., Shen, Y. Q., Li, L., Chen, N. X., Zhu, Z. C., Castellanos, F. X., & Yan, C. G. (2020). Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage, 206, 116287. https://doi.org/10.1016/j.neuroimage.2019.116287
